Published online Sep 26, 2016. doi: 10.4252/wjsc.v8.i9.279
Peer-review started: May 27, 2016
First decision: June 17, 2016
Revised: July 12, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: September 26, 2016
Processing time: 115 Days and 11.9 Hours
Over the past two decades there has been remarkable progress in cancer diagnosis, treatment and screening. The basic mechanisms leading to pathogenesis of various types of cancers are also understood better and some patients, if diagnosed at a particular stage go on to lead a normal pre-diagnosis life. Despite these achievements, racial disparity in some cancers remains a mystery. The higher incidence, aggressiveness and mortality of breast, prostate and colorectal cancers (CRCs) in African-Americans as compared to Caucasian-Americans are now well documented. The polyp-carcinoma sequence in CRC and easy access to colonic epithelia or colonic epithelial cells through colonoscopy/colonic effluent provides the opportunity to study colonic stem cells early in course of natural history of the disease. With the advent of metagenomic sequencing, uncultivable organisms can now be identified in stool and their numbers correlated with the effects on colonic epithelia. It would be expected that these techniques would revolutionize our understanding of the racial disparity in CRC and pave a way for the same in other cancers as well. Unfortunately, this has not happened. Our understanding of the underlying factors responsible in African-Americans for higher incidence and mortality from colorectal carcinoma remains minimal. In this review, we aim to summarize the available data on role of microbiome and cancer stem cells in racial disparity in CRC. This will provide a platform for further research on this topic.
Core tip: The role of microbial dysbiosis and cancer stem cells (CSCs) in colorectal cancer (CRC) has been studied extensively, however, their implication in racial disparity is not well known. A number of recent studies have shown that different dietary patterns affect gut microbiome. Likewise, dietary patterns also affect intracellular regulatory events which may affect the function of CSCs. Our objective is to consolidate the available data, on the role of gut microbiome and CSCs in racial disparity in CRC, explore a link between them and lay a foundation for further advances.
- Citation: Goyal S, Nangia-Makker P, Farhana L, Yu Y, Majumdar AP. Racial disparity in colorectal cancer: Gut microbiome and cancer stem cells. World J Stem Cells 2016; 8(9): 279-287
- URL: https://www.wjgnet.com/1948-0210/full/v8/i9/279.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i9.279
Colorectal cancer (CRC) remains the second leading cause of cancer related mortality in the United States. However, the incidence and mortality of colon cancer is different among various racial and ethnic groups. African Americans (AAs) share the largest burden of CRC in the United States. Data from Surveillance, Epidemiology and End Results (SEER) revealed that the age-adjusted incidence of CRC in AAs, based on cases diagnosed between 2008 and 2012 was 52.3 per 100000 for men and women combined per year, compared to 41.5 per 100000 for men and women combined per year among Caucasian Americans (CAs). Similarly, the age-adjusted mortality from CRC in AAs, based on cases diagnosed between 2008 and 2012 was 21.4 per 100000 for men and women per year, compared to 15 per 100000 for men and women per year in Whites/CAs[1]. AAs not only tend to be diagnosed at a younger age but also have a worse prognosis than CAs[2,3]. Many genetic, epigenetic and environmental factors have been reported that are responsible for this racial disparity.
In recent years, there has been an increased focus on differences between microbiota of colon of healthy individuals and of patients with CRC. A relationship between microbial dysbiosis and CRC is now well established[4-7].
The concept that pluripotent and self-renewing cancer stem cells (CSCs) have a pivotal role in the development and progression of many malignancies, including CRC is now well accepted. We have reported a higher proportion of CSCs (specifically CD44+ CD166- phenotype) in AAs, who also had a significantly higher number of adenomas, compared to CAs[8]. However, underlying regulatory mechanisms remain unknown.
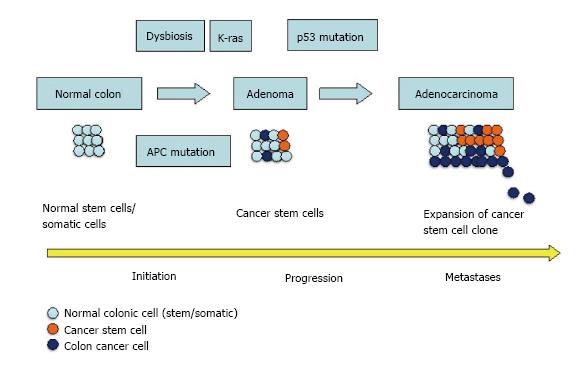
Recent studies have shown that host can alter gut microbiota through external (diet, obesity, etc.) and internal factors (microRNAs in intestinal epithelial cells)[9]. MicroRNAs (miRNAs) have also been reported to regulate CSCs[10]. Thus it is possible that the gut microbiota and CSCs are not entirely isolated domains and in AAs, the higher frequency of unfavorable mutations, through miRNAs, facilitates pathogenic microbiota over commensal bacteria (Figure 1).
Human gut is a major harbinger of a wide variety of microbial cells containing approximately 1014 cells estimating 1000 species. The dominant composition is bacteria with 90% of species belonging to Firmicutes and Bacteroides[11]. These bacteria are in a symbiotic relationship with the intestine, utilizing undigested nutrients as substrates and in return produce various vitamins, amino acids, transform bile salts and assist in maintenance of intestinal barrier, appropriate immune response against pathogens[12]. This homeostasis is altered in a state of dysbiosis, which is overgrowth of pathogenic bacteria that are normally inhibited by commensal bacteria.
Numerous studies have been performed to examine whether and to what extent the dietary changes may affect gut microbiota. In general, these studies suggest that changes in diet and their interaction with gut microbiota exert profound effects on intestinal homeostasis through various metabolites[13]. Emergence of metagenomic sequencing has enabled identification of microorganisms not possible with 16S ribosomal RNA gene (rRNA) sequence-based methods and traditional culture methods[13-15]. Collective genomes of the members of a microbial community are analyzed against widely available microbial databases, thus allowing identifying microbial communities, which are virtually uncultivable. This has led to discovery of hundreds of microbial genus not previously known to exist in the human gut.
Qin et al[11] published a comprehensive catalogue of human gut microbial genes in 2010. Among the various conclusions, one was that Fusobacterium genus is not an abundant constituent of the normal gut microbiota. It is a genus of anaerobic gram-negative bacilli and has been known to cause periodontal disease. Fusobacterium species esp. F. nucleatum has been isolated from colon and fecal samples of patients with CRC in multiple studies[16-18]. Castellarin et al[19] even found a positive association between Fusobacterium and lymph node metastases.
Gao et al[4] examined microbiota from cancerous tissues of CRC patients and found a significant abundance of Firmicutes and Fusobacteria compared to healthy individuals. Interestingly they also found that Proteobacteria was less abundant in patients with CRC. In the first large series sequencing of stool samples, Sobhani et al[5] reported that Bacteroides/Prevotella were markedly increased in patients with CRC.
Dietary components like vegetables, fiber, vitamin D are shown to be associated with a lower risk of colon cancer whereas red meat and a diet rich in saturated animal fat has been shown to be responsible for an increased risk of colon cancer[20,21]. Two major biotransformation pathways for dietary components mediated by microbiome have been reported.
A diet rich in fiber stimulates saccharolytic fermentation and production of short chain fatty acids (SCFAs) namely butyrate, acetate and propionate. These metabolites, particularly butyrate have anti-inflammatory, anti-proliferative and antineoplastic properties, while a fat rich diet stimulates the synthesis and release of bile acids in the gut[22,23].
In their study involving four racial groups, Hester et al[24] found that SCFA level was lower in stool from African-Americans than other racial groups. Interestingly, they also found a decreased prevalence of bacteria of Lachnospiraceae family in stool from African-American patients. Bacteria of Lachnospiraceae family have been previously shown to be associated with butyrate production in colon tissue[25]. A summary depicting bacteria, whose presence has been shown to have or probably has a positive or negative association with colon cancer in AAs has been shown in Table 1.
| Positive association | Negative association |
| Fusobacterium | Lactobacillus |
| Firmicutes | Lachnospiracea |
| Bacteroides | Eubacterium |
| Bifidobacterium |
It has been widely reported that the higher amount of butyrate is seen in stool of healthy controls than CRC patients[26]. On the other hand secondary bile acids have been postulated to have a carcinogenic role[27].
Ou et al[28] examined stool from healthy AAs and from age and sex-matched native Africans and found a higher concentration of fecal secondary bile acid in AAs and a higher concentration of short-chain fatty acids in native Africans. Although the reason(s) for these increases are not known, it is possible that changes in dietary habits are responsible for these differences.
Majority of the primary bile acids are returned to the liver by the enterohepatic circulation. A fraction of the primary bile acids escapes the enterohepatic circulation and reaches the colon. In the colon, 7-DHC (Dehydrocholesterol), converts primary bile acids into secondary bile acids, like deoxycholic acid and lithocholic acid. There is plenty of evidence to suggest that when exposed to high levels of bile acids, gastrointestinal cells undergo oxidative and nitrosative stress leading to anti-apoptotic and mutagenic properties[29]. De Boever et al[30] demonstrated the protective effect of Lactobacillus against bile salt cytotoxicity. Many studies have found that African-Americans have a lower prevalence of Lactobacillus, compared to other racial groups[31,32].
Moore and Moore[31] studied the stool microbial composition in populations with higher (CAs, patients with polyp) and lower CRC risk (South African blacks, native Japanese). They found a positive association of Bacteroides and Bifidobacterium, and a negative association of Lactobacillus and Eubacterium aerofaciens, with colon cancer risk.
These studies provide ample evidence that a variation in microbial composition between ethnic groups may partly be responsible in colorectal carcinogenesis and that diet plays a role in this microbial diversity.
According to the stem cell model of carcinogenesis, only some cells in a tissue possess the ability to initiate and sustain tumor growth. These cells, characterized as CSCs have two important properties: Indefinite proliferation and pleuripotency (ability to differentiate itself into more than one cell lineage)[33]. The critical role of CSCs in initiation, development and progression of CRC is now well established[34]. Mutations in normal stem, progenitor or terminally differentiated cells, are believed to be responsible for origin of CSCs, but the processes responsible are not completely known.
Colon stem cells are believed to exist as undifferentiated cells at the base of the crypt of lieberkuhn in the proliferative zone. The undifferentiated cells differentiate in to specialized cells as they move up the crypt-villic axis towards the luminal surface. It is estimated that, in human adults in every square centimeter of colon, there are about 14000 crypts and at a given rate of 5 d for colonic epithelium renewal; over 6 × 1014 colonocytes are produced during the individual lifetime[35,36]. The lifelong proliferation of the stem cells makes them more prone to accumulation of mutations than other short-lived cells.
Various pathways tightly regulate the processes involved in maintenance of a normal intestinal epithelium. The central among those is the canonical Wnt pathway. Canonical Wnt signals are transduced through an interaction with Frizzled family receptors (Fz) and LRP5/LRP6 (low-density lipid receptor) co-receptor to the β-catenin signaling pathway. In the absence of Wnt signaling, β-catenin becomes a part of a multiprotein degradation complex, containing tumor suppressor gene product adenomatous polyposis coli (APC), scaffold protein Axin and is phosphorylated by casein kinase Iα and glycogen synthase kinase 3β, and then ubiquinated for subsequent proteosome degradation.
In the presence of Wnt signaling, after signal transduction, Axin is recruited to cell membrane by a Fz-Disheveled (DVL) or LRP5/6 interaction. This leads to degradation of the degradation complex described above and β-catenin buildup in the nucleus. This stable nonphosphorylated β-catenin complexes with several factors and leads to activation of the transcription of several genes like c-Myc, CD44, CCND1, essential for DNA replication, cell cycle control and altered mitosis[37,38].
Characterization of CSCs: Identification of CSCs is a challenging task given the complexity of the cell surface markers, and their difference between various tissues, apart from the technical issues involved. One of the methods used to identify CSC is by the cell surface markers, also known as epitopes. Colonosphere formation, a functional assay is also used to characterize CSCs.
Colon CSCs have been identified by expression of numerous surface epitopes CD133, CD24, CD44, CD166, EPCAM (epithelial specific antigen/ESA), etc[39]. CD166 expression has been linked with shortened survival[40]. Similarly, CD44’s role in tumor invasiveness and progression has prompted it to be described as a potential CSC marker for CRC[41]. It has been shown that CSCs form tumors in SCID mice at much-diluted concentrations, which histologically resemble the primary tumor[42].
We studied the role of CD44, CD166 and ESA in CRC and reported their expression in premalignant adenomatous polyp[43] and also showed an age dependent increase in their expression, suggesting their role in tumor development and progression[43].
We have also recently observed that CD44+CD166- cell proportion in the colonic effluent as well as in the colonic mucosal cells is significantly increased in AAs with adenomas than CAs. We also observed that the colonic effluent from high risk AAs (more than 3 adenomas) contained markedly higher proportion of CD44+ CD166- cells than low risk AAs (subjects with no adenomas). We were not able to duplicate these results in colonic effluent from white population[8]. Taken together, the above observations point towards the substantial role of CSCs, not only in higher incidence, but also progression of CRC in AAs.
Racial disparity in miRNAs and signaling pathways regulating CSCs: According to the well-accepted Fearon and Vogelstein model of CRC progression, development of CRC is an outcome of accumulation of mutations in tumor suppressor genes, oncogenes; and accumulation of changes is more important than the sequence of changes[44]. This is also the basis of “adenoma-carcinoma model” in which APC gene mutation initiates the sporadic CRC, which accounts for 80%-85% CRC, followed by mutations in other genes-notably K-ras, deleted in colorectal carcinoma and p53[45]. Mutated APC, in association with β-catenin up regulates many oncogenes, notably CCND1 and c-myc[46,47]. We have recently reported that AAs had higher (48%) number of adenomas, recorded during colonoscopy, than CAs[8]. These findings confirm the data in separate studies by Corley et al[48] and Lebwohl et al[49]. In line with the Fearon and Vogelstein model, one of the reasons, in AAs, for a higher incidence of CRC could be the higher number of adenomas in them.
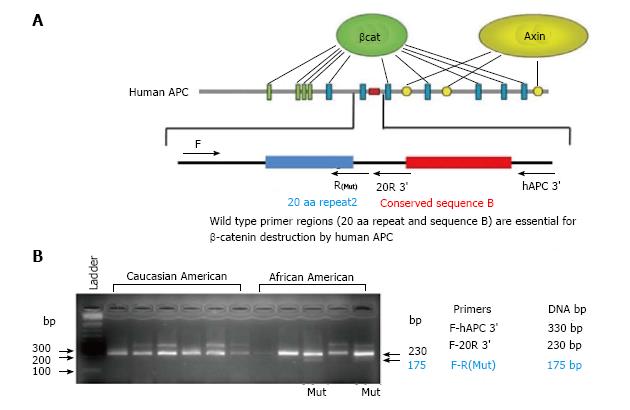
We also examined the rates of mutation of APC and β-catenin, in a small cohort of AAs (n = 10) and CAs (n = 10), The agarose gel electrophoresis of the PCR products of wild type and mutant APC gene (hAPC) in colonic biopsies from 5AAs and 6 CAs without adenomas is shown in Figure 2. Out of 10 AAs, 2 showed mutation in APC gene, whereas none of the CAs showed mutation in the gene. Similarly, 3 AAs showed mutation in β-catenin, as compared to none of the CAs. This preliminary data clearly supports the role of APC and β-catenin mutations in higher incidence of CRC in AAs.
MicroRNAs (miRNAs) are an expansive class of small non-coding RNAs, 18-23 nucleotides long, and regulate gene expression, either by translational repression or by mRNA degradation through cleavage. MiRNAs are atypically expressed in numerous pathological states, and depending on the target, may work as oncogenes or tumor suppressors. MiRNAs’ role in CRC regulation through CSCs is well researched[50-52]. We have examined the role of miRNAs 21 and 145 in regulating colon CSCs and reported that the expression of miR-21 is significantly increased and that of miR-145 markedly decreased in chemotherapy-resistant colon cancer cells, highly enriched in CSCs[53]. In colon cancer cells, forced expression of miR-145, significantly inhibits CSCs and tumor growth, whereas up-regulation of miR-21 augments the same[8]. We have also shown the role of miR-21 in age related rise of colon cancer. Upregulated miR-145 was associated with reduced levels of CD44, and β-catenin[53], both of which, we have been shown to be independently associated with racial disparity of CRC.
These observations led us to explore the role of miR-21 in ethnic differences in CRC and/or its precursor, adenoma. Ongoing studies (unpublished data) from our lab revealed that miR-21 levels in normal looking colon mucosa of AAs with adenomas were significantly higher than their CAs counterparts.
Mutation in K-ras gene, second most common in CRC progression, is not required for initiation of CRC. Reduced expression of miR-145 has been shown to contribute to CRC development through K-ras expression[54]. We have reported that K-RAS’ lack in chemo-resistant colon cancer cells upregulates miR-145, downregulates miR-21, as well as disrupts the negative cooperation among miR-21 and miR-145[53]. Epidermal growth factor receptor (EGFR) is another transmembrane protein, whose role is well established in CRC pathogenesis. We have reported that EGFR inhibitor Cetuximab decreases miR-21 expression, suggesting another link between stem cells and definitive mutations in CRC[55].
Mutation in p53 gene has been shown to facilitate not only growth, but also invasiveness in colorectal adenomas. Therefore, p53 is implicated in the adenoma-carcinoma sequence[56]. P53 mutations have also been associated with altered miRNA processing[57].
We have recently reported a significant increase in miR-1207-5p in colonic mucosal cells cultured in stem cell media (enriched for CSCs) and CD44+CD166-cells isolated via flow cytometry, from AAs with adenomas. Additionally, colon cancer cell lines HCT-116 and HT-29 showed a significant increase in miR-1207-5p, compared to normal colonic epithelial cells, HCoEpiC and CCD841[8]. This lays further weight to the role of miRNAs in promoting stem cell-like properties in colon epithelial cells.
A recent whole exome sequencing study on tissues from AAs with CRC identified somatic mutations in APC. This also supports the role of mutations in the key protein in Wnt/β-catenin signaling pathway-APC in pathogenesis of CRC[58].
Stemness and epithelial to mesenchymal transition: A tremendous problem in management of cancer is cancer recurrence. Inspite of modern breakthroughs, in CRC, the degree of recurrence is as high as 40%-60%[59].
In any cancer, the capacity of few cells to isolate themselves from an initial site and generate a secondary tumor after implantation at a second site, defines the property of recurrence and metastases. A variety of genetic changes take place via a process called EMT (epithelial-mesenchymal transition) that equips CSCs to invade other tissues and survive under attachment free conditions. In addition to mutations in APC, K-RAS, p53 described above, activation of signaling pathways like Wnt/β-catenin, TGF-β, notch, and hedgehog is a very critical step in EMT[60,61].
The Wnt/β-catenin signaling described above regulates EMT by downstream controlling of SNAIL, TWIST, SLUG, which in turn control the expression of effectors of EMT like Vimentin, E-cadherin, and N-cadherin[62-64].
TGF-β signaling is another key pathway regulating EMT progression and is affected by activation of certain transcription factors like TWIST, SNAIL, SLUG and ZEB. In addition to activation of canonical TGF-β signaling, it is also involved in downstream activation of other canonical pathways, including Hedgehog, Notch, and Wnt and for this reason, is considered to be the master switch of the EMT process[65-67].
Notch signaling is another central mechanism for EMT development. Bao et al[68] demonstrated that Notch pathway increases ZEB1 expression, which leads to EMT induction by inhibiting miRNA-200. Notch expression has also been correlated with the EMT markers such as, E-cadherin and Vimentin in prostate cancer[69].
There is ample evidence to suggest that cells that undergo EMT have CSC like properties. After invading the new site, these cells initiate secondary tumor, much like CSCs[70]. The regulatory role of miRNA-200 in Notch signaling further supports the CSC theory.
Although the specific differential expression of miRNA-200 in AAs and CAs is not yet elucidated, the direct association of notch signaling with miRNA-200 inhibition, opens avenues for further investigation in the area of racial disparity (see miRNA section). We have also shown that the induced overexpression of miR-1207-5p in normal human colonic epithelial cells (HCoEpiC and CCD841) induces stemness, as well as expression of EMT markers TGF-β, CTNNB1, MMP2, Slug, Snail, and Vimentin associated with an elongated cell morphology[8], indicating its role in regulating stem cell-like properties in colon mucosal cells.
TGF-β stimulation has been shown to cause increased motility in CD133+ cells as compared to CD133- cells in non-small-cell lung carcinoma. We have discussed the differential proportion of CD44+CD166- cells in the colonic effluent as well as in colonic mucosal cells of AAs and CAs[8]. CD44 has been shown to be associated with tumor progression and metastases in CRC in various studies[71].
The conventional therapies for colon cancer do not account for CSCs. This has been postulated as one of the reasons for recurrence. It is well known that the recurrence rates are higher in AAs than CAs. In various studies, racial disparity in survival/recurrence is not well explained by differences in socioeconomic conditions, and general patterns of treatment[72-74].
It is possible that the higher rate of recurrence in AAs is in part due to prevalence of those CSCs with a less favorable mutation.
The current cytotoxic therapies act by interfering with the cell cycle of rapidly growing cells. This provides selective advantage to the slow replicating stem cells, which in fact may be enriched after chemotherapy. Data from several studies suggest a pivotal role for CSCs in regulating many malignancies, including CRC. Numerous studies have reported that CSCs or CSC like cells are highly enriched in chemotherapy resistant cancer cells. These include glioma, breast cancer, and colon cancer[75]. Results from our own studies have demonstrated that although the combined therapy of 5-FU and Oxaliplatin inhibited the growth/proliferation of human colon cancer cells (HCT-116 or HT-29), the remaining cells showed enrichment of CSCs[76].
It is well known that butyrate induces differentiation of colon cancer cells[77,78]. Forced cell differentiation has not only been shown to deplete CSCs in colon cancer but also to sensitize colon cancer cells to chemotherapy[79,80]. When these findings are viewed in light of the observations of lower amount of butyrate in stool from AAs with colon cancer than other racial groups (see section on dietary regulation of microbiota and racial disparity), it provides an interesting link between racial disparity, CSCs and CRC.
In order to successfully tackle the disparity and recurrence issues in colon cancer, a better understanding of the biological pathways is needed. Further, the focus needs to be shifted from a uniform treatment approach to a more personalized medicine. An understanding of specific CSC markers responsible for differential initiation, progression and recurrence in AAs, will help develop therapies, which target the same.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Barreto S, Komatsu K, Meshikhes AWN, Takenaga K, Zhu YL S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | National Cancer Institute. Surveillance, Epidemiology and End Results Program. SEER Stat Fact Sheets: Colon and Rectum Cancer. [accessed 2016; May 6] Available from: http://seer.cancer.gov/statfacts/html/colorect.html. |
| 2. | Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gastroenterol. 2009;15:3734-3743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 3. | Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, Vernon SW, Cronin K, Edwards BK. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 385] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 5. | Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J, Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 621] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 6. | Wu N, Yang X, Zhang R, Li J, Xiao X, Hu Y, Chen Y, Yang F, Lu N, Wang Z. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol. 2013;66:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 343] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 7. | Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, Schloss PD. The gut microbiome modulates colon tumorigenesis. MBio. 2013;4:e00692-e00613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 535] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 8. | Farhana L, Antaki F, Anees MR, Nangia-Makker P, Judd S, Hadden T, Levi E, Murshed F, Yu Y, Van Buren E. Role of cancer stem cells in racial disparity in colorectal cancer. Cancer Med. 2016;5:1268-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock LE, Gandhi R, Weiner HL. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe. 2016;19:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 10. | Yu Y, Nangia-Makker P, Majumdar A. Overcoming drug resistance in colorectal cancer by microRNAs. Mirna targeted cancer therapy. New York: Springer Publishers 2014; 139-156. [DOI] [Full Text] |
| 11. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7837] [Article Influence: 522.5] [Reference Citation Analysis (4)] |
| 12. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3545] [Article Influence: 177.3] [Reference Citation Analysis (5)] |
| 13. | Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4098] [Cited by in RCA: 4541] [Article Influence: 324.4] [Reference Citation Analysis (1)] |
| 14. | Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2278] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 15. | Zoetendal EG, Akkermans AD, De Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. 1998;64:3854-3859. [PubMed] |
| 16. | Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 1914] [Article Influence: 159.5] [Reference Citation Analysis (0)] |
| 17. | McCoy AN, Araújo-Pérez F, Azcárate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8:e53653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 18. | Strauss J, White A, Ambrose C, McDonald J, Allen-Vercoe E. Phenotypic and genotypic analyses of clinical Fusobacterium nucleatum and Fusobacterium periodonticum isolates from the human gut. Anaerobe. 2008;14:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 20. | O'Keefe SJ, Kidd M, Espitalier-Noel G, Owira P. Rarity of colon cancer in Africans is associated with low animal product consumption, not fiber. Am J Gastroenterol. 1999;94:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Sharma S, O’Keefe SJ. Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J. 2007;83:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | O'Keefe SJ, Ou J, Aufreiter S, O’Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139:2044-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21:351-366. [PubMed] |
| 24. | Hester CM, Jala VR, Langille MG, Umar S, Greiner KA, Haribabu B. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015;21:2759-2769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 25. | Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654-1661. [PubMed] |
| 26. | Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8:e70803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 491] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 27. | Dongfeng D, An C, Shujia P, Jikai Y, Tao Y, Rui D, Kai T, Yafeng C, Jianguo L, Xilin D. Explanation of colon cancer pathophysiology through analyzing the disrupted homeostasis of bile acids. Afr Health Sci. 2014;14:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, Gaskins HR, O’Keefe SJ. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 462] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 29. | Ridlon JM, Bajaj JS. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics. Acta Pharm Sin B. 2015;5:99-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | De Boever P, Wouters R, Verschaeve L, Berckmans P, Schoeters G, Verstraete W. Protective effect of the bile salt hydrolase-active Lactobacillus reuteri against bile salt cytotoxicity. Appl Microbiol Biotechnol. 2000;53:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Moore WE, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol. 1995;61:3202-3207. [PubMed] |
| 32. | O'Keefe SJ, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J, Adada H, van der Merwe T. Why do African Americans get more colon cancer than Native Africans? J Nutr. 2007;137:175S-182S. [PubMed] |
| 33. | Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J Clin Oncol. 2008;26:2795-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 34. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3051] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 35. | Cheng H, Bjerknes M, Amar J. Methods for the determination of epithelial cell kinetic parameters of human colonic epithelium isolated from surgical and biopsy specimens. Gastroenterology. 1984;86:78-85. [PubMed] |
| 36. | Potten CS, Kellett M, Rew DA, Roberts SA. Proliferation in human gastrointestinal epithelium using bromodeoxyuridine in vivo: data for different sites, proximity to a tumour, and polyposis coli. Gut. 1992;33:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 38. | Katoh M, Katoh M. Notch signaling in gastrointestinal tract (review). Int J Oncol. 2007;30:247-251. [PubMed] |
| 39. | Sanders MA, Majumdar AP. Colon cancer stem cells: implications in carcinogenesis. Front Biosci (Landmark Ed). 2011;16:1651-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Weichert W, Knösel T, Bellach J, Dietel M, Kristiansen G. ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J Clin Pathol. 2004;57:1160-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2552] [Cited by in RCA: 2626] [Article Influence: 154.5] [Reference Citation Analysis (0)] |
| 42. | Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158-10163. [PubMed] |
| 43. | Patel BB, Yu Y, Du J, Levi E, Phillip PA, Majumdar AP. Age-related increase in colorectal cancer stem cells in macroscopically normal mucosa of patients with adenomas: a risk factor for colon cancer. Biochem Biophys Res Commun. 2009;378:344-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 45. | Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1271] [Article Influence: 90.8] [Reference Citation Analysis (1)] |
| 46. | Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327-3335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 47. | Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 694] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 48. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1559] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 49. | Lebwohl B, Capiak K, Neugut AI, Kastrinos F. Risk of colorectal adenomas and advanced neoplasia in Hispanic, black and white patients undergoing screening colonoscopy. Aliment Pharmacol Ther. 2012;35:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Hutvágner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1474] [Cited by in RCA: 1472] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 51. | Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 754] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 52. | Ziyan W, Shuhua Y, Xiufang W, Xiaoyun L. MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Med Oncol. 2011;28:1469-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 53. | Yu Y, Nangia-Makker P, Farhana L, G Rajendra S, Levi E, Majumdar AP. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol Cancer. 2015;14:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 54. | Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, Cai X, Wang K, Wang G, Ba Y. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 450] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 55. | Xu H, Yu Y, Marciniak D, Rishi AK, Sarkar FH, Kucuk O, Majumdar AP. Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol Cancer Ther. 2005;4:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Baker SJ, Preisinger AC, Jessup JM, Paraskeva C, Markowitz S, Willson JK, Hamilton S, Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990;50:7717-7722. [PubMed] |
| 57. | Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 898] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 58. | Ashktorab H, Daremipouran M, Devaney J, Varma S, Rahi H, Lee E, Shokrani B, Schwartz R, Nickerson ML, Brim H. Identification of novel mutations by exome sequencing in African American colorectal cancer patients. Cancer. 2015;121:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Aghili M, Izadi S, Madani H, Mortazavi H. Clinical and pathological evaluation of patients with early and late recurrence of colorectal cancer. Asia Pac J Clin Oncol. 2010;6:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Rattan R, Ali Fehmi R, Munkarah A. Metformin: an emerging new therapeutic option for targeting cancer stem cells and metastasis. J Oncol. 2012;2012:928127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Vries RG, Huch M, Clevers H. Stem cells and cancer of the stomach and intestine. Mol Oncol. 2010;4:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs. 2007;185:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 482] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 64. | Kwon CY, Kim KR, Choi HN, Chung MJ, Noh SJ, Kim DG, Kang MJ, Lee DG, Moon WS. The role of serum response factor in hepatocellular carcinoma: implications for disease progression. Int J Oncol. 2010;37:837-844. [PubMed] |
| 65. | Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991;5:1568-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 431] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 66. | Ponnusamy MP, Seshacharyulu P, Lakshmanan I, Vaz AP, Chugh S, Batra SK. Emerging role of mucins in epithelial to mesenchymal transition. Curr Cancer Drug Targets. 2013;13:945-956. [PubMed] |
| 67. | Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res. 2012;347:85-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 68. | Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 69. | Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2010;3:90-99. [PubMed] |
| 70. | Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 71. | Su YJ, Lai HM, Chang YW, Chen GY, Lee JL. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30:3186-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Albain KS, Unger JM, Crowley JJ, Coltman CA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 380] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 73. | Mayberry RM, Coates RJ, Hill HA, Click LA, Chen VW, Austin DF, Redmond CK, Fenoglio-Preiser CM, Hunter CP, Haynes MA. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87:1686-1693. [PubMed] |
| 74. | Yothers G, Sargent DJ, Wolmark N, Goldberg RM, O’Connell MJ, Benedetti JK, Saltz LB, Dignam JJ, Blackstock AW. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: an analysis of ACCENT adjuvant trials. J Natl Cancer Inst. 2011;103:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1451] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 76. | Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol. 2009;2:321-328. [PubMed] |
| 77. | Orchel A, Dzierzewicz Z, Parfiniewicz B, Weglarz L, Wilczok T. Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent. Dig Dis Sci. 2005;50:490-498. [PubMed] |
| 78. | Tanaka Y, Bush KK, Klauck TM, Higgins PJ. Enhancement of butyrate-induced differentiation of HT-29 human colon carcinoma cells by 1,25-dihydroxyvitamin D3. Biochem Pharmacol. 1989;38:3859-3865. [PubMed] |
| 79. | Wielenga MC, Colak S, Heijmans J, van Lidth de Jeude JF, Rodermond HM, Paton JC, Paton AW, Vermeulen L, Medema JP, van den Brink GR. ER-Stress-Induced Differentiation Sensitizes Colon Cancer Stem Cells to Chemotherapy. Cell Rep. 2015;13:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 80. | Dow LE, O’Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, Lowe SW. Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer. Cell. 2015;161:1539-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 412] [Article Influence: 41.2] [Reference Citation Analysis (0)] |