Published online Jan 26, 2016. doi: 10.4252/wjsc.v8.i1.1
Peer-review started: August 24, 2015
First decision: October 13, 2015
Revised: December 4, 2015
Accepted: December 18, 2015
Article in press: December 21, 2015
Published online: January 26, 2016
Processing time: 150 Days and 15.3 Hours
The extracellular matrix-associated bone morphogenetic proteins (BMPs) govern a plethora of biological processes. The BMPs are members of the transforming growth factor-β protein superfamily, and they actively participate to kidney development, digit and limb formation, angiogenesis, tissue fibrosis and tumor development. Since their discovery, they have attracted attention for their fascinating perspectives in the regenerative medicine and tissue engineering fields. BMPs have been employed in many preclinical and clinical studies exploring their chondrogenic or osteoinductive potential in several animal model defects and in human diseases. During years of research in particular two BMPs, BMP2 and BMP7 have gained the podium for their use in the treatment of various cartilage and bone defects. In particular they have been recently approved for employment in non-union fractures as adjunct therapies. On the other hand, thanks to their potentialities in biomedical applications, there is a growing interest in studying the biology of mesenchymal stem cell (MSC), the rules underneath their differentiation abilities, and to test their true abilities in tissue engineering. In fact, the specific differentiation of MSCs into targeted cell-type lineages for transplantation is a primary goal of the regenerative medicine. This review provides an overview on the current knowledge of BMP roles and signaling in MSC biology and differentiation capacities. In particular the article focuses on the potential clinical use of BMPs and MSCs concomitantly, in cartilage and bone tissue repair.
Core tip: Since their first identification, bone morphogenetic proteins (BMPs) have attracted the attention for their potential therapeutic use in tissue engineering and biomedical regenerative therapies. In particular, BMP2 and BMP7 have been successfully used in the treatment of a number of cartilage and bone defects, although these strategies present a certain number of concerning side effects. Also in the field of mesenchymal stem cell (MSC) biology there is a continually growing interest, especially in the regulation of their differentiation, and in demonstrating their utility in tissue engineering. The review focuses on the current knowledge of BMP physiological roles in MSC biology and differentiation capacities. In particular it highlights the potentialities of the concomitant clinical use of BMPs and MSCs in cartilage and bone tissue repair.
- Citation: Scarfì S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J Stem Cells 2016; 8(1): 1-12
- URL: https://www.wjgnet.com/1948-0210/full/v8/i1/1.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i1.1
The extracellular matrix (ECM)-associated bone morphogenetic proteins (BMPs) govern a plethora of biological processes[1]. The BMPs are members of the transforming growth factor-β (TGF-β) protein superfamily[2], and they actively participate to kidney development, digit and limb formation, angiogenesis, tissue fibrosis and tumor development[3]. In particular, these proteins are upregulated in the limb bud epithelium playing a crucial role in the proliferation and differentiation of resident mesodermal progenitors[4]. Thus, the dysregulation of the BMP signaling pathway has dramatic consequences for the development in mammals. As a matter of fact, mutations in BMP receptors impairing the BMP signaling are implicated in important vascular conditions and skeletal abnormalities[5]. On the other hand, since BMPs are important morphogens in embryogenesis and development, and also regulate the maintenance of adult tissue homeostasis, their mutations lead to a wide spectrum of both skeletal and extraskeletal abnormalities[3,6]. First of all BMP2 and 4 null mice are embryonic lethal demonstrating the fundamental role of these proteins in the early development. In general, mutations affecting BMPs are associated to various skeletal defects such as the short ear phenotype (BMP5), polydactylity (BMP4 and 7), abnormalities in rib formation (BMP7), smaller long bones (BMP6), chondrodysplasia (BMP14), bone fusions (BMP13) spontaneous fractures (BMP2 and 5) and osteogenesis imperfecta (BMP1)[3]. For what concerns extraskeletal abnormalities many BMPs are involved in the development of the brain (BMP2, 4, 5 and 11), while BMP4 defects lead to various organ abnormalities. Mutations in BMP7 lead to severe defects in kidney and eye development; BMP6 and BMP8 are associated to decreased fertility and BMP9 to an abnormal lymphatic development[6]. Because of these diverse functions in all organ systems, it has been suggested that BMPs deserve to be called body morphogenetic proteins[7].
BMPs can upregulate growth factors such as platelet-derived growth factor (PDGF), vascular endothelial growth factor and insulin-like growth factor 1 (IGF1)[8]. In particular, the expression of specific BMPs is induced during early recruitment of mesodermal progenitors, namely mesenchymal stem cells (MSCs) and is sustained throughout osteogenic and chondrogenic differentiation until formation of woven bone[4,9]. MSCs are multipotent cells resident in many tissues such as bone marrow, adipose tissue and periosteum[10]. Thanks to their potential biomedical applications there is a growing interest in studying MSC biology, mainly their differentiation capacities, and in testing their true abilities in tissue engineering[11,12]. In fact, the specific differentiation of MSCs into targeted cell-type lineages for transplantation into sites of injury is a primary goal of the regenerative medicine[12,13].
This review summarizes the current knowledge of BMP roles in MSC biology and lineage differentiation focusing in particular on the potential clinical use of BMPs and MSCs in cartilage and bone tissue repair.
BMPs were originally shown to induce cartilage formation and ectopic bone growth in vivo[14] and are known to set up, foster and support chondrogenesis and osteogenesis[15,16].
Approximately 20 members of the BMP family are known[17,18]. In particular they have been grouped into several subfamilies which members are often redundant: The bona fide BMP subfamily (from BMP1 to BMP15), the osteogenic protein (OP) subfamily (OP1, OP2 and OP3 alias BMP7, BMP8, and BMP8b, respectively), the growth differentiation factor subfamily (GDF1, GDF2/BMP9, GDF3, GDF5/BMP14, GDF6/BMP13, GDF7/BMP12, GDF8, GDF9, GDF10 and GDF11/BMP11) and finally the cartilage-derived morphogenetic proteins (CDMP1 and CDMP2 alias BMP14 and BMP13, respectively)[3,19]. BMPs are synthesized as large inactive precursors containing a N-terminal signal peptide followed by a prodomain controlling appropriate folding and a C-terminal mature polypeptide[20].
Once secreted, BMPs mainly act as homodimers[21] and they can be recognized by homodimeric antagonists like gremlin and noggin, which in turn restrict their biological activity[22].
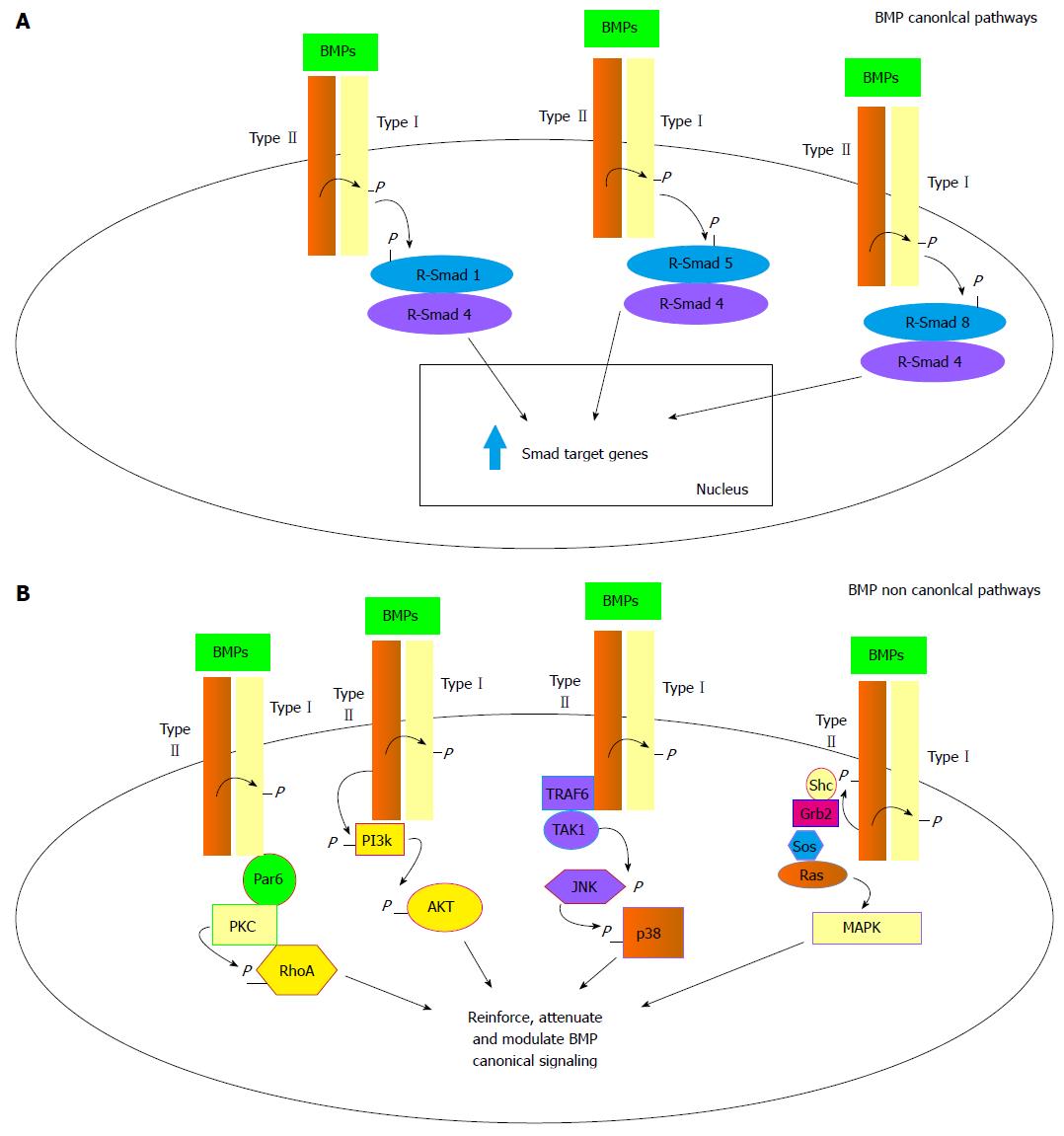
BMPs bind to two types of serine/threonine kinase receptors, namely type I (BMPR-I) and type II receptors (BMPR-II)[23]. BMPs preferentially engage three different type II receptors and also three different type I receptors[24]. Once bound to a BMPR-I, the ligand/receptor complex recruits BMPR-II, which in turn phosphorylates the BMPR-I on its cytoplasmic domain containing a glycine/serine rich domain (GS domain)[5]. Upon ligand binding, the BMP signal is transduced to target genes through the Smad-dependent (canonical pathway) or the Smad-independent pathways (Figure 1). The Smad proteins are homologues of D. melanogaster mothers against decapentaplegic and related C. elegans Sma gene[25]. They can be distinguished upon their functions or their activators. In particular, Smad1/5/8 (R-Smads) are so-called receptor Smads and are triggered by BMPs via BMPR-I recruitment and activation. Once the R-Smads have been phosphorylated they form a DNA binding heterodimer with the mediator Smad4[26,27] (Figure 1A). In the nucleus, the active dimer promotes the transcription of BMP target genes through recognition of Smad-binding sequences or GC-rich elements present in the promoters of such genes[5]. This specific transduction pathway is finely regulated both by extracellular and intracellular mediators and signals. Intracellular signals encompass proteasome-promoted degradation[28], inhibition by Smad6 and 7 factors which impair Smad4 mediator binding, and protein phosphorylation/dephosphorylation processes[29]. The BMP/Smad signaling pathway can be also strictly regulated by a group of extracellular protein antagonists that directly bind to the BMPs and prevent the interaction with their receptors such as gremlin, chordin, noggin and follistatin[1,17,30]. In most cases BMP antagonist expression is finely regulated in a temporospatial manner during the development. Their fundamental role as antagonists in BMP signaling is attested by a number of severe or lethal defects occurring in experimental animals lacking one of these proteins[1]. In addition to Smad canonical pathway several non canonical pathways have been described so far. Through their type I and type II receptors, the TGF family members have been shown to activate the JNK/p38, the MAPK/ERK, the PI3K/AKT and the PKC/RhoA transduction pathways (Figure 1B)[6,31]. The PI3K/AKT pathway seems to be recruited by the direct phosphorylation of a PI3K serine residue by a type II activated receptor. Alternatively the MAPK pathway can be activated through the docking of the Shc and Grb2 MAPK mediators recognizing three sites of rare autophosphorylated tyrosines on the type II activated receptors. Activated type II receptor can also bind to TRAF6 protein triggering the TGF-β-activated kinase 1 and leading to the JNK/p38 pathway activation. And finally an activated type I receptor can bind to the par6 scaffold protein able to recruit the PKC and RhoA proteins[6,31]. These pathways in addition to Smad signaling have been demonstrated to alternatively reinforce, attenuate or otherwise modulate downstream BMP cellular responses[32,33].
In vertebrates, bone formation can be achieved by direct differentiation of osteoblasts in membranous ossification, or starting from differentiation of chondrocytes in endochondral ossification[34,35]. These two processes are directed by BMPs, with BMP2 and BMP4 acting as the master differentiation triggers of osteoblast and chondrocyte phenotypes leading to bone and cartilage formation[35].
BMP2 and BMP4 drive bone formation through the Smad1/5/8 signaling pathway described earlier. This pathway is common to osteoblasts and chondrocytes and its precursors and is strictly regulated in these cells[1]. BMPs are released in a mature form from osteoblasts and may interact with their cell surface receptors or bind to proteins of the ECM. In the latter case the ECM acts as a “reservoir” of BMPs for future paracrine signaling[5]. In regard to this, a number of transcription factors necessary to cartilage and bone formation have been acknowledged regulating downstream BMP signaling[35].
The chondrogenic potential of different BMPs has been tested because of the eventual clinical applications in cartilage repair tissue engineering (Table 1). BMP2, 4, 6, 7 and 9 have been reported to induce in vitro chondrogenesis of human MSCs[36-42].
| BMP type and conditions | Cell type | Ref. |
| Chondrogenesis | ||
| BMP2 (micromass + TGF-β3) | BM MSCs | [41,45,46] |
| BMP2 (3D alginate beads) | BM MSCs | [37] |
| BMP4 (micromass + TGF-β3) | BM MSCs | [41] |
| BMP6 (micromass + TGF-β3) | BM MSCs | [41] |
| BMP7 (micromass + TGF-β3) | BM MSCs | [45,46] |
| BMP9 (3D alginate beads) | BM MSCs | [37] |
| BMP2 (3D agarose) | Synovial explant MSCs | [43] |
| BMP7 (3D agarose) | Synovial explant MSCs | [43] |
| BMP7 (monolayer) | Adipose derived MSCs | [44] |
| BMP7 (monolayer + Asc) | C3H10T1/2 (multipotent fibroblasts) | [54] |
| BMP2 (monolayer) | MC615 (chondrocyte precursors) | [53] |
| BMP4 (monolayer) | MC615 (chondrocyte precursors) | [53] |
| Osteogenesis | ||
| BMP2 (monolayer) | Adipose derived MSCs | [44] |
| BMP2 (monolayer + Dexa and Asc) | BM MSCs | [56,62] |
| BMP3 (monolayer + Dexa and Asc) | BM MSCs | [62] |
| BMP4 (monolayer + Dexa and Asc) | BM MSCs | [62] |
| BMP5 (monolayer + Dexa and Asc) | BM MSCs | [62] |
| BMP6(monolayer + Dexa and Asc) | BM MSCs | [62] |
| BMP6 (monolayer) | BM MSCs | [58] |
| BMP7 (monolayer + Dexa and Asc) | BM MSCs | [62] |
| BMP8a (monolayer + Dexa and Asc) | BM MSCs | [62] |
| BMP7 (monolayer + Asc) | C3H10T1/2 (multipotent fibroblasts) | [55] |
| BMP7 (monolayer + Asc) | MC3T3-E1 (committed osteoblasts) | [54] |
In human bone marrow-derived (BM) MSCs, BMP2 (in the presence of TGF-β3) was the most efficient inducer of chondrogenesis with the production of a proteoglycan rich cartilage over BMP4 and BMP6[41] while in synovial explants, BMP7 was a more effective trigger of chondrocyte differentiation than BMP2[43]. BMP7 could also stimulate chondrogenic differentiation in adipose tissue-derived stem cells[44] while in other studies Shen et al[45,46] demonstrated that both BMP2 and BMP7 enhance TGF-β3-mediated chondrogenic phenotype of BM MSCs in vitro. In another study, BMP9 and BMP2 used separately and in absence of TGF-β stimulation enhanced the expression of cartilage transcription factor Sox-9 followed by induction of type II collagen, aggrecan and cartilage oligomeric matrix protein in BM MSCs[37]. In addition, BMP13 and 14, also called CDMP2 and 1 respectively, demonstrated to be necessary for stimulation of early chondrogenesis and chondrocyte differentiation (BMP14/CDMP1) as well as in the terminal differentiation of chondrocytes in the final stage of hypertrophy and mineralization in vivo[19]. In vivo, BMP7 has also shown a marked anabolic activity in cartilage and bone[47,48] and it has demonstrated to act synergistically with microfractures to boost cartilage repair[49]. Related to this, Mishima and Lotz[50] have more recently demonstrated that BMP4 and 7 elicit a significant chemotactic in vitro response from human MSCs suggesting that the use of these factors in vivo promotes directed cell migration in sites of injury for cartilage repair in transplanted engineered tissues.
For what concerns osteoinduction, studies of pre-natal bone development as well as of fracture repair[9,51,52] showed the expression of a plethora of BMP genes with temporospatial variability. In particular, early experiments using human recombinant BMP2, BMP4 BMP6 or BMP7 demonstrated that such proteins are able to individually stimulate osteoblastic (or chondrogenic) phenotypes in a variety of mesenchymal precursor cell lines (Table 1)[53-58]. However, differently from in vitro studies, in vivo investigations indicate that BMPs work in a coordinated fashion[52,59]. In particular, BMP2 can be described as a necessary constituent orchestrating the signaling pathway that regulates fracture repair[60-62]. Differently, BMP7 is undetectable in the MSC differentiating system, but when exogenously added may play the same function of one of the endogenous BMPs physiologically produced by the cells[62].
As a matter of fact, both BMP2 and BMP7 are now approved in clinics for the treatment of non-union fractures as adjunct therapies[63]. In particular human recombinant BMP2 is sold from Medtronic (Minneapolis, MN, United States) with the acronym of In FUSE®, while hrBMP7 is sold from Stryker (Kalamazoo, MI, United States) with the acronym OP-1.
Although the use of these molecules in fracture healing has been welcomed by physicians with great enthusiasm, it must be emphasized that several, clinically relevant, adverse effects have been reported especially at BMP high dosages. The most frequently described effect is the development of antibodies against BMPs even if this event does not seem to have real adverse consequences[64]. Differently, serious concerns raised from the observation that application of BMPs to a fracture site could result in increased bone resorption as a primary event. As a consequence a higher nonunion rate has been observed in a number of patients leading to termination of BMP use in several clinical settings[65]. Furthermore, local inflammatory responses have also been reported at several anatomical sites, with different degrees of severity[66]. Finally BMP use has also been associated to wound healing complications[66], hematoma formation[67] and several cases of heterotopic bone formation[67]. Thus, we can conclude that the dosage of these powerful molecules needs to be finely calibrated in each clinical setting and in any case reserved to patients in which the risks associated to BMP use are clearly outweighed by the higher risks of fracture healing failure.
Repair of adult bone involves BM MSCs which serve as a source of osteochondral progenitors able to invade the fracture site, proliferate and differentiate into cartilage and bone. MSCs are multipotent adult cells that have the ability to self-renew and differentiate into multiple lineages[10] that were discovered in 1980[68] but only fully recognized in 1994[69]. MSCs have recently gained increasing attention for their potential in the regenerative medicine. The main reasons for this interest are the relative ease of isolation from several adult tissues and suitable expansion in culture and the high degree of plasticity of these cells. Currently, at least 198 registered MSC clinical trials are ongoing (http://www.clinicaltrials.gov), as well as autologous and allogeneic MSC products accepted for use in bone repair in a number of international jurisdictions (Mesoblast_Media_Release by Mesoblast Ltd., Melbourne, Australia; Osteocel by Osiris therapeutics Inc., Columbia, MD, United States)[70]. Despite their apparent therapeutic potential, clinical applications of MSCs have been restricted due to the limited understanding of the factors that regulate their fate and activity. Another limiting factor is the lack of knowledge of the complex interplay between these cells and the components of their niche or immediate microenvironment. Due to the disposition of MSC to differentiate into osteoblasts and chondrocytes, and their attested clinical potential in bone tissue engineering, a great amount of research has been centered on the identification of the factors governing osteogenesis in vitro and in vivo (i.e., TGF-β1, 2 and 3, BMPs and PDGF)[71,72].
The chondrogenic differentiation occurs when MSCs are seeded in serum-free, 3D culture format in the presence of one or more TGF-β superfamily members[73]. In this asset, cells abandon the typical fibroblastic morphology and start producing cartilage-specific matrix components. In vitro chondrogenesis is usually obtained by the micromass pellet culture system, allowing the necessary cell-cell interactions which resemble what occurs in pre-chondrogenic condensations in the embryonic development[74]. In these conditions cells usually differentiate in no more than 2-3 wk into chondrocyte-like cells secreting proteoglycans. Pellets are bordered by a narrow capsule of connective tissue, almost cell-free and rich in type IIA collagen. The advancement to terminal differentiation is attested by accumulation of type X collagen and matrix mineralization[75]. When BMPs are added in this experimental setting, namely MSCs in micromass culture and in the presence TFG-β, they enhance chondrogenic differentiation and cartilage formation significantly (see Table 1 for the various BMP employed). The 3D culture and the concomitant presence of TGF-β seem to be necessary to attain a real chondrocytic phenotype. Thus, it is possible that in the mesenchymal precursor chondrocyte differentiation occurs only when strict cell-cell interactions are established and when the parallel activation of different R-Smad pathways is achieved by different members of the TGF-β superfamily. In particular, the TGF-β members activating the Smad2/3 and the BMP members activating the Smad1/5/8 (see Figure 1A).
Differently, MSCs undergo an osteogenic differentiation when cultured with the opportune osteoinduction factors on two dimensional substrates. In this case, osteogenesis is promoted by a large spread area, while in the same conditions the reduction of the spread area induces adipogenesis[76,77]. In this experimental setting, namely MSCs in 2D wide spread areas, several BMPs used alone or in the presence of ascorbic acid have demonstrated to promote significant osteoblast differentiation (see Table 1 for the various BMP employed). In the presence of BMPs, progenitor cells achieve an osteoblastic phenotype expressing several bone-characterizing ECM proteins. In particular they express type I collagen, osteopontin, osteocalcin and bone sialoprotein, and produce high levels of the alkaline phosphatase (ALP) ecto-enzyme. Sustained expression of ALP is required for mineralization of skeletal tissues[78,79], and is induced early during osteoblast differentiation[80,81].
Several studies have explored the use of MSCs encapsulated in osteoinductive scaffolds or morphogenic biomaterials to enhance the natural healing process of bone and cartilage in vivo[82-86]. They overall suggest that these multipotent cells seem both able to differentiate themselves within the scaffolds as well as to secrete factors attracting neighboring autologous progenitors. This behavior can accomplish fracture healing faster and with a superior quality of the resulting new bone respect to the osteoinductive or chondrogenic scaffolds used alone[13]. Thus, these promising results have prompted the accomplishment of several studies exploring the concomitant use of MSCs and of the most promising members of the BMP family. Namely BMP2 and 7, embedded in suitable scaffolds or carriers, have been used to heal several cartilage defects and bone fractures in experimental animal models hopefully soon to be transferred to human beings.
Cartilage defects such as degeneration of intervertebral discs and knee joints are ordinary causes of joint disabilities able to affect the quality of life of many people all over the world[87]. It is well known that articular cartilage has a limited capacity of spontaneous repair after damage[88]. Treatments for articular surface lesions usually encompass various clinical approaches like conservation therapies as well as invasive surgery comprising abrasion, debridement and perichondral grafting[87,89]. In recent times also autologous chondrocyte regeneration has been used. Grafting of autologous chondrocytes to promote cartilage resurfacing has some benefits over allogeneic chondrocyte or solid tissue grafting and other procedures[90]. Unfortunately, its application is hindered by chondrocyte de-differentiation during in vitro expansion and the necessity of large amounts of cartilage samples[91]. Recently, the appearance of MSCs in the landscape of the cellular sources for cartilage repair available in quite large quantities raised a great interest and optimism for the treatment of these defects by tissue engineering and cell therapy approaches[92]. As already mentioned, both BMP2 and BMP7 have plenty demonstrated the ability to enhance cartilage repair in vivo as well as the capacity to promote chondrogenic differentiation of MSCs cultured in appropriate inducing media in vitro. Although the outcome of the combined use of precursor cells and BMPs in suitable scaffolds for cartilage repair could be a research field actively persecuted in these years, to date a limited number of studies are present in the literature (Table 2). In particular, one of the major unresolved problems is a durable integration between cartilage and the scaffold[93]. Thus, the presence of chondrogenic precursors releasing chemoattractant factors and of appropriate BMPs stimulating said precursors could ensure the ultimate scaffold remodeling with new cartilaginous tissue formation. Furthermore, both MSCs and BMPs seem able to stimulate endogenous cells to migrate and colonize the artificial graft further promoting the final healing.
| Conditions | Scaffold | Ref. |
| MSCs and BMPs in cartilage defects | ||
| MSCs transfected with BMP7 | Bioresorbable polimer scaffold | [94] |
| MSCs transfected with BMP7 and TGF-β1 | Bilayered osteochondral scaffold | [95] |
| MSCs + TGF-β1, PDGF and BMP2 | Bilayer scaffold with platelet rich plasma | [96] |
| MSCs + TGF-β1, TGF-β3, BMP2, 4 and 7 | Osteochondral allograft with extracellular matrix proteins | [98] |
| MSCs and BMPs in bone defects | ||
| MSCs transfected with BMP2 | Animal models of ectopic and orthotopic bone formation | [103-110] |
| MSCs transfected with BMP2 | Alginate or type I collagen hydrogels | [111] |
| MSCs transfected with BMP2 | Injectable chitosan biopolymer and inorganic phosphate | [112] |
| MSCs + BMP2 | Macroporous β-tricalcium phosphate deposited by robocasting | [72] |
| MSCs + BMP7 | Natural bone mineral particles | [113] |
| MSCs + BMP7 | 3D collagen nanofiber implant | [114] |
In early studies Grande et al[94] transfected MSCs from periosteum with human BMP7 or sonic hedgehog and then seeded them on bioresorbable polymer scaffolds. These implants were used to fill full-thickness osteochondral defects created in the mid-trochlear region of New Zealand white rabbits. The authors observed that, for both genes, their addition significantly enhanced the quality of the repaired tissue, also noticing that the subchondral compartment in the animal group receiving the BMP7-transfected cells seemed to remodel with bone much faster than the sonic hedgehog group.
In another study Chen et al[95] formulated a bilayered gene-activated osteochondral scaffold containing a TGF-β1 plasmid for the chondrogenic layer and a BMP2 plasmid for the osteogenic layer. MSCs seeded in each layer were able to differentiate to chondrocytes and osteoblasts both in vitro and in vivo supporting the articular cartilage and subchondral bone regeneration in the rabbit knee ostechondral defect model.
In a recent study Seo et al[96] investigated the use of bilayer scaffolds embedded with MSCs and platelet rich plasma (PRP) containing TGF-β1 and PDGF for the chondrogenic layer, and MSCs and BMP2, for the osteogenic layer, on the osteochondral defect in an equine model. The defects were produced at the lateral trochlear ridge of the talus, where osteochondrosis is commonly found, and bilayered scaffolds were inserted. Tissue repair was then evaluated showing that implantation of the scaffolds significantly improved osteochondral tissue regeneration respect to controls. Differently, non-ameliorative results were obtained by Gulotta et al[97] testing the use of BMP13-expressing MSCs to improve regeneration of the tendon-bone insertion site in a rat rotator cuff repair model. This study was prompted by the observation that BMP13 has been implicated in tendon and cartilage repair and thus may augment rotator cuff repair. The results showed that new cartilage formation and collagen fiber deposition was observable in both experimental groups (MSCs expressing or non-expressing BMP13) with no significant differences between the two.
Finally, a recently published study from Geraghty et al[98] describes a novel, viable osteochondral allograft containing ECM proteins and chondrogenic growth factors (i.e., TGF-β1 and 3, BMP2, 4, 7, bFGF and IGF1) able to stimulate MSC migration and chondrocyte differentiation in vitro as well as cartilage repair in vivo in a goat microfracture model.
Taken together all the above mentioned studies show that the use of BMPs associated to MSCs to promote articular cartilage repair has brought limited favorable results. Differently, the use of other chondrogenic induction factors such as TGF-β proteins, or heterogeneous cocktails of factors such as PRP or the ones embedded in the new osteochondral allograft described by Geraghty et al[98] have demonstrated more positive outcomes. This has happened likely because these cocktails of factors, also containing BMPs, hold more promising results than the use of BMPs alone in cartilage repair. Indeed, BMPs together with MSCs have shown a higher osteoinductive ability in vivo more than chondrogenic.
Over one million surgical procedures, and in the United States only, each year deal with bone replacement[99]. Skeletal diseases, tumor resection, trauma and congenital malformations are the main reasons for bone defects requiring bone reconstruction. For decades, autologous bone graft has been the gold standard for treatment of bone defects in clinic. Due to limited availability of autologous bone grafts and morbidity of donor sites, stem cell-based tissue engineering strategies are very promising as an alternative therapeutic approach. The use of allogeneic transplantation is restricted due to immunological rejection, premature resorption and possible transmission of infections. Bone generated from human recombinant BMPs alone[100], or embedded in a demineralized bone powder[101], has a limited volume. In addition, biocompatible bone substitutes[102] are subjected to infection and require osteoinductive molecules or tissues for large bone defects. Recently, the use of progenitor MSCs embedded in biocompatible and biodegradable scaffolds, possibly in the presence of growth or osteoinductive factors, has allowed the creation of functional tissues (Table 2).
In early studies a number of researchers showed that autologous or allogeneic MSCs engineered with BMP2 were capable of differentiating into the osteoblast lineage and inducing bone formation in several animal models in both ectopic and orthotopic sites in mice, rats, rabbits and pigs[103-107]. In all these systems the authors concluded that combining MSC implantation with BMP2 gene transfer more effectively induced bone formation than MSC implantation alone.
With similar results, but using a different modular expression system approach Moutsatsos et al[108] used a tetracycline-regulated expression vector encoding human BMP2 to transfect a MSC cell line. With such expression system the authors were able to demonstrate that doxycycline controlled BMP2 expression and thus controlled MSC osteogenic differentiation both in vitro and in vivo in a mouse ectopic bone model. Moreover, they showed increased angiogenesis accompanied by bone formation whenever genetically engineered MSCs were induced to express BMP2 in vivo.
In other studies, Chang et al[109] demonstrated the usefulness of BMP2-expressing MSCs in bone repair of large cranial defect in two different animal models: The rabbit model and the swine model[110]. The authors clearly demonstrated near-complete repair of the large cranial defects by the tissue engineered bone containing BMP2-expressing MSCs in the three months of the experiment both in the rabbit[109] and in the swine[110] with respect to the controls.
Thus, the use BMP2 together with MSCs in bone repair, either exogenously added to cells either enabling cells to directly express the protein, has been in the years thoroughly validated by the above mentioned studies. Consequently, the attention has been focused on the use of different scaffolds able to support the MSC colonization and differentiation as well as the temporospatially controlled delivery of the BMPs to quicken bone reconstruction and healing. Thus, in this contest were alternatively tested: (1) alginate or type I collagen hydrogels as scaffolds loaded with MSCs expressing BMP2 for bone regeneration in a large cranial defect repair in the swine demonstrating the superiority of BMP2-MSC/collagen type I construct over the alginate counterpart[111]; (2) an injectable biopolymer of chitosan and inorganic phosphate seeded with MSCs and BMP2 in a rat calvarial critical size defect demonstrating the superiority of the MSC/BMP2 coupling over the controls[112]; and (3) a macroporous β-tricalcium phosphate (β-TCP) system fabricated by robocasting loaded with MSCs and with BMP2 embedded in microspheres to provide a prolonged BMP release in a critical rat calvarial defect[72]. In the latter case only a minor synergistic effect was demonstrated in the BMP2-MSC group with respect to the BMP2 group alone.
Alternative to these studies only a limited number of works have focused on the concomitant use of BMP7 and MSCs in bone repair (Table 2). In particular Burastero et al[113] used the association of human MSCs and BMP7, with natural bone mineral particles as a scaffold to fill the bone loss, to improve bone regeneration in a rat model of critical size segmental bone defect. Indeed a significantly higher score in bone regeneration was observed in the rats treated with MSCs and BMP7 compared to controls, receiving either MSCs or BMP-7. The data indicated that the association of the two provided a better osteoinductive graft compared to MSCs or BMP7 alone. Finally, Schiavi et al[114] tested a novel 3D collagen nanofiber implant functionalized with BMP7 nanoreservoirs and equipped with human MSC microtissues. The implant was optimized for cell colonization, differentiation and growth. The group clearly demonstrated an acceleration of ectopic bone growth in vivo of the coupled BMP7/MSC microtissues respect to the controls using either BMP7 or MSC microtissues alone.
Since their first identification, BMPs have demonstrated great potentialities in the regenerative medicine and tissue engineering fields. They have been tested in numerous preclinical and clinical studies exploring their chondrogenic or osteoinductive potential in several animal model defects and in human diseases. During the years two BMP members in particular, BMP2 and BMP7, have been thoroughly used in the treatment of a number of cartilage and bone defects and have been recently approved for employment in protocols of nonunion fractures as adjunct therapies.
On the other hand, to date the scientific literature provides extensive in vitro evidence of the improvement of the osteoblastic and chondrogenic potential of MSCs, now obtained from many tissues, by treatment with BMPs. Thus, it was just a matter of time for the two, BMPs and MSCs, to be investigated together hopefully to finally achieve the goal of producing the ideal graft for bone replacement. Besides, recently the grafts have evolved including more and more sophisticated scaffolds, appropriate cell precursors and optimal differentiating factors. As outlined in this review, the growing literature in this field and the promising results in recent years suggest that this goal indeed can be achieved and that both BMPs and MSCs in the future will take part to the production of successful avant-garde implants especially designed for bone tissue engineering.
P- Reviewer: Huang W, Jun Y, Liu J, Liu KY S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Walsh DW, Godson C, Brazil DP, Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends Cell Biol. 2010;20:244-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ. The Concise Guide to PHARMACOLOGY 2013/14: catalytic receptors. Br J Pharmacol. 2013;170:1676-1705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 324] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Sakou T. Bone morphogenetic proteins: from basic studies to clinical approaches. Bone. 1998;22:591-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 260] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 787] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 6. | Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 772] [Cited by in RCA: 741] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 7. | Wagner DO, Sieber C, Bhushan R, Börgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;3:mr1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Nolan K, Thompson TB. The DAN family: modulators of TGF-β signaling and beyond. Protein Sci. 2014;23:999-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 545] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15187] [Article Influence: 584.1] [Reference Citation Analysis (0)] |
| 11. | Scarfì S. Purinergic receptors and nucleotide processing ectoenzymes: Their roles in regulating mesenchymal stem cell functions. World J Stem Cells. 2014;6:153-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Krampera M, Pizzolo G, Aprili G, Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Sun H, Yang HL. Calcium phosphate scaffolds combined with bone morphogenetic proteins or mesenchymal stem cells in bone tissue engineering. Chin Med J (Engl). 2015;128:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Urist MR. Bone: formation by autoinduction. Science. 1965;150:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4079] [Cited by in RCA: 3689] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 15. | Pizette S, Niswander L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol. 2000;219:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 209] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2955] [Cited by in RCA: 2822] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 17. | Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 432] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 18. | Weiskirchen R, Meurer SK. BMP-7 counteracting TGF-beta1 activities in organ fibrosis. Front Biosci (Landmark Ed). 2013;18:1407-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Chang SC, Hoang B, Thomas JT, Vukicevic S, Luyten FP, Ryba NJ, Kozak CA, Reddi AH, Moos M. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227-28234. [PubMed] |
| 20. | Xiao YT, Xiang LX, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Commun. 2007;362:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23:609-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 514] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 22. | Guo J, Wu G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012;23:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA. 1995;92:7632-7636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 400] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 401] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 25. | Riggins GJ, Thiagalingam S, Rozenblum E, Weinstein CL, Kern SE, Hamilton SR, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mad-related genes in the human. Nat Genet. 1996;13:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 265] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Xu L, Chen YG, Massagué J. The nuclear import function of Smad2 is masked by SARA and unmasked by TGFbeta-dependent phosphorylation. Nat Cell Biol. 2000;2:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Shi W, Chang C, Nie S, Xie S, Wan M, Cao X. Endofin acts as a Smad anchor for receptor activation in BMP signaling. J Cell Sci. 2007;120:1216-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687-693. [PubMed] |
| 29. | Shi W, Sun C, He B, Xiong W, Shi X, Yao D, Cao X. GADD34-PP1c recruited by Smad7 dephosphorylates TGFbeta type I receptor. J Cell Biol. 2004;164:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Nakamura J, Yanagita M. Bmp modulators in kidney disease. Discov Med. 2012;13:57-63. [PubMed] |
| 31. | Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1451] [Cited by in RCA: 1427] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 32. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3797] [Cited by in RCA: 4294] [Article Influence: 195.2] [Reference Citation Analysis (0)] |
| 33. | Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573-3584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 877] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 34. | Karsenty G. The complexities of skeletal biology. Nature. 2003;423:316-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 303] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem. 2012;151:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Palmer GD, Steinert A, Pascher A, Gouze E, Gouze JN, Betz O, Johnstone B, Evans CH, Ghivizzani SC. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol Ther. 2005;12:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 40. | Schmitt JM, Hwang K, Winn SR, Hollinger JO. Bone morphogenetic proteins: an update on basic biology and clinical relevance. J Orthop Res. 1999;17:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Xu D, Gechtman Z, Hughes A, Collins A, Dodds R, Cui X, Jolliffe L, Higgins L, Murphy A, Farrell F. Potential involvement of BMP receptor type IB activation in a synergistic effect of chondrogenic promotion between rhTGFbeta3 and rhGDF5 or rhBMP7 in human mesenchymal stem cells. Growth Factors. 2006;24:268-278. [PubMed] |
| 43. | Shintani N, Hunziker EB. Chondrogenic differentiation of bovine synovium: bone morphogenetic proteins 2 and 7 and transforming growth factor beta1 induce the formation of different types of cartilaginous tissue. Arthritis Rheum. 2007;56:1869-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Knippenberg M, Helder MN, Zandieh Doulabi B, Wuisman PI, Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Shen B, Wei A, Tao H, Diwan AD, Ma DD. BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A. 2009;15:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Shen B, Wei A, Whittaker S, Williams LA, Tao H, Ma DD, Diwan AD. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem. 2010;109:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81:710-718. [PubMed] |
| 48. | Chubinskaya S, Kuettner KE. Regulation of osteogenic proteins by chondrocytes. Int J Biochem Cell Biol. 2003;35:1323-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. 2006;14:1126-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26:1407-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 51. | Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 329] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev. 1996;57:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 202] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Valcourt U, Ronzière MC, Winkler P, Rosen V, Herbage D, Mallein-Gerin F. Different effects of bone morphogenetic proteins 2, 4, 12, and 13 on the expression of cartilage and bone markers in the MC615 chondrocyte cell line. Exp Cell Res. 1999;251:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem. 2003;90:1112-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 56. | Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol. 1994;161:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 397] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 57. | Dosch R, Gawantka V, Delius H, Blumenstock C, Niehrs C. Bmp-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development. 1997;124:2325-2334. [PubMed] |
| 58. | Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98:538-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | Macias D, Gañan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM. Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development. 1997;124:1109-1117. [PubMed] |
| 60. | Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977-2986. [PubMed] |
| 61. | Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 628] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 62. | Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007;40:1389-1398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 309] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 64. | Lissenberg-Thunnissen SN, de Gorter DJ, Sier CF, Schipper IB. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011;35:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 65. | Delimar D, Smoljanovic T, Bojanic I. Could the use of bone morphogenetic proteins in fracture healing do more harm than good to our patients? Int Orthop. 2012;36:683; author reply 685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1049] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 67. | Boraiah S, Paul O, Hawkes D, Wickham M, Lorich DG. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. 2009;467:3257-3262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Friedenstein AJ. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Haematol Blood Transfus. 1980;25:19-29. [PubMed] |
| 69. | Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429-435. [PubMed] |
| 70. | Parson AB. Stem cell biotech: seeking a piece of the action. Cell. 2008;132:511-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009;218:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 72. | Del Rosario C, Rodríguez-Évora M, Reyes R, Delgado A, Évora C. BMP-2, PDGF-BB, and bone marrow mesenchymal cells in a macroporous β-TCP scaffold for critical-size bone defect repair in rats. Biomed Mater. 2015;10:045008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 705] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 74. | Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1828] [Cited by in RCA: 1733] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 75. | Taipaleenmäki H, Suomi S, Hentunen T, Laitala-Leinonen T, Säämänen AM. Impact of stromal cell composition on BMP-induced chondrogenic differentiation of mouse bone marrow derived mesenchymal cells. Exp Cell Res. 2008;314:2400-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9969] [Cited by in RCA: 9659] [Article Influence: 508.4] [Reference Citation Analysis (0)] |
| 77. | Kabiri M, Kul B, Lott WB, Futrega K, Ghanavi P, Upton Z, Doran MR. 3D mesenchymal stem/stromal cell osteogenesis and autocrine signalling. Biochem Biophys Res Commun. 2012;419:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, Waymire K, Narisawa S, Millán JL, MacGregor GR. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14:2015-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 280] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 79. | Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, Millán JL. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 263] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 80. | Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 268] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 81. | Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130:1318-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Wang T, Dang G, Guo Z, Yang M. Evaluation of autologous bone marrow mesenchymal stem cell-calcium phosphate ceramic composite for lumbar fusion in rhesus monkey interbody fusion model. Tissue Eng. 2005;11:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Lee LT, Kwan PC, Chen YF, Wong YK. Comparison of the effectiveness of autologous fibrin glue and macroporous biphasic calcium phosphate as carriers in the osteogenesis process with or without mesenchymal stem cells. J Chin Med Assoc. 2008;71:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Jafarian M, Eslaminejad MB, Khojasteh A, Mashhadi Abbas F, Dehghan MM, Hassanizadeh R, Houshmand B. Marrow-derived mesenchymal stem cells-directed bone regeneration in the dog mandible: a comparison between biphasic calcium phosphate and natural bone mineral. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e14-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Chen W, Liu J, Manuchehrabadi N, Weir MD, Zhu Z, Xu HH. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials. 2013;34:9917-9925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 86. | Espitalier F, Vinatier C, Lerouxel E, Guicheux J, Pilet P, Moreau F, Daculsi G, Weiss P, Malard O. A comparison between bone reconstruction following the use of mesenchymal stem cells and total bone marrow in association with calcium phosphate scaffold in irradiated bone. Biomaterials. 2009;30:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487-504. [PubMed] |
| 88. | Campbell CJ. The healing of cartilage defects. Clin Orthop Relat Res. 1969;64:45-63. [PubMed] |
| 89. | Browne JE, Branch TP. Surgical alternatives for treatment of articular cartilage lesions. J Am Acad Orthop Surg. 2000;8:180-189. [PubMed] |
| 90. | Brittberg M. Autologous chondrocyte transplantation. Clin Orthop Relat Res. 1999;367s:S147-S155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 91. | Hunziker EB. Articular cartilage repair: problems and perspectives. Biorheology. 2000;37:163-164. [PubMed] |
| 92. | Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 93. | Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Nöth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 94. | Grande DA, Mason J, Light E, Dines D. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:111-116. [PubMed] |
| 95. | Chen J, Chen H, Li P, Diao H, Zhu S, Dong L, Wang R, Guo T, Zhao J, Zhang J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011;32:4793-4805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 96. | Seo JP, Tanabe T, Tsuzuki N, Haneda S, Yamada K, Furuoka H, Tabata Y, Sasaki N. Effects of bilayer gelatin/β-tricalcium phosphate sponges loaded with mesenchymal stem cells, chondrocytes, bone morphogenetic protein-2, and platelet rich plasma on osteochondral defects of the talus in horses. Res Vet Sci. 2013;95:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Gulotta LV, Kovacevic D, Packer JD, Ehteshami JR, Rodeo SA. Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 98. | Geraghty S, Kuang JQ, Yoo D, LeRoux-Williams M, Vangsness CT, Danilkovitch A. A novel, cryopreserved, viable osteochondral allograft designed to augment marrow stimulation for articular cartilage repair. J Orthop Surg Res. 2015;10:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8352] [Cited by in RCA: 6638] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 100. | Zegzula HD, Buck DC, Brekke J, Wozney JM, Hollinger JO. Bone formation with use of rhBMP-2 (recombinant human bone morphogenetic protein-2). J Bone Joint Surg Am. 1997;79:1778-1790. [PubMed] |
| 101. | Niederwanger M, Urist MR. Demineralized bone matrix supplied by bone banks for a carrier of recombinant human bone morphogenetic protein (rhBMP-2): a substitute for autogeneic bone grafts. J Oral Implantol. 1996;22:210-215. [PubMed] |
| 102. | Furukawa T, Matsusue Y, Yasunaga T, Nakagawa Y, Okada Y, Shikinami Y, Okuno M, Nakamura T. Histomorphometric study on high-strength hydroxyapatite/poly(L-lactide) composite rods for internal fixation of bone fractures. J Biomed Mater Res. 2000;50:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 103. | Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA, Berk AJ, Witte ON. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905-917. [PubMed] |
| 104. | Lou J, Xu F, Merkel K, Manske P. Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 105. | Riew KD, Wright NM, Cheng S, Avioli LV, Lou J. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif Tissue Int. 1998;63:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Cheng SL, Lou J, Wright NM, Lai CF, Avioli LV, Riew KD. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif Tissue Int. 2001;68:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 107. | Tsuchida H, Hashimoto J, Crawford E, Manske P, Lou J. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. J Orthop Res. 2003;21:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 108. | Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, Kelley P, Stumm N, Mi S, Müller R. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 109. | Chang SC, Chuang H, Chen YR, Yang LC, Chen JK, Mardini S, Chung HY, Lu YL, Ma WC, Lou J. Cranial repair using BMP-2 gene engineered bone marrow stromal cells. J Surg Res. 2004;119:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | Chang SC, Lin TM, Chung HY, Chen PK, Lin FH, Lou J, Jeng LB. Large-scale bicortical skull bone regeneration using ex vivo replication-defective adenoviral-mediated bone morphogenetic protein-2 gene-transferred bone marrow stromal cells and composite biomaterials. Neurosurgery. 2009;65:75-81; discussion 81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Chang SC, Chung HY, Tai CL, Chen PK, Lin TM, Jeng LB. Repair of large cranial defects by hBMP-2 expressing bone marrow stromal cells: comparison between alginate and collagen type I systems. J Biomed Mater Res A. 2010;94:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Stephan SJ, Tholpady SS, Gross B, Petrie-Aronin CE, Botchway EA, Nair LS, Ogle RC, Park SS. Injectable tissue-engineered bone repair of a rat calvarial defect. Laryngoscope. 2010;120:895-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Burastero G, Scarfì S, Ferraris C, Fresia C, Sessarego N, Fruscione F, Monetti F, Scarfò F, Schupbach P, Podestà M. The association of human mesenchymal stem cells with BMP-7 improves bone regeneration of critical-size segmental bone defects in athymic rats. Bone. 2010;47:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Schiavi J, Keller L, Morand DN, De Isla N, Huck O, Lutz JC, Mainard D, Schwinté P, Benkirane-Jessel N. Active implant combining human stem cell microtissues and growth factors for bone-regenerative nanomedicine. Nanomedicine (Lond). 2015;10:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |