Published online Feb 26, 2016. doi: 10.4252/wjsc.v8.i2.56
Peer-review started: August 21, 2015
First decision: September 30, 2015
Revised: December 17, 2015
Accepted: January 8, 2016
Article in press: January 11, 2016
Published online: February 26, 2016
Processing time: 190 Days and 2.2 Hours
There are two types of human pluripotent stem cells: Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), both of which launched themselves on clinical trials after having taken measures to overcome problems: Blocking rejections by immunosuppressants regarding ESCs and minimizing the risk of tumorigenicity by depleting exogenous gene components regarding iPSCs. It is generally assumed that clinical applications of human pluripotent stem cells should be limited to those cases where there are no alternative measures for treatments because of the risk in transplanting those cells to living bodies. Regarding lifestyle diseases, we have already several therapeutic options, and thus, development of human pluripotent stem cell-based therapeutics tends to be avoided. Nevertheless, human pluripotent stem cells can contribute to the development of new therapeutics in this field. As we will show, there is a case where only a short-term presence of human pluripotent stem-derived cells can exert long-term therapeutic effects even after they are rejected. In those cases, immunologically rejections of ESC- or allogenic iPSC-derived cells may produce beneficial outcomes by nullifying the risk of tumorigenesis without deterioration of therapeutic effects. Another utility of human pluripotent stem cells is the provision of an innovative tool for drug discovery that are otherwise unavailable. For example, clinical specimens of human classical brown adipocytes (BAs), which has been attracting a great deal of attention as a new target of drug discovery for the treatment of metabolic disorders, are unobtainable from living individuals due to scarcity, fragility and ethical problems. However, BA can easily be produced from human pluripotent stem cells. In this review, we will contemplate potential contribution of human pluripotent stem cells to therapeutic development for lifestyle diseases.
Core tip: Clinical application of human embryonic stem cells (ESCs)/induced pluripotent stem cells (iPSCs) is currently limited to remediless diseases due to risk of tumorigenesis. However, application of these cells to therapeutic purposes and drug discovery for lifestyle diseases is promising. Because a short-term presence of human ESC/iPSC-derived vascular endothelial cells reportedly exerts long-term therapeutic effects on injured stenotic arteries, immunologically rejections can nullify risk of tumorigenesis without deteriorating therapeutic effects. Another utility is to produce high-scarcity-valued cells such as brown adipocytes, which are unobtainable from living bodies and commercially available sources, as a new tool for drug discovery for lifestyle diseases.
- Citation: Nishio M, Nakahara M, Yuo A, Saeki K. Human pluripotent stem cells: Towards therapeutic development for the treatment of lifestyle diseases. World J Stem Cells 2016; 8(2): 56-61
- URL: https://www.wjgnet.com/1948-0210/full/v8/i2/56.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i2.56
Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are the only pluripotent stem cells that are applicable to therapeutic purposes (Table 1). The first clinical trial of human ESCs (hESCs) was launched in 2010 by Geron Corporation in the Unites States[1-4], aiming for the safety evaluation of transplanting hESC-derived oligodendrocyte progenitor cells for the treatment of spinal cord injury. Although the program was shut down due to fund shortage in 2011, no severe side effects were reported from all four cases. Another clinical trial was started in 2010 by Ocata Therapeutics in the Unites States (named Advanced Cell Technology, Incorporated until 2014), aiming for the evaluation of safety and efficacy of hESC-derived retinal pigment cells for the treatment of macular degeneration[5]. Up till now, positive results were reported from two open-label phase 1/2 studies although we have to wait for the final evaluation. Regarding human iPSCs (hiPSCs), a clinical trial was started in 2014 by RIKEN and Foundation for Biomedical Research and Innovation in Japan, aiming for the safety evaluation of hESC-derived retinal pigment cells for the treatment of macular degeneration. No severe side effects have been reported so far.
| Human pluripotent stem cells | Sources | Ethical hurdle | Safety |
| ESCs | Embryos | High | Relatively high1 |
| Allogenic iPSCs (with immunosuppression) | Cell banks | Low | Not yet evaluated |
| Allogenic iPSCs (without immunosuppression) | Low | High | |
| Autologous iPSCs | Patient samples | Low | Under evaluation |
Although it was expected that the invention of hiPSCs had completely resolved the issue of immunological hurdles, it has turned out that the situation is not so simple. Up till now, two concerns have been raised. One is regarding the differentiation propensity of hiPSCs. It is known that there are marked differences in differentiation propensity among human pluripotent stem cell lines[6], and thus, it is necessary to establish scores of hiPSC lines to obtain an appropriate line for the preparation of differentiated cells of the intended lineage. In some cases, however, an appropriate line may not be obtained and, as a result, transplantation materials would be unavailable from patients. In addition, genetic mutations may possibly occur during the process of hiPSC establishment. In such cases, usage of mutated hiPSC lines should be avoided. Thus, there may be cases where transplantation materials are unavailable from patients themselves. This actually occurred in the second patient during the clinical trial in Japan: The authorities announced that they resigned the utilization of autologous hiPSCs but decided to use allogenic hiPSCs instead in this case. It seems, however, allogenic hiPSCs are less advantageous than hESCs from the viewpoint of safety although they have merits from the perspective of ethics and labor. The second concern is regarding an issue of possible acquisition of immunogenicity of autologous iPSCs due to spontaneous mutations in the mitochondrial DNA, which is five to ten times more prone to be mutated than the chromosomal DNA. In addition, alterations of mitochondrial DNA reportedly occur upon an induction of pluripotency in hiPSCs[7]. Because cells that contain allogenic mitochondria are rejected by innate immune system[8], autologous hiPSC-derived cells with mutated mitochondrial DNA may possibly be immunologically rejected, dissipating the effects of transplantation. These two concerns should be deeply reflected for the success of hiPSC-based cell therapies in near future.
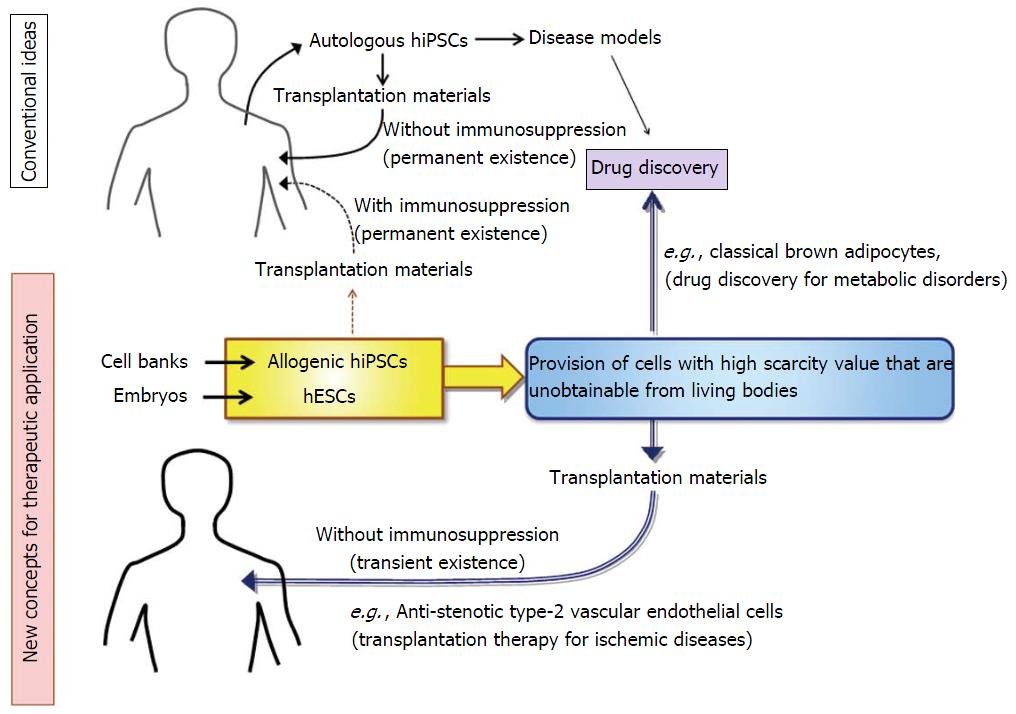
Currently, the application of human pluripotent stem-derived cells is limited to such diseases that have no other therapeutic options because there is a certain level of risk including tumorigenesis in the transplantation of human pluripotent stem cells. Nevertheless, the application range will be extended if the safety is secured. Regarding lifestyle diseases such as obesity-associated metabolic disorders and ischemic diseases, we already have various therapeutic options including medications and surgeries. In addition, a large number of candidate drugs are currently in the process of research and development. Thus, development of human pluripotent stem cell-base therapies for lifestyle diseases has not been eagerly sought thus far. Nevertheless, there is ample potential for hESCs/hiPSCs to effectively be utilized towards therapeutic development in this field. In this review, we suggest two cases as examples. One is a transplantation therapy for the treatment of ischemic diseases: hESC/hiPSC-derived vascular endothelial cells (VECs) having anti-stenotic capacities, which we termed as type-II VECs[9-11], can exert their full effects within a short time (< 1 wk) to produce long-term beneficial outcomes even after they are rejected[11]. In those cases, risk of tumorigenesis may be nullified because hESC- or allogenic hiPSC-derived cells are promptly rejected by immune systems. The second one is utilization of human pluripotent stem cells as a novel tool to provide cells that have high scarcity value but are unavailable from living individuals. Actually, anti-stenotic VECs is an example of such high-scarcity-valued cells[9]. As another example, we will describe human ESC/iPSC-derived classical brown adipocyte (BA), which has been much awaited as a new target of drug discovery for the treatment of obesity-associated metabolic disorders.
According to the report by World Health Organization, the top two leading causes of death in the world in 2012 are ischemic heart disease and stroke, both which are considered as lifestyle diseases. Ischemia is caused by narrowing of arteries (i.e., arteriostenosis), whose pathological basis is hyperproliferation of vascular smooth muscle cells (VSMCs). Stent revascularization is one of the most effective therapies, where a meshed tube made of shape-memory alloy is inserted into the affected artery (i.e., the coronary artery for ischemic heart disease and the carotid artery for stroke) to mechanically expand the stenotic region. Nevertheless, a comparative study in India in 2010 reported that 23.1% of patients with drug-eluting stents and 48.8% of patients with bare metal stents developed restenosis[12]. Therefore, development of new therapeutics is required for the control of ischemic diseases.
Regarding the etiology of arteriostenosis, involvements of VSMCs and macrophages are well understood. By contrast, roles for VECs remained controversial for long time. Recently, we have clarified that there are two types of human VECs: Pro-stenotic VECs (type-I) and anti-stenotic VECs (type-II)[9-11]. We also showed that the vast majority of human VECs that are obtainable from commercially available sources such as biopsy samples and bone marrow- or umbilical cord blood-derived endothelial progenitor cells (EPCs) belong to type-I VECs, which promote VSMC proliferation and exacerbate the development of stenosis in injured arteries[9,11]. By contrast, type-II VECs, which suppress VSMC proliferation and prevent arteriostenosis[9,11], are rarely obtained from commercially available sources. Because type-II VECs are convert into type-I VECs by oxidative stress and aging[9], it seems that type-I VECs are in a generative state. Intriguingly, hESCs/hiPSCs easily produce type-II VECs, although they convert to type-I VECs after repetitive subcultures. Thus, hESCs/hiPSCs provide an excellent tool to produce high scarcity-valued cells that are otherwise unavailable.
There is still another merit in utilizing hESC/hiPSC-derived type-II VECs as a transplantation material: They can generate beneficial outcomes by their anti-stenotic effects although they are immunologically rejected shortly after the transplantation (< 1 wk)[11]. A transient existence of hESC/hiPSC-derived type-II VECs on the luminal surface of the injured artery effectively blocks injury-associated VSMC hyperproliferation. After immunological rejection of hESC/hiPSC-derived type-II VECs, host VECs take over the role of hESC/hiPSC-derived type-II VECs[11]. If hESC/hiPSC-derived type-I VECs cover the injured luminal surface, development of arteriostenosis is highly accelerated and, in most cases, injured arteries undergo total stenosis[11]. Thus, the critical point that determines the fate of injured arteries is which type of VECs, type-I or type-II, covers the luminal surface immediately after the arterial injury. Because hESCs/hiPSCs can steadily provide type-II anti-stenotic VECs, which are extremely unobtainable from commercially available sources or clinical samples of patients, hESC/hiPSC-derived type-II VECs will make a large contribution of therapeutic development of ischemic diseases (Figure 1). It should be remembered that any surgical operations which mechanically dilate stenotic arteries would more or less injure endothelial layers, causing the injury-mediated stenosis. In this sense, endothelial cell-transplanting therapies may become an indispensable mean for the control of ischemic diseases.
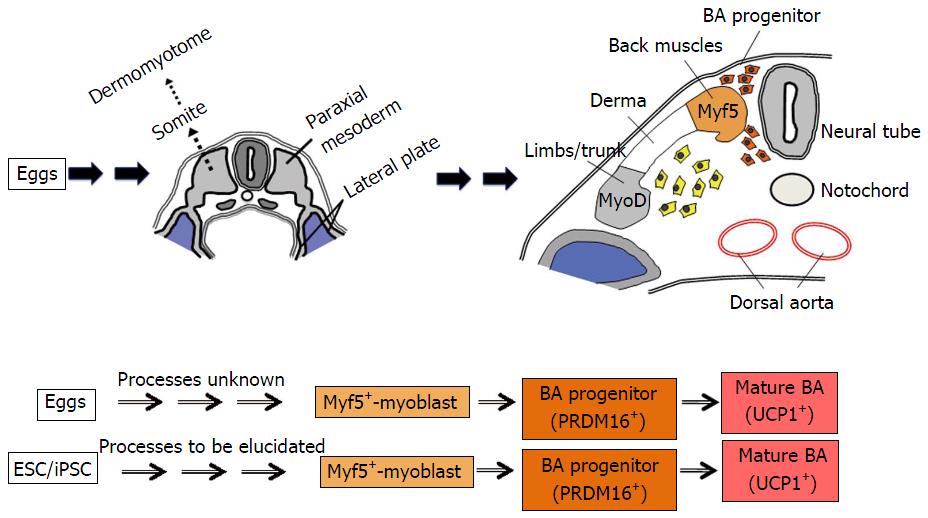
Brown adipose tissue (BAT) is a unique adipose tissue that has high calorigenic capacities, thus contributing thermogenesis under cold environments. It is distributed in specific areas including interscapular spaces (mice and newborn humans) and deep neck regions (mice and humans). BA is derived from myf5-positive myoblast[13] although the developmental process prior to the myoblast stage remains elusive. It is also known that BA-like cells called beige cells emerge in white adipose tissue under cold-acclimated conditions. To distinguish BA from beige cells, it is also called as classical BA. In addition to heat production, BAT plays crucial roles in metabolic regulation as demonstrated by murine studies: It contributes to prevention of obesity[14,15] and improvements of glucose[16-18] and lipid[16,19] metabolisms.
The existence of classical BAT in humans was first reported in 2009[20-23]. After a minor dispute in 2012[24,25], the presence of classical BAT in adult humans was reconfirmed in 2013[26]. Clinical studies have supported that the findings obtained from murine studies are also the case with humans[27-30]. Thus, human classical BA is attracting great attention as a new therapeutic target for obesity-associated lifestyle diseases. However, it is hardly possible to obtain high-quality human BA samples because of economical, technical and ethical problems. First, visualization of BA-distributing sites requires an expensive medical apparatus called positron emission and computer tomography (PET/CT). Secondly, PET/CT examinations impose gamma ray irradiations on young individuals (approximately early twenties), whose BATs are visualized by PET/CT at a high probability. Thirdly, biopsy-mediated removal of BAT, whose amount is assumed to be less than 150 g/body[31], may possibly increase the risk of obesity-associated lifestyle diseases. Fourthly, BAT is known as a very fragile tissue to handle. Indeed, BioGPS database[32] shows that murine BAT expresses RNase1 and various chymotrypsin family peptidase genes at high levels. Therefore, it is extremely difficult to obtain high-quality BA samples even from mice, which have abundant BATs. Lastly, techniques for long-term cultures, expansions and frozen storage of BA do not currently exist.
All those problems have been overcome by the establishment of a method for a directed differentiation of hESCs/hiPSCs into classical BA[33,34]. hESC/hiPSC-derived BAs possess high capacities to improve glucose/lipid metabolisms in vivo as proven by transplantation experiments[33]. Moreover, this technique correctly reproduces in vivo developmental process of BAT because hESCs/hiPSCs were differentiated into classical BAs via myoblast stage[33]. This innovative method has opened an avenue to the implementation of BA-based drug discovery (Figure 1). Moreover, it provides a groundbreaking system for basic studies to strip BAT of its aura of mystery. Although the developmental process of BA prior to the myoblast stage is currently unknown, it will be elucidated by using the method for the differentiation of hESC/hiPSC into classical BA (Figure 2). The elucidation of an early BA process will even provide new molecular targets for the drug discovery of obesity-associated lifestyle diseases.
P- Reviewer: Chhabra CA, Li ZJ S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Bretzner F, Gilbert F, Baylis F, Brownstone RM. Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell. 2011;8:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Wirth E, Lebkowski JS, Lebacqz K. Response to Frederic Bretzner et al. “Target populations for first-in-human embryonic stem cell research in spinal cord injury”. Cell Stem Cell. 2011;8:476-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Solbakk JH, Zoloth L. The tragedy of translation: the case of “first use” in human embryonic stem cell research. Cell Stem Cell. 2011;8:479-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Lebkowski J. GRNOPC1: the world’s first embryonic stem cell-derived therapy. Interview with Jane Lebkowski. Regen Med. 2011;6:11-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 877] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 6. | Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 629] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 7. | Prigione A, Lichtner B, Kuhl H, Struys EA, Wamelink M, Lehrach H, Ralser M, Timmermann B, Adjaye J. Human induced pluripotent stem cells harbor homoplasmic and heteroplasmic mitochondrial DNA mutations while maintaining human embryonic stem cell-like metabolic reprogramming. Stem Cells. 2011;29:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Ishikawa K, Toyama-Sorimachi N, Nakada K, Morimoto M, Imanishi H, Yoshizaki M, Sasawatari S, Niikura M, Takenaga K, Yonekawa H. The innate immune system in host mice targets cells with allogenic mitochondrial DNA. J Exp Med. 2010;207:2297-2305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Nishio M, Nakahara M, Sato C, Saeki K, Akutsu H, Umezawa A, Tobe K, Yasuda K, Yuo A, Saeki K. New categorization of human vascular endothelial cells by pro- vs anti-proliferative phenotypes. World J Transl Med. 2015;4:88-100. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 10. | Nakahara M, Nishio M, Saeki K, Yuo A, Saeki K. p38 MAPK regulates type-I vs type-II phenotyping of human vascular endothelial cells. World J Transl Med. 2015;4:101-112. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Nishio M, Nakahara M, Saeki K, Fujiu K, Iwata H, Manabe I, Yuo A, Saeki K. Pro- vs anti-stenotic capacities of type-I versus type-II human iPS-derived endothelial cells. World J Transl Med. 2015;4:113-122. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Mohan S, Dhall A. A comparative study of restenosis rates in bare metal and drug-eluting stents. Int J Angiol. 2010;19:e66-e72. [PubMed] |
| 13. | Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1947] [Cited by in RCA: 1806] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 14. | Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740-742. [PubMed] |
| 15. | Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 1040] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 16. | Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137:21-29. [PubMed] |
| 17. | Guerra C, Navarro P, Valverde AM, Arribas M, Brüning J, Kozak LP, Kahn CR, Benito M. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest. 2001;108:1205-1213. [PubMed] |
| 18. | Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 907] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 19. | Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1265] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 20. | van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2587] [Cited by in RCA: 2632] [Article Influence: 164.5] [Reference Citation Analysis (0)] |
| 21. | Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3598] [Cited by in RCA: 3329] [Article Influence: 208.1] [Reference Citation Analysis (0)] |
| 22. | Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2311] [Cited by in RCA: 2362] [Article Influence: 147.6] [Reference Citation Analysis (0)] |
| 23. | Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1443] [Cited by in RCA: 1488] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 24. | Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2276] [Cited by in RCA: 2555] [Article Influence: 196.5] [Reference Citation Analysis (0)] |
| 25. | Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 510] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 26. | Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 553] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 27. | Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, Saito M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring). 2011;19:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 28. | Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 2011;19:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 29. | Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 732] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 30. | Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686-3698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 313] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 31. | Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783-1790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 32. | Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1039] [Cited by in RCA: 1132] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 33. | Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394-406. [PubMed] |
| 34. | Nishio M, Saeki K. Differentiation of human pluripotent stem cells into highly functional classical brown adipocytes. Methods Enzymol. 2014;537:177-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Sadler TW, Langman J. Langman’s medical embryology. 8th ed. Philadelphia: Lippincott Williams & Wilkins, c2000. . |