Published online Sep 26, 2016. doi: 10.4252/wjsc.v8.i9.297
Peer-review started: May 27, 2016
First decision: July 6, 2016
Revised: July 12, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: September 26, 2016
Processing time: 116 Days and 4.2 Hours
Mesenchymal stem cells (MSCs) have been used to treat patients suffering from acute myocardial infarction (AMI) and subsequent heart failure. Although it was originally assumed that MSCs differentiated into heart cells such as cardiomyocytes, recent evidence suggests that the differentiation capacity of MSCs is minimal and that injected MSCs restore cardiac function via the secretion of paracrine factors. MSCs secrete paracrine factors in not only naked forms but also membrane vesicles including exosomes containing bioactive substances such as proteins, messenger RNAs, and microRNAs. Although the details remain unclear, these bioactive molecules are selectively sorted in exosomes that are then released from donor cells in a regulated manner. Furthermore, exosomes are specifically internalized by recipient cells via ligand-receptor interactions. Thus, exosomes are promising natural vehicles that stably and specifically transport bioactive molecules to recipient cells. Indeed, stem cell-derived exosomes have been successfully used to treat cardiovascular disease (CVD), such as AMI, stroke, and pulmonary hypertension, in animal models, and their efficacy has been demonstrated. Therefore, exosome administration may be a promising strategy for the treatment of CVD. Furthermore, modifications of exosomal contents may enhance their therapeutic effects. Future clinical studies are required to confirm the efficacy of exosome treatment for CVD.
Core tip: Exosomes are membrane vesicles that contain and transport specific bioactive molecules, such as proteins, messenger RNAs, and microRNAs, to recipient cells. In this review, we describe the mechanisms of exosome biogenesis, selective sorting of bioactive molecules into exosomes, and exosome secretion. We also discuss preclinical studies in which stem cell-derived exosomes were successfully used to treat cardiovascular disease (CVD). Finally, we discuss the future possibility of exosome-based clinical treatment of CVD.
- Citation: Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J Stem Cells 2016; 8(9): 297-305
- URL: https://www.wjgnet.com/1948-0210/full/v8/i9/297.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i9.297
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide. Owing to recent advances in the treatment of acute myocardial infarction (AMI) using percutaneous coronary intervention or bypass surgery, the survival of patients with AMI has substantially improved. However, many of these survivors develop heart failure (HF) as a result of the death of cardiomyocytes and subsequent tissue remodeling. As the induction of the proliferation and differentiation of the remaining cardiac tissue to regenerate heart structure remains challenging, heart transplantation is still the only treatment option for fatal HF. The development of new therapies for AMI and HF is thus required to improve the outcome in these patients.
Recently, many attempts have been made to improve the outcome of AMI and ischemic HF (IHF) using stem cells in preclinical[1-4] and clinical[5-10] studies. Among of the various stem cells, mesenchymal stem cells (MSCs), particularly bone marrow-derived MSCs, have been used to treat patients with AMI and IHF in clinical trials, with their safety and efficacy demonstrated in some studies[5-10]. The earliest preclinical studies suggested that MSCs have the potential to differentiate into multiple cardiac cell types including cardiomyocytes, vascular endothelial cells, and vascular smooth muscle cells[1-3]. However, subsequent studies did not demonstrate this remarkable differentiation capacity of MSCs. Rather, it was reported that most intravenously injected cells are trapped in the lung rather than engrafted in the heart[11,12]. Even when MSCs are administered to the swine heart via the coronary artery following AMI induction, only 6% of the injected cells remained in the infarct zones 14 d after AMI induction[11]. Furthermore, the supernatant of MSC cultures reportedly improves cardiac function[13-15]. These results suggest that MSCs improve cardiac function via the secretion of paracrine factors rather than via the direct differentiation of MSCs into cardiac cell types. Furthermore, MSC transplantation has several problems such as low survival rate and stem cell tumorigenesis[16]. However, if MSC-secreted paracrine factors can efficiently repair and regenerate cardiac tissues, cell-free therapy is possibly a safer alternative in the future.
Recently, a variety of cell types, including stem cells, have been shown to secrete paracrine factors in not only naked forms but also membrane vesicles, such as exosomes, microvesicles, ectosomes, membrane particles, exosome-like vesicles, and apoptotic bodies[17]. Exosomes are one of the secreted vesicles (also referred to as extracellular vesicles or EVs) that are 30-100 nm in diameter and contain a variety of biologically active molecules, such as proteins, messenger RNAs (mRNAs), and microRNAs (miRs)[18]. In this manuscript, we review the characteristics of exosomes and their possible applications in CVD treatment.
Several strategies have been used to isolate exosomes from tissues. These strategies utilize ultracentrifugation, size-based purification, precipitation using polymers, and immunoaffinity purification as reviewed in some reports[19-21]. Ultracentrifugation is the most established method of exosome isolation which employs sequential centrifugation combined with sucrose density gradient ultracentrifugation. Size-based purification includes ultrafiltration and gel filtration methods. Alternatively, polymers such as polyethylene glycol, widely used to precipitate proteins and viruses, can also be used to precipitate exosomes. As exosomes express specific proteins and lipids on their surface, antibodies recognizing these molecules (frequently conjugated with magnetic beads) are also used in their isolation.
Identification of exosomes is usually achieved by evaluating their morphology and size, their motion in a solution, and the specific molecules they express, as previously reviewed[22,23]. Electron microscopy is commonly employed to measure the size and assess the morphology of exosomes. The number of particles corresponding to exosome size can be counted by nanoparticle tracking analysis. This method utilizes the phenomenon of Brownian motion in a liquid suspension to measure particle size. Because exosomes are derived from endosomes and are finally released from cells as described in the following section, molecules involved in exosome formation, such as tetraspanins (CD81, CD9, and CD63), are expressed in exosomes. These markers can be used to identify exosomes.
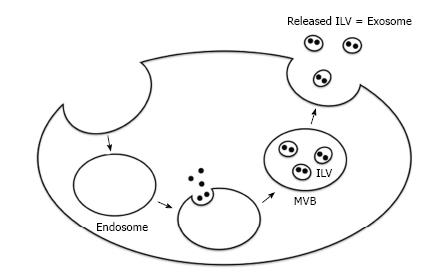
Exosomes are derived from endosomes that are formed by the inward budding of the plasma membrane (Figure 1)[18]. The subsequent inward budding of the endosomal membrane results in the formation of intraluminal vesicles (ILVs) into which cytoplasmic molecules, such as proteins, mRNAs, and miRs are sorted[24,25]. These endosomes containing ILVs, or multivesicular bodies (MVBs)[18], fuse with the plasma membrane and release ILVs into the extracellular environment by exocytosis. These secreted ILVs containing biologically active molecules are referred to as exosomes.
The mechanisms of exosome formation and processing are just starting to be revealed. The formation of MVBs is reportedly mediated by the endosomal sorting complexes required for transport (ESCRT) system or by systems independent of the ESCRT machinery as summarized in some reviews[26-28]. The ESCRT machinery comprises four protein complexes, ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III, together with accessory proteins. ESCRT-0 recognizes ubiquitinated proteins and is recruited to the endosomal membrane, where it initiates processes leading to the uptake of ubiquitinated proteins into ILVs. ESCRT-0 subsequently recruits ESCRT-I to the endosomal membrane, which in turn recruits ESCRT-II and ESCRT-III. ESCRT-III induces the inward budding of the endosomal membrane and formation of ILVs, while accessory proteins (particularly the vacuole protein sorting gene 4 ATPase or VPS4) are implicated in the dissociation and recycling of the ESCRT machinery. In addition, other molecular pathways mediate ESCRT-independent MVB formation including tetraspanins[29] such as CD81, CD9, and CD63, and proteolipid proteins such as ceramide[30].
The docking and fusion of MVBs to the plasma membrane appear to be mediated by soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) proteins such as vesicle-associated membrane protein 7 (VAMP7)[31]. The release of ILVs (exosomes) from cells following the fusion of MVBs to the plasma membrane is mediated by several mechanisms. The small GTPases of the Rab family (Rab27a/b, Rab11, and Rab35) are the most studied molecules involved in exosome release[32-34]. Other pathways include WNT5A, glycosphingolipids, flotillins, and stress-induced stimuli such as the increase in intracellular calcium concentration, DNA damage, heat shock, and hypoxia[35-39]. In addition, an acidic environment has been shown to trigger the secretion of exosomes from cells[40].
Once released from cells, exosomes bind to target cells via ligand-receptor interactions. Molecules, such as integrins, intercellular adhesion molecules, and tetraspanins seem to be implicated in the binding of exosomes to recipient cells[41-43]. After binding, exosomal contents are reportedly internalized by recipient cells via two major mechanisms as summarized in some reviews[23,44]: (1) exosome fusion with the plasma membrane of recipient cells and direct release of contents into the cytoplasm; or (2) internalization by endocytosis into recipient cells. It has been demonstrated that bioactive molecules in exosomes are not only transferred to recipient cells but also exert functional effects[45-47].
Although the precise mechanism remains unknown, a specific set of proteins, mRNAs, and miRs are selectively accumulated within exosomes[48]. It has also been demonstrated that exosomes contain a distinct set of mRNAs compared to the donor cells[49]. Ubiquitination appears to be required for the uptake of some proteins into exosomes[50], although ubiquitination-independent accumulation of proteins has also been reported[51]. The accumulation of miRs into the exosomes of T cells appears to require the recognition of a GGAG sequence located in miRs by the heterogeneous nuclear ribonucleoprotein hnRNPA2B1[49].
Taken together, accumulating evidence indicates that exosomes are a natural vehicle for the efficient and specific transport of biologically active cargo into recipient cells. These properties may be exploited for the delivery of bioactive molecule such as miRs and chemical compounds such as drugs. For instance, stem cell-derived exosomes may be useful for CVD treatment. We review the potential utility of stem cell-derived exosomes for CVD treatment in the following section.
Several preclinical studies have demonstrated the efficacy of MSC-derived exosomes for CVD treatment (Table 1). Lai et al[52] found that the supernatant of human embryonic stem cell (ESC)-derived MSCs contained small particles (50-100 nm in diameter) corresponding to exosomes. When administered to a mouse model of myocardial ischemia/reperfusion injury, these exosomes remarkably reduced infarct size. The same group also administered exosomes secreted from human ESC-derived MSCs to a mouse model of AMI and demonstrated improved cardiac function[53]. In addition, they found that the tissue levels of ATP and nicotinamide adenine dinucleotide were significantly increased, while those of reactive oxygen species were significantly decreased after exosome administration. Furthermore, they demonstrated that the phosphorylation of Akt and glycogen synthase kinase 3 (that has anti-apoptotic effects) significantly increased and that of c-jun N-terminal kinase (that has proapoptotic effects) significantly decreased in cardiac tissue following exosome administration. Bian et al[54] demonstrated the proliferation and migration of human umbilical vein endothelial cells in response to EVs (100 nm in diameter) collected from human MSCs. They also administered MSC-derived EVs to a rat model of AMI and showed that MSC-derived EV administration significantly reduced infarct size, restored cardiac function, and stimulated angiogenesis in the ischemic zone. Feng et al[55] demonstrated that exosomes secreted from mouse MSCs following ischemic preconditioning contained a large amount of miR-22. When administered to mice with AMI, these miR-22-enriched exosomes exerted an anti-apoptotic effect on cardiomyocytes via the downregulation of methyl-CpG-binding protein 2. Yu et al[56] used MSCs overexpressing the transcription factor GATA-4 (MSC_GATA-4) and demonstrated that the administration of MSC_GATA-4-derived exosomes restored cardiac function and reduced infarct size in a rat model of AMI. The authors also showed that MSC_GATA-4-derived exosomes expressed a greater amount of miRs, particularly miR-19a, than control MSCs and that miR-19a appeared to be involved in the cardioprotective effect of MSC_GATA-4-derived exosomes via the downregulation of phosphatase and tensin homolog (PTEN) and subsequent activation of anti-apoptotic Akt and extracellular signal-regulated kinase.
| Origin of exosomes | Experimental model | Findings | Ref. |
| Human ESC-derived MSCs | AMI | Reduction in infarct size Recovery of cardiac function Decreased oxidative stress Activation of Akt and GSK3 Inhibition of c-JNK | Lai et al[52,53] |
| Human MSCs | AMI | Reduction in infarct size Recovery of cardiac function Increased angiogenesis | Bian et al[54] |
| Mouse MSCs | AMI | Exosomes were enriched in miR-22 miR22 was implicated in the anti-apoptotic effect of exosomes | Feng et al[55] |
| Rat MSCs overexpressing GATA-4 | AMI | Reduction in infarct size Recovery of cardiac function Exosomes were enriched in miR-19a | Yu et al[56] |
| Rat MSCs | Stroke | Recovery of neurological function Stimulation of neurogenesis and angiogenesis | Xin et al[57] |
| Rat MSCs overexpressing miR-133b and those whose expression of miR-133b was knocked down | Stroke | Recovery of neurological function was mediated by miR-133b expressed in exosomes | Xin et al[58] |
| Mouse MSCs | Pulmonary hypertension | Reduction in the progression of pulmonary hypertension and right ventricular hypertrophy | Lee et al[59] |
| Mouse CPCs | AMI | Suppression of apoptosis | Chen et al[60] |
| Human CPCs | AMI | Recovery of cardiac function Suppression of apoptosis Stimulation of angiogenesis | Barile et al[61] |
| Human CPCs | AMI | Recovery of cardiac function Suppression of apoptosis Stimulation of angiogenesis miR-146a was enriched in exosomes and partially mediated their function | Ibrahim et al[62] |
| Mouse ESCs | AMI | Recovery of cardiac function Stimulation of angiogenesis and cardiomyocyte survival Stimulation of the survival and proliferation of CPCs miR-294 was enriched in exosomes and miR-294 promoted the survival and proliferation of CPCs | Khan et al[63] |
| Human CD34+ cells | Matrigel plug assay Corneal angiogenesis assay | Promotion of angiogenesis | Sahoo et al[64] |
| Human CD34+ cells expressing SHH | AMI | Recovery of cardiac function SHH was enriched in exosomes and transferred to recipient cells | Mackie et al[66] |
Preclinical studies have also reported favorable effects of exosome administration on neurological recovery following stroke induction. Xin et al[57] found that the systemic administration of rat MSC-derived exosomes following the induction of stroke by the ligation of the middle cerebral artery significantly accelerated neurological recovery and stimulated neurogenesis and angiogenesis at the border zone between normal and ischemic tissues. The same group also demonstrated that the administration of MSCs overexpressing miR-133b (MSCs_miR-133b+) enhanced the recovery of neurological function in a rat stroke model whereas MSCs with miR-133b knockdown (MSCs_miR-133b-) did not[58]. Furthermore, they showed that the level of miR-133b in exosomes isolated from cerebrospinal fluid was higher in the group that received MSCs_miR-133b+. They also demonstrated that MSC-derived exosomes could be transferred to neighboring cells. Finally, they showed that the expression of connective tissue growth factor (CTGF), a target for miR-133b, was significantly reduced in the ischemic boundary zone following MSCs_miR-133b+ administration, while CTGF expression remained unchanged after MSCs_miR-133b- administration. They concluded that miR-133b derived from exosomes was implicated in MSC-mediated recovery of neurological function in this model.
The beneficial effects of MSC-derived exosome administration have also been reported in a mouse model of hypoxic pulmonary hypertension. Lee et al[59] demonstrated that the administration of MSC-derived exosomes significantly ameliorated the progression of pulmonary hypertension and right ventricular hypertrophy, possibly via the suppression of signal transducer and activator of transcription 3 (STAT3).
Chen et al[60] demonstrated that the injection of exosomes isolated from murine cardiac progenitor cells (CPCs) into the murine heart following ischemia/reperfusion injury significantly suppressed apoptosis. Barile et al[61] demonstrated that the administration of EVs (most of which were exosomes) isolated from human CPCs significantly suppressed apoptosis, stimulated angiogenesis, and improved cardiac function in a rat model of AMI. They also showed that specific miRs, such as miR-210, miR-132, and miR-146a-3p, were enriched in CPC-derived exosomes. Ibrahim et al[62] reported that the administration of human CPC-derived exosomes in a mouse model of AMI significantly suppressed apoptosis, stimulated angiogenesis, and restored cardiac function. They also demonstrated that miR-146a was enriched in CPC-derived exosomes and that miR-146a administration partially mimicked the beneficial effects of CPC-derived exosomes on cardiac function.
Khan et al[63] reported that ESC-derived exosomes from mouse stimulated neovascularization, enhanced cardiomyocyte survival, and restored cardiac function in a mouse model of AMI. Furthermore, ESC-derived exosomes augmented the survival and proliferation of CPCs. miR-294 was enriched in ESC-derived exosomes and the treatment of CPCs with miR-294 promoted the progression of the cell cycle to the S phase, suggesting that ESC-derived exosomes transferred miRs, such as miR-294, to CPCs, which promoted the proliferation and survival of CPCs.
Sahoo et al[64] isolated exosomes from human CD34+ stem cells (which include endothelial progenitor cells[65]) and examined their proangiogenic activity. CD34+ stem cell-derived exosomes stimulated tube formation from cultured endothelial cells in Matrigel (in vitro assay), and promoted angiogenesis in vivo, as assessed by the Matrigel plug assay and the corneal angiogenesis assay. Mackie et al[66] demonstrated that CD34+ stem cells expressing the pro-angiogenic factor sonic hedgehog (SHH) restored cardiac function in a mouse model of AMI. They also showed that SHH was enriched in exosomes secreted from stem cells and that it was transferred to and expressed functionally in recipient cells, suggesting that exosome-mediated transfer of SHH to recipient cells accounts for the beneficial effects of stem cell administration in this model of AMI.
Collectively, these studies provide compelling evidence that exosomes derived from a variety of stem cells exert beneficial effects on animal models of CVD.
Although clinical trials using exosomes for CVD treatment have not yet started, exosome administration in humans has been tested, particularly for cancer immunotherapy[67-69]. Phase I and phase II studies have been performed and the safety of the treatment has been confirmed. Future clinical studies will be required to test the safety and efficacy of exosome treatment for CVD.
Given the low toxicity, high stability in the circulation, and high efficiency of transport to donor cells demonstrated by exosomes, several studies have attempted to augment the therapeutic efficacy by modifying exosomal content. For instance, small RNAs such as small interfering RNAs and miRs have been loaded into exosomes during exosome formation using lipofection or following exosome formation using electroporation[70-74]. These modified exosomes reportedly exerted biological effects in recipient cells[70-74]. Exosomes have also been used as vehicles to transport exogenous chemical compounds to recipient cells stably and efficiently, because some drugs are condensed in the exosomes of donor cells and transferred to recipient cells. Exosomes enriched in curcumin, an anti-inflammatory agent, or chemotherapeutic agents, such as paclitaxel and doxorubicin, have been used to transport these compounds to recipient cells, with their beneficial biological effects confirmed[75-78]. Another strategy that has been examined is the modification of exosomal membrane proteins to improve the efficiency of uptake by recipient cells. Alvarez-Erviti et al[70] prepared dendritic cells that expressed Lamp2b, an exosomal membrane protein, fused to a peptide fragment of neuron-specific rabies viral glycoprotein so that exosomes would be accumulated specifically in the brain. The authors demonstrated that these modified exosomes were specifically taken up by brain tissues when intravenously administered. Therefore, the modification of exosome structure will enhance the specificity and efficiency of transport and the modification of exosome content (for example, by inclusion of specific miRs) will enhance the therapeutic effect in the future.
It has been reported that MSC-derived exosomes promote tumor growth in vivo via the stimulation of vascular endothelial growth factor expression in tumor cells[79]. In most cases, the stimulation of angiogenesis appears to be favorable for the regeneration of cardiomyocytes after AMI. However, angiogenesis may stimulate tumor growth in other tissues. Therefore, it is desirable to explore a strategy to specifically deliver exosomes to target tissues.
Exosomes are one of the secreted vesicles that contain bioactive molecules, such as proteins, mRNAs, and miRs. Exosomes transfer these bioactive molecules to recipient cells, thus exerting biological effects. Preclinical studies have suggested that exosomes can be used for the treatment of CVD such as AMI and stroke. Future clinical studies are warranted to confirm the efficacy of exosome administration for CVD treatment. Furthermore, modifications of exosomal structure and content will enhance the efficacy of exosome administration for such treatments in the future.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: de Carvalho KAT, Georgescu A, Louboutin JP, Sumi S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919-1925; discussion 1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 610] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 2. | Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1548] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 3. | Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474-11479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 777] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 4. | Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 827] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 6. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 995] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 7. | Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, Herlihy JP, Fernandes MR, Cheong BY, Flamm SD. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF). Am Heart J. 2011;161:1078-1087.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 903] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 9. | Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 374] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 10. | Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Kwon W, Hong IS, Lee K. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29:23-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 455] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 12. | Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 925] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 13. | Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1261] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 14. | Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 883] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 15. | Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 16. | Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 301] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 17. | Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2703] [Cited by in RCA: 3103] [Article Influence: 193.9] [Reference Citation Analysis (0)] |
| 18. | Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta. 2015;1852:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 19. | Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M. Isolation of extracellular vesicles: Determining the correct approach (Review). Int J Mol Med. 2015;36:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 20. | Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 260] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 21. | van der Pol E, Böing AN, Gool EL, Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 22. | Kastelowitz N, Yin H. Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. Chembiochem. 2014;15:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997-5000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 705] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 25. | Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 1060] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 26. | Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 27. | Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 1048] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 28. | Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1376] [Article Influence: 125.1] [Reference Citation Analysis (0)] |
| 29. | Perez-Hernandez D, Gutiérrez-Vázquez C, Jorge I, López-Martín S, Ursa A, Sánchez-Madrid F, Vázquez J, Yáñez-Mó M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288:11649-11661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 387] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 30. | Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 2677] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 31. | Fader CM, Sánchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793:1901-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 32. | Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115:2505-2515. [PubMed] |
| 33. | Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 634] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 34. | Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19-30; sup pp 1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1525] [Cited by in RCA: 1957] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 35. | Savina A, Furlán M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083-20090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 654] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 36. | Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol. 2011;186:2219-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 37. | Ekström EJ, Bergenfelz C, von Bülow V, Serifler F, Carlemalm E, Jönsson G, Andersson T, Leandersson K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol Cancer. 2014;13:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 38. | Giricz Z, Varga ZV, Baranyai T, Sipos P, Pálóczi K, Kittel Á, Buzás EI, Ferdinandy P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 39. | Phuyal S, Hessvik NP, Skotland T, Sandvig K, Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014;281:2214-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 40. | Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211-34222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 1190] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 41. | Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci USA. 2003;100:6670-6675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 811] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 43. | Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zöller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 44. | Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 589] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 45. | Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8246] [Cited by in RCA: 9762] [Article Influence: 542.3] [Reference Citation Analysis (0)] |
| 46. | Ekström K, Valadi H, Sjöstrand M, Malmhäll C, Bossios A, Eldh M, Lötvall J. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracell Vesicles. 2012;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 48. | de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BW. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 490] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 49. | Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1504] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 50. | Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1091] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 51. | Marsh M, van Meer G. Cell biology. No ESCRTs for exosomes. Science. 2008;319:1191-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1675] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 53. | Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 866] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 54. | Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 539] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 55. | Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 56. | Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 371] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 57. | Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 751] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 58. | Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 580] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 59. | Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 60. | Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 61. | Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 560] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 62. | Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 662] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 63. | Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 590] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 64. | Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 497] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 65. | Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6624] [Cited by in RCA: 6337] [Article Influence: 226.3] [Reference Citation Analysis (1)] |
| 66. | Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111:312-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 67. | Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 1005] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 68. | Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 678] [Cited by in RCA: 882] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 69. | Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 637] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 70. | Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3883] [Cited by in RCA: 3564] [Article Influence: 254.6] [Reference Citation Analysis (0)] |
| 71. | Wahlgren J, De L Karlson T, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 552] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 72. | Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1269] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 73. | Cooper JM, Wiklander PB, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, Schapira AH, Simons JP, El-Andaloussi S, Alvarez-Erviti L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord. 2014;29:1476-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 403] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Li L, Yu J, Zhu D, Zhang Y, Li X, Gu H, Zhang CY, Zen K. Microvesicle-mediated delivery of transforming growth factor β1 siRNA for the suppression of tumor growth in mice. Biomaterials. 2014;35:4390-4400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1184] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 76. | Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1072] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 77. | Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia SM. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 676] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 78. | Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 1340] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 79. | Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |