Published online Jan 26, 2015. doi: 10.4252/wjsc.v7.i1.11
Peer-review started: July 27, 2014
First decision: August 14, 2014
Revised: September 15, 2014
Accepted: September 18, 2014
Article in press: December 16, 2014
Published online: January 26, 2015
Outcomes following peripheral nerve injury remain frustratingly poor. The reasons for this are multifactorial, although maintaining a growth permissive environment in the distal nerve stump following repair is arguably the most important. The optimal environment for axonal regeneration relies on the synthesis and release of many biochemical mediators that are temporally and spatially regulated with a high level of incompletely understood complexity. The Schwann cell (SC) has emerged as a key player in this process. Prolonged periods of distal nerve stump denervation, characteristic of large gaps and proximal injuries, have been associated with a reduction in SC number and ability to support regenerating axons. Cell based therapy offers a potential therapy for the improvement of outcomes following peripheral nerve reconstruction. Stem cells have the potential to increase the number of SCs and prolong their ability to support regeneration. They may also have the ability to rescue and replenish populations of chromatolytic and apoptotic neurons following axotomy. Finally, they can be used in non-physiologic ways to preserve injured tissues such as denervated muscle while neuronal ingrowth has not yet occurred. Aside from stem cell type, careful consideration must be given to differentiation status, how stem cells are supported following transplantation and how they will be delivered to the site of injury. It is the aim of this article to review current opinions on the strategies of stem cell based therapy for the augmentation of peripheral nerve regeneration.
Core tip: Outcomes following peripheral nerve injury remain poor. Stem cells may increase Schwann cell numbers and longevity, prolonging their ability to support regeneration. At the level of the cell body, they may have the ability to rescue populations of neurons destined for apoptosis. They may also be used in non-physiologic ways in order to protect muscle targets from the detrimental effects of denervation. Aside from stem cell type, consideration must be given to differentiation status, scaffolding support and the mechanism of delivery. This article reviews current opinion on the strategies of stem cell-based therapy for the augmentation of peripheral nerve regeneration.
- Citation: Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: A review of current opinion. World J Stem Cells 2015; 7(1): 11-26
- URL: https://www.wjgnet.com/1948-0210/full/v7/i1/11.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i1.11
Following nerve transection, the cell bodies of axons swell in anticipation of the increased metabolic demand of regeneration. Some nerve cells, particularly in response to proximal sectioning of axons, will then go on to apoptosis, lowering the number of regenerating neurons permanently. The Schwann cells (SCs), the principal glial cells of the peripheral nervous system and orchestrators of regeneration, switch from a myelinating to a phagocytic phenotype and, through chemotactic signaling, recruit circulating macrophages into the zone of injury. This heralds the beginning of Wallerian degeneration and serves to clear the distal stump of axonal and myelin debris in preparation for the reception of the regenerating growth cone. Through complex and incompletely understood cell signaling, arborized daughter axons extending from the proximal stump are attracted to columns of SCs known as the Bands of Büngner. These scaffolds nurture and guide regenerating axons towards their distal basal lamina tubes. Once SCs and the regenerating axon have been reunited, a growth promoting symbiosis is established. Any interruption in this process results in chronic SC senescence, down-regulation, a loss of axonal support and compromised recovery.
The gold standard method of nerve repair involves re-approximation using suture. Even with meticulous tissue handling and accurate, tensionless re-orientation of fascicular anatomy, outcomes are limited by suture inflammation, intra-and extra neural scar tissue, escape of axons and neurotrophic factors into the surrounding tissues, axonal mis-direction and the extremely slow rates of regeneration. These limitations are amplified following proximal limb injuries and in nerve gap reconstructions when axons must traverse two coaptation sites bridged by nerve grafts.
Autologous nerve grafts represent the gold standard bridging material for nerve gaps but these are limited by the morbidity of harvesting an “expendable” nerve. In addition, following severe trauma involving multi-limb amputation, demand for autologous material may exceed supply. In these situations, alternatives such as conduits or acellular nerve allograft (ANA) must be considered. The efficacy of hollow conduits is limited to short distances due to the lack of cellular components and extracellular matrix (ECM) to aid axonal guidance. Despite increasingly elaborate designs, these fail to replicate the efficacy of autogenous nerve. The use of ANA provides the most accurate representation of ECM, however, these grafts lack SCs and must be repopulated with endogenous SCs migrating from adjacent nerve. As a result, outcomes following the use of these grafts for large gaps have typically been poor in comparison to autografts, though better than conduits. Improving the performance of conduits and allografts by adding stem cells, ECM components and growth factors is an area of intensive research, one that may have enormous clinical implications.
The overarching goal of any stem cell based approach to peripheral nerve injury is to establish a more favorable environment for regenerating axons and perhaps more importantly, maintain this support for an extended period of time. Repopulation of the depleted motor or sensory neuron pools with stem cells would potentially provide higher numbers of neurons for regeneration, which could aid regeneration in an additive fashion to the creation of a favorable environment. Preventing SC senescence and down-regulation during prolonged recovery periods, such as occurs in proximal injuries and long nerve gaps, and expediting the re-innervation of distal targets should translate to improvements in recovery. Finally, stem cell transplantation into denervated muscle may provide a means of preventing the expected sequelae of denervation, preventing atrophy and leaving the tissue more receptive to reinnervation over longer periods. It is the aim of this article to discuss how stem cell-based strategies have attempted to overcome these problems and how they may provide solutions in the future.
The ideal stem cell for clinical application, including peripheral nerve repair, must be easily accessible, rapidly expandable in culture, capable of in vivo survival and integration into host tissue and must be amenable to stable transfection and expression of exogenous genes[1]. If the process of nerve regeneration is deconstructed into a sequence of individual events, a strategy for optimizing outcome can be formulated. Emphasis has been placed on the importance of stem cell type, differentiation, cell scaffold and method of cell delivery[2]. The influence on regeneration of each of these components has been thoroughly investigated. An overview of each of these, in addition to proposed mechanisms of action behind the therapeutic effect, will now be provided. Table 1 supplements the section on stem cell type, summarizing outcomes following the application of different stem cells in animal models.
| Stem cell source | Author | Experimental model | Stemcell Diff. | Scaffold | Delivery system | Outcome |
| Embryonic | Cui et al[71] | Rat sciatic transection (10 mm gap) | D | Culture medium | Epineurium natural conduit | Cell survival and differentiation into SCs after 3-mo; superior regeneration of myelinated axons in comparison to culture media alone |
| Lee et al[75] | Mouse sciatic transection (2 mm gap) | U | Matrigel | Direct injection of microspheres | ESC-derived MSC sphere-treated nerves recovered significantly greater CMAP, SFI and histological parameters than ESC-MSC single cell suspension | |
| Kubo et al[77] | Mouse tibial | D | PBS | Direct injection into gastrocnemius muscles following nerve transection and repair | Co-culture identified formation of new NMJs; muscle transplanted with stem cells experienced less atrophy 7 and 21-d post injury; cells transplanted after 2-wk were unable to provide any protective effect; motor recovery following repair superior in those muscles receiving stem cells | |
| Craff et al[76] | Rat sciatic + gastrocnemius muscle | D | PBS | Direct injection into gastrocnemius muscles following nerve transection | Muscles injected with stem cells retained muscle weight preservation and myocyte cross sectional area in comparison to control muscle after 7-d post injury. New NMJs observed. Benefits lost after 21 d | |
| Neural | Fu et al[15] | Rat sciatic transection (15 mm gap; gene transfection) | U (gene therapy) | Culture medium (cells seeded directly onto conduit wall) | Poly(D,L-Lactide) conduit | Enhanced expression of BDGF and GDNF in transfected cells; conduits with transfected cells led to larger myelinated axons and improved functional and electrophysiological outcome |
| Zhang et al[62] | Rabbit facial nerve transection (5 mm gap) | U | Collagen medium | HA-collagen composite conduit | Conduits with NSCs and NT-3 experienced superior outcomes in comparison to NSC alone; results equivalent to normal nerves after 12-wk | |
| Murakami et al[86] | Rat sciatic transection (15 mm gap) | U | Collagen gel | Silicone conduit | NSCs differentiated into astrocytes, oligodendrocytes and schwann cell-like cells; dNCSs implanted into 15 mm defects; Improved axon number, diameter and myelination compared with controls; labeled cells present after 10-wk; expressed markers of SC phenotype | |
| Guo et al[87] | Rabbit facial nerve transection (10 mm gap) | U | Collagen sponge | Chitosan conduit | Electrophysiological and histological outcomes and immunohistochemistry superior in chitosan conduits seeded with NSCs and NGF in comparison to conduit+NGF alone. Results comparable to standard autograft | |
| Liard et al[88] | Pig nervis cruralis transection (30 mm gap) | U | Neurosphere in culture medium | Autologous vein graft | Grafts containing NSCs recovered superior EMG recording and immunohistochemistry profiles in comparison to empty conduits; NSCs identifiable after 240 d follow-up. | |
| Johnson et al[89] | Rat sciatic crush, transection, (10 mm gap) | U (C17.2) | Culture medium | Direct injection | 12/45 rodents developed neuroblastomas | |
| Bone marrow | Zhao et al[160] | Rat sciatic transection (15 mm gap) | U | Fibrin glue | ANA | Survival of BMSCs within fibrin glue; growth factor secretion preserved (NGF, BDNF); equivalent results when cells injected directly into nerve compared with around nerve |
| Hu et al[165] | Monkey median transection (50 mm gap) | U | Culture medium | Chitosan conduit with longitudinally aligned PGLA fibers | Functional and electrophysiological recovery and FG retrograde tracing after 1-year with BMSC-laden conduits equivalent to autograft and superior to empty conduits | |
| Dezawa et al[19] | Rat sciatic transection (15 mm gap) | U + D | Matrigel | Matrigel graft | Successful differentiation into SC phenotype; Axon number and elongation superior with dBMSCs | |
| Jia et al[32] | Rat sciatic transection (10 mm gap) | U | Gelatin | Acellular xenograft | Neurotrophic factor expression elevated in BMSC xenografts; regeneration and functional recovery significantly better than empty xenografts and equivalent to autograft | |
| Mohammadi et al[34] | Rat sciatic transection (10 mm gap) | U | Culture medium | Vein graft | Veins filled with uBMSCs had significantly improved functional, histological and immunohistochemical outcomes compared with veins filled with PBS | |
| Nijhuis et al[36] | Rat sciatic transection (15 mm gap) | U | Culture medium | Vein graft +/- muscle | BMSC identifiable after 6 and 12-wk follow-up; vein graft + muscle + BMSCs outperformed vein graft+muscle but inferior to autograft | |
| Salomone et al[39] | Rat facial nerve transection (3 mm gap) | U + D | Matrigel | Silicone conduit | Histological outcomes superior in conduits containing uBMSCs and dBMSCs compared to empty and matrigel containing conduits; functional outcomes superior using uBMSCs | |
| Wang et al[41] | Rat sciatic transection (15 mm gap) | U | Culture medium | Direct injection into ANA | BMSC produced NGF and BDNF; CSPGs reduced in grafts treated with ChABC Allograft containing BMSCs and ChABC resulted in superior functional, electrophysiological and histological outcome compared with BMSC alone | |
| Adipose | di Summa et al[28] | Rat sciatic transection (10 mm gap) | D | Culture medium | Fibrin glue conduit | Reduced muscle atrophy in autograft, dADSC and dBMSC groups in comparison to empty conduits; dADSCs recovered greatest axon and fiber diameter, evoked potentials and regeneration of motorneurons; results comparable to autograft |
| Tomita et al[21] | Rat common peroneal nerve transection (no gap) | D | Culture medium | Direct injection into distal nerve | dADSCs survived for at least 10 wk in vivo; dADSCs associated with axons and participated in re-myelination; dADSCs resulted in regeneration superior to cultured SCs | |
| Zhang et al[31] | Rat sciatic transection (10 mm gap) | D | Collagen gel | Xenogeneic acellular graft | dADSCs formed columns resembling bands of Büngner and expressed NGF, BDNF and GDNF; axon regeneration, retrograde labeling and electrophysiology were similar between dADSCs and SC supplemented grafts, superior to empty grafts but inferior to standard autograft | |
| Mohammadi et al[33] | Rat sciatic transection (10 mm gap) | U | Culture medium | Vein graft | No difference in functional, morphometric or immunohistochemistry between ADSCs and BMSCs | |
| Erba et al[45] | Rat sciatic transection (10 mm gap) | U | Fibrin | PHB conduit | Lack of sufficient quantities of viable cells 14-d after transplantation; conclusion that regenerative effect due to initial growth factor boost or paracrine effect on resident cells | |
| Sun et al[51] | Rat facial transection (8 mm gap) | D | Matrigel | Decellularized allogeneic artery | dADSCs persisted at repair site and integrated with regenerated tissue; conduits containing dADSCs achieved results comparable to those of SC-containing conduits and superior to matrigel-containing conduits alone; results inferior to autograft | |
| Fetal | Pan et al[106] | Rat sciatic crush | U | Fibrin glue | Direct injection at site | High expression of BDNF, CNTF, NGF and NT-3 found in AFMSCs; motor function, CMAP and conduction velocity improved in those nerves augmented with AFMSCs; high levels of S-100 and GFAP and reduced fibrosis found at repair site |
| Pan et al[111] | Rat sciatic crush | U | Fibrin glue | Direct injection at site | HBO therapy reduced production of inflammatory cytokines and macrophage chemokines following crush injury; when administered with AFMSCs, HBO reduced apoptosis of AFMSCs in comparison to AFMSCs alone; myelination and motor recovery superior in HBO + AFMSC group | |
| Pan et al[110] | Rat sciatic crush | U | Fibrin glue | Direct injection at site | Anti-apoptotic, anti-inflammatory agent G-CSF, when administered with AFMSCs, reduced crush-induced inflammation, and apoptosis in comparison to AFMSCs alone; myelination and motor function superior with AFMSCs + G-CSF in comparison to AFMSCs alone | |
| Matsuse et al[109] | Rat sciatic nerve transection (8 mm gap) | U + D | Matrigel | “Transpermeable” tube | Differentiated UC-MSCs regenerated greater number of myelinated axons and thicker nerve fibers compared with undifferentiated UC-MSCs; number of labeled cells greater in dUC-MSC nerves; results comparable to SC group | |
| Cheng et al[113] | Rat sciatic crush | U | Matrigel | Direct injection at site | AFMSCs successfully transfected; high expression of GDNF detected for 4 wk before subsiding; GDNF-modified AFMSCs recovered greatest SFI, conduction velocity, CMAP and muscle weight in comparison to AFMSCs alone | |
| Gärtner et al[108] | Rat sciatic crush | D | Culture medium | Cells seeded onto PLC wrap | Wraps seeded with UC-MSCs resulted in superior increased myelin thickness, motor and sensory function in comparison to unseeded wraps | |
| Skin | McKenzie et al[120] | Mouse sciatic crush | D/U | Culture medium | Direct injection at site | SKPs successfully induced into SKP-SCs; SKP and SKP-SCs associated with and myelinated axons |
| Marchesi et al[123] | Rat sciatic transection (16 mm gap) | D | PBS | (1) Synthetic co-polymer L-lactide and trimethylene carbonate; and (2) collagen conduit | SFI and CMAPs were significantly better in conduits filled with SDSCs; number of regenerated myelinated axons significantly greater in SDSC conduits; no significant difference in neurotrophic factor expression | |
| Walsh et al[124] | Rat sciatic transection (12 mm gap) | D | Culture medium | Direct injection into acellular freeze-thawed nerve graft | SKP-SCs maintained differentiation up to 8-wk; outcomes significantly improved in comparison to cell free grafts and comparable to cultured SCs; neurotrophic factor release greater in SKP-SCs | |
| Walsh et al[121] | Rat CP/tibial (Immediate vs chronic repair; no gap) | D | Culture medium | Direct injection into distal nerve | Muscle weight and CMAPs superior in SKP-SC group in comparison to media injected controls; significantly higher counts of axon regeneration in SKP-SC group equivalent to immediate suture group | |
| Walsh et al[22] | Rat sciatic transection [acute vs chronic vs ANA (12 mm gap)] | U/D | Culture medium | Direct injection into nerve ends and ANA | SKP-SCs maintained in vivo viability and differentiation better than uSKP; viability poorest in normal nerve, best in acutely injured nerve; SKP-SCs remain differentiated over time and myelinate axons; neuregulin able to prevent apoptosis following transplantation | |
| Khuong et al[122] | Rat sciatic and tibial (12 mm gap) | D | Culture medium | Direct injection into ANA | SKP-SCs containing allografts resulted in superior functional and histological outcomes in both acute and delayed injury models compared with SCs and media controls | |
| Hair follicle | Amoh et al[135] | Mouse sciatic and tibial transection (no gap) | U | Culture medium | Direct injection at site | HFSC transplanted nerves recovered significantly greater function compared with untreated nerves; GFP-labeled cells differentiated into GFAP positive schwann cells and were involved with myelination |
| Amoh et al[133] | Mouse sciatic crush | U | Culture medium | Direct injection at site | HFSCs transplanted around crushed nerve differentiated into SC-like cells and participated in myelination; gastrocnemius muscle contraction significantly greater compared with untreated crushed nerves | |
| Amoh et al[134] | Mouse sciatic transection (2 mm gap) | U | Culture medium | Direct injection at site | HFSCs differentiated into GFAP expressing SCs and were able to myelinate axons; gastrocnemius muscle contraction significantly greater compared with untreated nerves | |
| Lin et al[136] | Rat sciatic transection (40 mm gap) | D | PBS | Direct injection into acellular xenograft | Differentiation into neurons and SCs maintained for 52-wk; number of regenerated axons, myelin thickness and ratio of myelinated axons to total nerve count significantly higher in dHFSCs compared with acellular grafts; conduction velocity slower in dHFSC nerves | |
| Induced pluripotent stem cell | Ikeda et al[146] | Mouse sciatic nerve (5 mm gap) | D | Microsphere seeded into conduit | Mixed PLA/PCL conduit +/- iPSC microspheres +/- bFGF | Regeneration was accelerated by combination of iPSCs + bFGF within conduits in comparison to iPSCs and bFGF alone; outcomes remained inferior to autograft controls; empty conduits performed least well |
| Uemura et al[148] | Mouse sciatic nerve (5 mm gap) | D | Microsphere seeded into conduit | Mixed PLA/PCL conduit +/- iPSC microspheres | Motor and sensory recovery was superior in iPSC group at 4, 8 and 12 wk in comparison to empty conduits. Axonal regeneration superior in iPSC group. Conduit structurally stable after 12 wk | |
| Wang et al[149] | Rat sciatic nerve (12 mm gap) | D | Matrigel | PLCL/PPG/sodium acetate copolymer electrospun nanofiber conduit | Conduits filled with either (1) matrigel; (2) matrigel + NCSCs differentiated from ESCs; and (3) matrigel + NCSCs differentiated from iPSCs; NCSC differentiated into SCs and integrated into myelin sheaths; electrophysiology and histology showed equivalent regeneration in all NCSC containing conduits; no teratoma formation observed after 1-yr |
Stem cells have the potential to replace lost neurons or increase the number of glial support cells. Replacing lost neurons has been widely demonstrated in vitro and has great potential in the CNS. Although not yet demonstrated in the dorsal root ganglia or ventral horn of the spinal cord, stem cells have the potential to rescue and replenish axotomized neurons as well as to manipulate the microenvironment of the neurons during regeneration. In the peripheral nervous system, emphasis has been placed mostly on increasing SC number and activity. This approach is driven by the pragmatic difficulties associated with autologous SC culture. Achieving sufficient autologous SC numbers requires explantation of healthy nerve, extended periods of purification and expansion and consequently, delays to repair. Prolonged axotomy and denervation only exacerbate central cell body apoptosis and loss of SC-mediated axonal support in the distal nerve[3,4]. As a result, the use of cultured SCs is considered impractical.
Exogenous stem cells differentiated into SC-like phenotype may integrate into Bands of Büngner and aid axonal guidance and re-myelination. Transplanted cells enhance growth factor secretion and ECM production. These factors may be released directly or, through cell-to-cell contact and paracrine signaling, can stimulate endogenous SCs to upregulate secretory activity[5]. Increased recruitment of circulating macrophages into the area can potentiate this effect[6].
ECM proteins such as collagen I, collagen IV, fibronectin and laminin, and neurite guidance proteins such as netrin and ninjurin-2 have pro-regenerative effects[7,8]. Other ECM components such as chondroitin sulphate proteoglycan are potent inhibitors of axonal regeneration and the degradation of this protein by liberated matrix metalloproteinase-2 has been shown to support regeneration[9,10].
The production of nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), ciliary neurotrophic factor and neurotrophin-3 (NT-3) by endogenous SCs and transplanted stem cells has been described[5,7,8,11-17]. Angiogenic factors such as vascular endothelial growth factor, basic fibroblast growth factor (bFGF), hepatocyte growth factor and angiopoietin-1 may also be released[12]. Hypoxia has been found to potentiate the angiogenic and neurogenic influence of some stem cells, leading to improved blood vessel and nerve fiber formation[7]. This has led some investigators to attempt pre-conditioning and differentiation in hypoxic environments[7]. The expression of leukemia inhibitory factor and insulin-like growth factor has also been detected[8]. These factors are thought to improve neuronal survival, promote corticospinal tract growth, increase astrogliosis and potentiate the inflammatory response[8,18]. The observed immunomodulatory effects of stem cells are believed to be due to the secretion of factors such as granulocyte and macrophage colony stimulating factor (G-CSF, M-CSF), interleukin-6, 7, 8, and 11 (IL-6, IL-7, IL-8, IL-11) and TNF-α[8]. Suppression of the host immune response may help reduce the detrimental impact of inflammation and fibrosis following nerve repair and is obviously desirable in the context of allotransplantation of cells. The relative contribution of each of these mechanisms and therefore the levels of ECM protein and growth factor production may vary not only with stem cell type but also with the differentiation status of transplanted cells.
Stem cells can be transplanted in their undifferentiated state or can undergo a period of in vitro differentiation into SC-like cells. This is commonly achieved with exposure to β-mercaptoethanol, all-trans retinoic acid, fetal bovine serum, forskolin, recombinant human bFGF, recombinant human platelet derived growth factor-AA, and recombinant human heregulin β-1[19,20]. The purported benefits of differentiation include superior in vivo viability and an enhancement of neurotrophic factor secretion and myelinating ability[14,21,22]. Many investigators have shown the beneficial effects on regeneration of these cells[19-21,23-31]. Some claim that transplanting undifferentiated cells risks in vivo differentiation along unwanted, non-neuronal lines in response to other dominant cells in the area.
Interestingly, however, the positive effects of undifferentiated cells have also been widely reported[5,7,13,32-51]. These investigators claim that in vitro differentiation incurs an unnecessary delay, limiting clinical applicability. In one study, the process of differentiation actually reduced the secretion of neurotrophic factors[52]. Others have reported difficulties with maintaining in vivo neuronal differentiation, claiming that once in vitro differentiation cues are removed, cells tend to revert back to their original phenotype[53]. Evidence suggests that undifferentiated cells may undergo in vivo differentiation in response to local stimuli[20,40,54-56], although others have been unable to show this[45,49,57]. A small number of direct comparisons have been performed and have shown either a small advantage for differentiated cells[20,23], a small advantage for undifferentiated cells[39] or no significant difference[45,48].
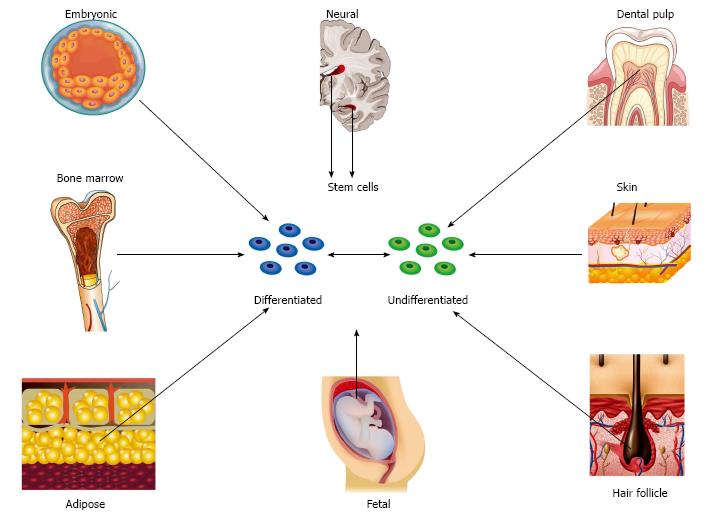
Embryonic stem cells: Thomson et al[58] described the isolation of pluripotent cell lines from human blastocysts in 1998. Since this discovery, stem cells have been harvested from a variety of different fetal and adult tissues (Figure 1). Embryonic stem cells (ESCs) can form derivatives of all three embryonic germ layers and can provide an unlimited source of cells with superior differentiation potential and long-term proliferation capacity in comparison to mesenchymal stem cells (MSCs)[59]. Unlike adult sources, these cells are homogenous, are not susceptible to the detrimental impact of age and disease and can be harvested without physical discomfort[60]. However, differentiation down specialized neural cell lines is challenging and established protocols only exist for a limited number of lines[61,62]. Additional disadvantages include immunogenicity, tumourogenicity and potential ethical controversy due to the fact that cells are harvested during the blastocyst stage of development and consequently result in the destruction of embryos.
ESCs have been shown to migrate and differentiate into neurons, and glial cells of the central and peripheral nervous system and have the capacity for in vivo myelination[61,63-70]. Studies reporting the successful application of these cells to peripheral nerve repair are limited, perhaps reflecting the lack of facile clinical translatability[71]. In recognition of the limitations, investigators have derived mesenchymal precursors from ESC[59,72-74]. Augmentation of peripheral nerve regeneration following the transplantation of spheres of human MSCs derived from ESCs has been reported[75]. Our group has shown in small animal models of sciatic and tibial nerve transection that ESCs transplanted directly into muscle can preserve short-term muscle mass and myocyte cross-sectional area following injury and repair[76,77].
Nerve stem cells: Nerve stem cells (NSCs) naturally differentiate into neurons and glial cells. However, the process of neurogenesis almost exclusively occurs during embryogenesis and continues in the adult in response to injury only within very limited locations of the CNS[78]. NSCs were first isolated from adult murine brain in the early 1990s[79,80]. This was followed by similar discoveries in humans and non-human primates[81,82]. The subventricular zone and the subgranular zone in the brain represent the primary sites of NSC differentiation in adult mammalians (Figure 1). Most recently, populations of NSCs and neurogenesis have been discovered in adjacent areas of the adult human striatum[83]. Neural crest stem cells have been isolated from fetal rat sciatic nerve but the existence of such cells in peripheral nerves of the adult human is unknown[84,85].
Many have reported positive results following NSC implantation into peripheral nerve injury[15,62,86-88]. Heine et al[9] showed that the beneficial effects of NSC transplantation are not confined to acute peripheral nerve injury. Transplantation of NSCs into chronically denervated nerve had a beneficial effect on morphological and electrophysiological recovery in comparison to controls. Immortalized murine C17.2 NCSs have become commercially available and are commonly used for in vivo animal studies. However, experience with C17.2 NSCs in the PNS has not been uniformly positive. Following the implantation of these cells into rat models of crush, transection and graft, high rates of neuroblastoma formation have been encountered[89]. In addition to these concerns, pragmatic difficulties associated with cell harvest have limited their use.
MSCs: MSCs are multipotent stromal cells that originate from the bone marrow and a variety of non-marrow sources such as adipose tissue, fetal tissue, skin, hair follicle and dental pulp (Figure 1). Although the potency of these cells was originally considered to be limited to tissues of mesodermal origin, differentiation into non-mesodermal lineage is now generally accepted. It is precisely this characteristic that endows MSCs with the ability to support neuroregeneration. An overview of the main sub-types of MSC and the evidence behind their application to peripheral nerve regeneration will now be discussed.
Bone marrow derived stem cells: In contrast to embryonic and NSCs, bone marrow derived stem cells (BMSCs) are readily accessible and have no potential ethical concerns. Under appropriate conditions BMSCs can differentiate into non-mesodermal lineages such as neurons, astrocytes and SC-like cells[90]. Many studies have shown that the addition of BMSCs to conduits and acellular grafts results in superior outcomes when compared with empty or cell deplete channels[24,36-38,40,41]. Although some have failed to show that BMSCs can match outcomes achieved with cultured SCs[20], the majority of reports show that performance is at least equivalent[27,43,91]. There is a dearth of studies showing superiority over gold standard autograft although some report equivalent outcomes[42,44]. Evidence suggests that these outcomes may be dose dependent[92].
Although BMSCs are more clinically applicable than ESCs, NSCs and cultured SCs, stem cell fraction is low and in comparison with other sources, BMSCs generally have inferior proliferation capacity and differentiation potential. In addition, harvest is invasive and painful. This has driven the pursuit of less invasive MSC sources such as fetal tissue, skin, hair follicle and adipose tissue.
Adipose derived stem cells: Adipose tissue is abundant, easily harvested and is a frequent waste product of commonly performed procedures such as liposuction. It has a superior stem cell fraction in comparison to bone marrow and adipose derived stem cells (ADSCs) have superior proliferation and differentiation potential in comparison to BMSCs[93,94]. Unlike BMSCs, donor age and site of harvest does not seem to significantly influence the therapeutic effect of ADSCs[50].
Multiple neuronal and SC markers have been expressed from differentiated ADSCs[7,30,95-97]. The expression of myelin protein zero, peripheral myelin protein-22 and myelin basic protein also suggest the retention of myelinating capability[98]. Although some reports have found no difference in outcome between ADSC-filled conduits and cell deplete products[49], the vast majority of studies show an augmented effect[13,28,29,31,99]. Several studies have shown that ADSCs are at least as effective as autologous SCs[21,45,51]. As with BMSCs, a small number of investigators have reported equivalent outcomes in comparison to autograft[13,28,29] with even fewer reporting superior outcomes[48]. Studies directly comparing the performance of ADSCs to BMSCs have found no significant differences between the two[33,45]. Low morbidity of harvest, wide availability and superior stem cell characteristics have helped establish ADSCs as the preferred option for pre-clinical studies.
Fetal derived stem cells: Fetal tissues represent the most primitive source of MSCs. These tissues are ordinarily discarded following birth and are therefore in abundant supply. Tissue age rarely exceeds 42-wk and therefore, in comparison to other adult sources, cells have accumulated less genetic damage due to age, environment and disease[100]. Stem cells can be harvested from amniotic membrane, amniotic fluid, umbilical cord cells, umbilical cord blood and Wharton’s jelly. These cells are readily expandable in culture and possess the ability to differentiate into neural phenotype[101,102].
Wharton’s jelly derived mesenchymal stem cells can differentiate into functional SC-like cells, produce neurotrophic factors such as NGF, BDNF and NT-3 and stimulate neurite growth in vitro[103]. Amniotic fluid derived stem cells (AFDSCs) express characteristics of both mesenchymal and NSCs[104], can differentiate into neural tissue[105] and, following transplantation into rat sciatic nerve gap models, have been shown to promote peripheral nerve regeneration[106,107]. Umbilical cord-derived mesenchymal stem cells have also been shown to improve outcomes following crush and transection injuries in rodent models[108,109].
Survival of AFDSCs following transplantation can be limited and as a result, efforts have been made to improve in vivo survivability. Inhibition of inflammatory mediators can attenuate the apoptotic cascade[110-112]. Cells can be genetically modified in order to over-express therapeutic genes. GDNF-modified human AFMSCs have been shown to improve viability, regeneration and motor outcomes in rodent models[113]. Stromal cell-derived factor-1α (SDF-1α) is chemotactic towards MSCs and is expressed in several tissues in the rat, including injured nerve. By timing intravenous administration of AFDSCs with peak expression of SDF-1α, this novel approach has been shown to improve AFDSC numbers at the repair site and nerve regeneration[114].
Fetal tissue represents a promising alternative source of stem cells. However the use of autologous cells following injury is currently impractical. The use of allogeneic cells and their associated immunoreactivity are obstacles that are not encountered with other adult sources such as adipose tissue. Widespread banking of fetal products following birth offers a solution to this problem.
Skin derived precursors: Skin derived precursors (SKPs) reside in the dermis and are an accessible source of adult multipotent cells that are easily expandable in culture. SKPs exhibit a remarkable similarity to embryonic neural crest cells, a migratory population of multipotent cells that gives rise to a diverse array of cell types such as melanocytes, craniofacial cartilage, bone and connective tissue, smooth muscle of vasculature, endocrine cells, and neurons and glial cells of the autonomic and peripheral nervous system[115]. Neuregulin is known to promote proliferation and differentiation of SCs from embryonic neural crest precursors[115-117] and is also believed to be mediate SC proliferation during Wallerian degeneration[118]. When cultured with neuregulin-1β, rodent and human SKPs express markers consistent with functioning SCs[115-120]. Exogenous neureglins and heregulin-1β may also improve in vivo viability by reducing apoptosis[22].
Both undifferentiated and differentiated SKPs (SKP-SCs) have been shown to have positive effects on regeneration. In rodent models, SKP-SCs have been shown to maintain differentiation and viability, myelinate axons, and exhibit superior outcomes following de-myelination and crush injury[117,120], acute and chronic transection injury[22,120-122] and following implantation into ANAs[122-124].
Hair follicle stem cells: Hair follicles are dynamic dermal appendages and are abundant and readily accessible sources of pluripotent stem cells. The hair follicle is derived from the neural crest and harbors NCSC-like cells[125]. Hair follicle stem cells (HFSCs) were discovered following the detection of nestin, a maker for neural progenitor cells, in the cells of the hair follicle bulge in mice[126] and humans[127]. They are readily expanded in culture, although, as with SKPs, clinical applicability may be limited by prolonged periods of cell expansion. HFSCs are positive for ESC transcription factors Nanog, Oct4 and nestin, and have the ability to differentiate into adipocytes, smooth muscle cells, melanocytes, neurons and glial cells[116,128,129]. Differentiation into epithelial and vascular cells emphasizes the important role HFSCs have in vascularizing the skin and dermis[130-132].
A small number of studies have reported the use of undifferentiated HFSCs in murine models of sciatic and tibial nerve crush and transection injuries[133-135]. HFSCs were found to differentiate into glial fibrillary acidic protein positive SC-like cells and participated in myelination. Functional outcomes were significantly improved in those nerves receiving HFSCs. The efficacy of differentiated HFSCs seeded into acellular xenografts has also been investigated[136]. Cells were able to maintain differentiation long term and the number of regenerated axons and myelination in cell-supplemented xenografts was higher than cell-free xenografts.
Dental pulp stem cells: Dental pulp is neural crest-derived tissue that can be harvested from exfoliated deciduous teeth and arguably represents the most convenient source of multipotent stem cells. As with fetal tissue, widespread application of autologous cells would require harvest and storage of teeth at an early age. Although experience is limited with this newly described population of cells, evidence shows that these cells may have a role in supporting peripheral nerve regeneration. In avian embryonic models, dental pulp stem cells (DPSCs) have been shown to chemo-attract trigeminal ganglion axons[137]. DPSCs have also been shown to successfully differentiate into SCs in vitro and were able to support DRG neurite outgrowth[138]. When implanted into a collagen gel matrix, DPSCs were able to form aligned columns and had the ability to guide and myelinate neurites[138]. Rat DPSCs have also been shown to provide axonal support following hemisection of the spinal cord[139,140].
Induced pluripotential stem cells: In 2006, Takahashi et al[141] described the derivation of induced pluripotency in somatic mouse cells following ectopic co-expression of transcription factors[141]. This ability to reprogram cells questioned the stability of cellular identity and injected new optimism into the quest to develop a patient specific pluripotent stem cell that could circumvent the limitations of ESCs. Although multiple similarities between ESCs and induced pluripotential stem cells (iPSCs) exist, subtle differences have also been detected[142]. Currently it is believed that these cells are neither identical nor distinct populations and instead represent cells with overlapping identity. These differences do not abrogate their potential. Since their discovery, iPSCs have furthered the understanding of many disease mechanisms and have advanced in vitro drug screening and the assessment of therapeutic efficacy following pharmaceutical and cell-based interventions[142].
In addition to differentiation into various somatic cell lines, established protocols now exist for the in vitro differentiation of iPSCs along neural lineages[143,144]. Although evidence suggests that differentiation occurs with reduced efficiency and increased variability[145], iPSCs have been shown to have a pro-regenerative effect in small animal models of central and peripheral nervous system injury[146-149].
These cells possess several advantages over embryonic sources such as the avoidance of ethical and immunosuppressive issues. However, concerns surrounding these cells exist. Retained epigenetic “memory” from their somatic cells of origin, coupled with chromosomal aberrations resulting from re-programming, can render these cells even more tumorogenic than ESCs[150].
The preferred option: Adipose derived stem cells represent the most practical source of cells for transplantation. In light of easier harvest, superior stem cell fraction, differentiation potential and proliferation capacity, they have supplanted bone marrow derived cells. Other alternatives such as fetal, hair follicle and tooth pulp have great potential but are currently limited by the lack of autogenous supply and the requirements for cell expansion. Whether these cells are best differentiated prior to transplantation is a contentious issue although the use of undifferentiated cells obviates the requirement for in vitro differentiation. The beneficial effect of these cells on regeneration has been widely reported and, until clear evidence emerges that differentiation is superior, the use of undifferentiated cells is the most pragmatic and clinically translatable option. Optimizing the effect of the preferred option is heavily reliant on appropriate delivery and support.
Cell scaffolds aim to maintain cell viability, support proliferation and permit intercellular communication and growth factor elution. N-methacrylate glycol chitosan, alginate, hyaluronic acid, polyethylene glycol, fibrin and Matrigel have all been shown to successfully fulfill these requirements[151-157]. In addition to their supportive role, scaffolds also help prevent unwanted cell dissipation from the site of injury and, when used in combination with nerve conduits, have emerged as useful adjuncts for improving the delivery of cells.
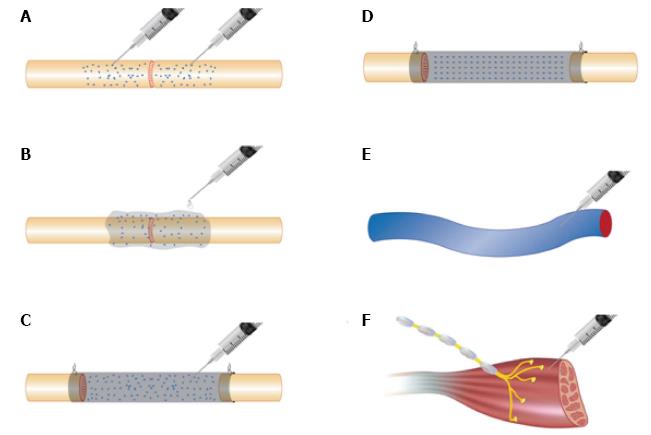
In cases where nerves are repaired primarily or when autograft or allograft nerve is going to be used, stem cells can be delivered by a variety of different methods (Figure 2). Cells suspended in culture medium can be microinjected into nerve ends or grafts (Figure 2A)[28,29,32,40,158-160]. Although this delivers cells directly to the site of injury, the process of micro-injection can be traumatic to the delicate intra-neural architecture and can result in unpredictable cell distribution. Evidence suggests that intra-neural injection may be unnecessary. Cells suspended in fibrin matrix that were injected around repair sites maintained neurotrophic factor secretion and cell migration and resulted in outcomes that were not significantly different in comparison to injected nerves (Figure 2B)[160].
Cells can be injected within the lumen of a conduit (Figure 2C) or can be seeded onto conduit matrix (Figure 2D). Natural conduits including vein and artery grafts are rich in ECM proteins such as collagen and laminin and provide a useful substrate for cell adhesion, though only at the periphery of the constructs[33,34,37,161-163]. Vessels can remain empty or can be pre-filled with tissue such as muscle to support axonal guidance[36]. Commercially available natural conduits are usually composed of ECM components such as collagen[38] and fibrin[28]. Synthetic materials include polyglycolic acid[57], Poly(lactic-co-glycolic) acid[164], silicone tube[39,43], polyhydroxybutyrate[20], polytetrafluoroethylene[24] silk fibroin[42] and chitosan[91,165]. The degradation profile of conduits must be carefully considered when used with cell therapy. Natural conduits tend to degrade in a predictable, non-toxic fashion, though the rate of degradation may not be sufficiently slow in some cases to allow for adequate regeneration time. Some synthetic polymers can acidify the microenvironment during degradation and can have a detrimental impact on cellular activity[166].
In recognition of the importance of basal lamina and other ECM framework for axonal guidance, conduits with internal structure have become more popular than hollow, single lumen tubes. These structures may be organized arrangements of multiple fibers[23,164,165] or less orderly structures such as collagen sponge[24]. However, in the absence of cells, attempts to replicate the structural complexity of the nerve have led to disappointing results.
Recent evidence exists that systemic administration of stem cells following peripheral nerve injury can be efficacious (Figure 2E)[5,114]. With further refinement, it may become possible to sustain regenerative support through regular systemic dosing. There is also evidence that stem cells may be efficacious when administered at the level of neuromuscular junction (NMJ) (Figure 2F) or when distributed within denervated muscle[76,77,167].
Nerve regeneration and re-innervation is a protracted and dynamic process. Any attempts to improve the speed and integrity of this process must be equally enduring and migratory. Local delivery of biochemical mediators to the site of repair is a common approach, though this provides only finite support that is unable to follow the advancing growth cone, not to mention recapitulate the elegant temporal coordination of myriad different factors. Development of systemic administration and cell homing to bring these cells to the advancing neural growth cone and the regenerating axons may help sustain regenerative support.
A fusion of cell based therapy and optogenetics may also hold promise by restoring motor function in those that have lost central control. Murine embryonic stem cell derived motor neurons have been induced to express the light-sensitive ion channel channelrhodopsin-2. When engrafted into mouse sciatic nerve, not only did axons re-innervate hind limb muscles, but function was restored in a controllable manner through optical stimulation and did not require connection to the central nervous system[168].
Despite in vitro and pre-clinical success, the application of stem cells to peripheral nerve repair has yet to make an impact in the clinical arena. Translation is currently limited by justified concerns regarding genetic manipulation, cell instability and the risks of tumorigenesis. However, perhaps the most pertinent issue is that despite great effort to manipulate the regenerative process, the use of stem cells simply has not led to outcomes that significantly and consistently surpass those achieved with conventional techniques. Augmentation of peripheral nerve repair with stem cells must consider stem cell type, differentiation, cell scaffold and method of delivery in order to offer effective new therapies. These factors, in addition to animal and injury models, vary widely in the current literature, making it very difficult to draw definitive conclusions as to which combination is most efficacious.
The ideal strategy would involve administration of stem cells immediately, or as soon as possible, following injury and for this to be sustained throughout the period of regeneration and recovery. This may require a shift away from the development of methods of local delivery and a re-focus on developing cells that can be administered systemically and that have the ability to specifically target and support not only the migrating growth cone, but also centrally located cell bodies and peripherally located NMJs.
P- Reviewer: Tanabe S, Wang LS S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908-3913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 744] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 2. | Widgerow AD, Salibian AA, Kohan E, Sartiniferreira T, Afzel H, Tham T, Evans GR. “Strategic sequences” in adipose-derived stem cell nerve regeneration. Microsurgery. 2014;34:324-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886-3895. [PubMed] [Cited in This Article: ] |
| 4. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995;15:3876-3885. [PubMed] [Cited in This Article: ] |
| 5. | Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, Constantin G, Bedogni G, Bedogni A, Bonetti B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012;18:1264-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Ribeiro-Resende VT, Pimentel-Coelho PM, Mesentier-Louro LA, Mendez RM, Mello-Silva JP, Cabral-da-Silva MC, de Mello FG, de Melo Reis RA, Mendez-Otero R. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience. 2009;159:540-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;6:e17899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 8. | Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 9. | Heine W, Conant K, Griffin JW, Höke A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004;189:231-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 449] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 11. | Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, Wang CC, Wang WY, Huang YS, Hsu SH. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014;23:741-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia H, Wang Y, Tong L, Tong X. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med. 2011;28:565-572. [PubMed] [Cited in This Article: ] |
| 14. | Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Fu KY, Dai LG, Chiu IM, Chen JR, Hsu SH. Sciatic nerve regeneration by microporous nerve conduits seeded with glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor gene transfected neural stem cells. Artif Organs. 2011;35:363-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in F6Publishing: 611] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 17. | Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in F6Publishing: 650] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 18. | Hawryluk GW, Mothe A, Wang J, Wang S, Tator C, Fehlings MG. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21:2222-2238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14:1771-1776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 422] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Tomita K, Madura T, Mantovani C, Terenghi G. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res. 2012;90:1392-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Walsh SK, Kumar R, Grochmal JK, Kemp SW, Forden J, Midha R. Fate of stem cell transplants in peripheral nerves. Stem Cell Res. 2012;8:226-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun. 2007;359:915-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Wakao S, Hayashi T, Kitada M, Kohama M, Matsue D, Teramoto N, Ose T, Itokazu Y, Koshino K, Watabe H. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp Neurol. 2010;223:537-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (4)] |
| 25. | Keilhoff G, Goihl A, Stang F, Wolf G, Fansa H. Peripheral nerve tissue engineering: autologous Schwann cells vs. transdifferentiated mesenchymal stem cells. Tissue Eng. 2006;12:1451-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Keilhoff G, Stang F, Goihl A, Wolf G, Fansa H. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol. 2006;26:1235-1252. [PubMed] [Cited in This Article: ] |
| 27. | Ladak A, Olson J, Tredget EE, Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol. 2011;228:242-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 28. | di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63:1544-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 30. | Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, Xu J, Yang W, Fan X, Du B. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials. 2010;31:5312-5324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Jia H, Wang Y, Tong XJ, Liu GB, Li Q, Zhang LX, Sun XH. Sciatic nerve repair by acellular nerve xenografts implanted with BMSCs in rats xenograft combined with BMSCs. Synapse. 2012;66:256-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Mohammadi R, Azizi S, Delirezh N, Hobbenaghi R, Amini K. Comparison of beneficial effects of undifferentiated cultured bone marrow stromal cells and omental adipose-derived nucleated cell fractions on sciatic nerve regeneration. Muscle Nerve. 2011;43:157-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Mohammadi R, Azizi S, Delirezh N, Hobbenaghi R, Amini K, Malekkhetabi P. The use of undifferentiated bone marrow stromal cells for sciatic nerve regeneration in rats. Int J Oral Maxillofac Surg. 2012;41:650-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Goel RK, Suri V, Suri A, Sarkar C, Mohanty S, Sharma MC, Yadav PK, Srivastava A. Effect of bone marrow-derived mononuclear cells on nerve regeneration in the transection model of the rat sciatic nerve. J Clin Neurosci. 2009;16:1211-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Nijhuis TH, Bodar CW, van Neck JW, Walbeehm ET, Siemionow M, Madajka M, Cwykiel J, Blok JH, Hovius SE. Natural conduits for bridging a 15-mm nerve defect: comparison of the vein supported by muscle and bone marrow stromal cells with a nerve autograft. J Plast Reconstr Aesthet Surg. 2013;66:251-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Nijhuis TH, Brzezicki G, Klimczak A, Siemionow M. Isogenic venous graft supported with bone marrow stromal cells as a natural conduit for bridging a 20 mm nerve gap. Microsurgery. 2010;30:639-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Pereira Lopes FR, Camargo de Moura Campos L, Dias Corrêa J, Balduino A, Lora S, Langone F, Borojevic R, Blanco Martinez AM. Bone marrow stromal cells and resorbable collagen guidance tubes enhance sciatic nerve regeneration in mice. Exp Neurol. 2006;198:457-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Salomone R, Bento RF, Costa HJ, Azzi-Nogueira D, Ovando PC, Da-Silva CF, Zanatta DB, Strauss BE, Haddad LA. Bone marrow stem cells in facial nerve regeneration from isolated stumps. Muscle Nerve. 2013;48:423-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, Yang LM, Wang HG, Yi JH. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008;1188:44-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Wang Y, Jia H, Li WY, Tong XJ, Liu GB, Kang SW. Synergistic effects of bone mesenchymal stem cells and chondroitinase ABC on nerve regeneration after acellular nerve allograft in rats. Cell Mol Neurobiol. 2012;32:361-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Yang Y, Yuan X, Ding F, Yao D, Gu Y, Liu J, Gu X. Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2011;17:2231-2244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Zarbakhsh S, Bakhtiyari M, Faghihi A, Joghataei MT, Mehdizadeh M, Khoei S, Mansouri K, Yousefi B, Pirhajati V, Moradi F. The effects of schwann and bone marrow stromal stem cells on sciatic nerve injury in rat: a comparison of functional recovery. Cell J. 2012;14:39-46. [PubMed] [Cited in This Article: ] |
| 44. | Zheng L, Cui HF. Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J Mater Sci Mater Med. 2012;23:2291-2302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg. 2010;63:e811-e817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Ghoreishian M, Rezaei M, Beni BH, Javanmard SH, Attar BM, Zalzali H. Facial nerve repair with Gore-Tex tube and adipose-derived stem cells: an animal study in dogs. J Oral Maxillofac Surg. 2013;71:577-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Mohammadi R, Azizi S, Amini K. Effects of undifferentiated cultured omental adipose-derived stem cells on peripheral nerve regeneration. J Surg Res. 2013;180:e91-e97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg. 2012;65:657-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Santiago LY, Clavijo-Alvarez J, Brayfield C, Rubin JP, Marra KG. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009;18:145-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 50. | Sowa Y, Imura T, Numajiri T, Nishino K, Fushiki S. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. 2012;21:1852-1862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Sun F, Zhou K, Mi WJ, Qiu JH. Repair of facial nerve defects with decellularized artery allografts containing autologous adipose-derived stem cells in a rat model. Neurosci Lett. 2011;499:104-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Hong SQ, Zhang HT, You J, Zhang MY, Cai YQ, Jiang XD, Xu RX. Comparison of transdifferentiated and untransdifferentiated human umbilical mesenchymal stem cells in rats after traumatic brain injury. Neurochem Res. 2011;36:2391-2400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Keilhoff G, Goihl A, Langnäse K, Fansa H, Wolf G. Transdifferentiation of mesenchymal stem cells into Schwann cell-like myelinating cells. Eur J Cell Biol. 2006;85:11-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 54. | Chen X, Wang XD, Chen G, Lin WW, Yao J, Gu XS. Study of in vivo differentiation of rat bone marrow stromal cells into schwann cell-like cells. Microsurgery. 2006;26:111-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Cuevas P, Carceller F, Dujovny M, Garcia-Gómez I, Cuevas B, González-Corrochano R, Diaz-González D, Reimers D. Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res. 2002;24:634-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Hu J, Zhu QT, Liu XL, Xu YB, Zhu JK. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol. 2007;204:658-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Costa HJ, Ferreira Bento R, Salomone R, Azzi-Nogueira D, Zanatta DB, Paulino Costa M, da Silva CF, Strauss BE, Haddad LA. Mesenchymal bone marrow stem cells within polyglycolic acid tube observed in vivo after six weeks enhance facial nerve regeneration. Brain Res. 2013;1510:10-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11399] [Cited by in F6Publishing: 10061] [Article Influence: 387.0] [Reference Citation Analysis (0)] |
| 59. | Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 60. | Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 483] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 61. | Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 871] [Cited by in F6Publishing: 749] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 62. | Zhang H, Wei YT, Tsang KS, Sun CR, Li J, Huang H, Cui FZ, An YH. Implantation of neural stem cells embedded in hyaluronic acid and collagen composite conduit promotes regeneration in a rabbit facial nerve injury model. J Transl Med. 2008;6:67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA. 2000;97:6126-6131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 448] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 64. | Ziegler L, Grigoryan S, Yang IH, Thakor NV, Goldstein RS. Efficient generation of schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev. 2011;7:394-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Brokhman I, Gamarnik-Ziegler L, Pomp O, Aharonowiz M, Reubinoff BE, Goldstein RS. Peripheral sensory neurons differentiate from neural precursors derived from human embryonic stem cells. Differentiation. 2008;76:145-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Mizuseki K, Sakamoto T, Watanabe K, Muguruma K, Ikeya M, Nishiyama A, Arakawa A, Suemori H, Nakatsuji N, Kawasaki H. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:5828-5833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 67. | Pomp O, Brokhman I, Ben-Dor I, Reubinoff B, Goldstein RS. Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells. 2005;23:923-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 68. | Hotta R, Pepdjonovic L, Anderson RB, Zhang D, Bergner AJ, Leung J, Pébay A, Young HM, Newgreen DF, Dottori M. Small-molecule induction of neural crest-like cells derived from human neural progenitors. Stem Cells. 2009;27:2896-2905. [PubMed] [Cited in This Article: ] |
| 69. | Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009;18:1059-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 70. | Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 71. | Cui L, Jiang J, Wei L, Zhou X, Fraser JL, Snider BJ, Yu SP. Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells. 2008;26:1356-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Lee EJ, Lee HN, Kang HJ, Kim KH, Hur J, Cho HJ, Lee J, Chung HM, Cho J, Cho MY. Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng Part A. 2010;16:705-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 74. | Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914-1922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | Lee EJ, Xu L, Kim GH, Kang SK, Lee SW, Park SH, Kim S, Choi TH, Kim HS. Regeneration of peripheral nerves by transplanted sphere of human mesenchymal stem cells derived from embryonic stem cells. Biomaterials. 2012;33:7039-7046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Craff MN, Zeballos JL, Johnson TS, Ranka MP, Howard R, Motarjem P, Randolph MA, Winograd JM. Embryonic stem cell-derived motor neurons preserve muscle after peripheral nerve injury. Plast Reconstr Surg. 2007;119:235-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Kubo T, Randolph MA, Gröger A, Winograd JM. Embryonic stem cell-derived motor neurons form neuromuscular junctions in vitro and enhance motor functional recovery in vivo. Plast Reconstr Surg. 2009;123:139S-148S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Paspala SA, Murthy TV, Mahaboob VS, Habeeb MA. Pluripotent stem cells - a review of the current status in neural regeneration. Neurol India. 2011;59:558-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565-4574. [PubMed] [Cited in This Article: ] |
| 80. | Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 742] [Cited by in F6Publishing: 708] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 81. | Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4410] [Cited by in F6Publishing: 4164] [Article Influence: 160.2] [Reference Citation Analysis (0)] |
| 82. | Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999;96:5263-5267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 544] [Cited by in F6Publishing: 590] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 83. | Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 631] [Cited by in F6Publishing: 639] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 84. | Le Douarin N, Dulac C, Dupin E, Cameron-Curry P. Glial cell lineages in the neural crest. Glia. 1991;4:175-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 597] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 86. | Murakami T, Fujimoto Y, Yasunaga Y, Ishida O, Tanaka N, Ikuta Y, Ochi M. Transplanted neuronal progenitor cells in a peripheral nerve gap promote nerve repair. Brain Res. 2003;974:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 87. | Guo BF, Dong MM. Application of neural stem cells in tissue-engineered artificial nerve. Otolaryngol Head Neck Surg. 2009;140:159-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Liard O, Segura S, Sagui E, Nau A, Pascual A, Cambon M, Darlix JL, Fusai T, Moyse E. Adult-brain-derived neural stem cells grafting into a vein bridge increases postlesional recovery and regeneration in a peripheral nerve of adult pig. Stem Cells Int. 2012;2012:128732. [PubMed] [Cited in This Article: ] |
| 89. | Johnson TS, O’Neill AC, Motarjem PM, Nazzal J, Randolph M, Winograd JM. Tumor formation following murine neural precursor cell transplantation in a rat peripheral nerve injury model. J Reconstr Microsurg. 2008;24:545-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Tohill M, Terenghi G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem. 2004;40:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, Shum DK. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 92. | Raheja A, Suri V, Suri A, Sarkar C, Srivastava A, Mohanty S, Jain KG, Sharma MC, Mallick HN, Yadav PK. Dose-dependent facilitation of peripheral nerve regeneration by bone marrow-derived mononuclear cells: a randomized controlled study: laboratory investigation. J Neurosurg. 2012;117:1170-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 360] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 94. | Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 676] [Cited by in F6Publishing: 629] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 95. | Safford KM, Safford SD, Gimble JM, Shetty AK, Rice HE. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 96. | Xu Y, Liu L, Li Y, Zhou C, Xiong F, Liu Z, Gu R, Hou X, Zhang C. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res. 2008;1239:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 97. | Xu Y, Liu Z, Liu L, Zhao C, Xiong F, Zhou C, Li Y, Shan Y, Peng F, Zhang C. Neurospheres from rat adipose-derived stem cells could be induced into functional Schwann cell-like cells in vitro. BMC Neurosci. 2008;9:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 98. | Mantovani C, Mahay D, Kingham M, Terenghi G, Shawcross SG, Wiberg M. Bone marrow- and adipose-derived stem cells show expression of myelin mRNAs and proteins. Regen Med. 2010;5:403-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Liu GB, Cheng YX, Feng YK, Pang CJ, Li Q, Wang Y, Jia H, Tong XJ. Adipose-derived stem cells promote peripheral nerve repair. Arch Med Sci. 2011;7:592-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 100. | Fairbairn NG, Randolph MA, Redmond RW. The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg. 2014;67:662-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 101. | Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 102. | Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 428] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 103. | Peng J, Wang Y, Zhang L, Zhao B, Zhao Z, Chen J, Guo Q, Liu S, Sui X, Xu W. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84:235-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 104. | Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee JL, Chang YJ. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod. 2006;74:545-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 105. | Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450-1456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 418] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 106. | Pan HC, Cheng FC, Chen CJ, Lai SZ, Lee CW, Yang DY, Chang MH, Ho SP. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J Clin Neurosci. 2007;14:1089-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 107. | Pan HC, Yang DY, Chiu YT, Lai SZ, Wang YC, Chang MH, Cheng FC. Enhanced regeneration in injured sciatic nerve by human amniotic mesenchymal stem cell. J Clin Neurosci. 2006;13:570-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 108. | Gärtner A, Pereira T, Alves MG, Armada-da-Silva PA, Amorim I, Gomes R, Ribeiro J, França ML, Lopes C, Carvalho RA. Use of poly(DL-lactide-ε-caprolactone) membranes and mesenchymal stem cells from the Wharton’s jelly of the umbilical cord for promoting nerve regeneration in axonotmesis: in vitro and in vivo analysis. Differentiation. 2012;84:355-365. [PubMed] [Cited in This Article: ] |
| 109. | Matsuse D, Kitada M, Kohama M, Nishikawa K, Makinoshima H, Wakao S, Fujiyoshi Y, Heike T, Nakahata T, Akutsu H. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J Neuropathol Exp Neurol. 2010;69:973-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 110. | Pan HC, Chen CJ, Cheng FC, Ho SP, Liu MJ, Hwang SM, Chang MH, Wang YC. Combination of G-CSF administration and human amniotic fluid mesenchymal stem cell transplantation promotes peripheral nerve regeneration. Neurochem Res. 2009;34:518-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Pan HC, Chin CS, Yang DY, Ho SP, Chen CJ, Hwang SM, Chang MH, Cheng FC. Human amniotic fluid mesenchymal stem cells in combination with hyperbaric oxygen augment peripheral nerve regeneration. Neurochem Res. 2009;34:1304-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Pan HC, Yang DY, Ho SP, Sheu ML, Chen CJ, Hwang SM, Chang MH, Cheng FC. Escalated regeneration in sciatic nerve crush injury by the combined therapy of human amniotic fluid mesenchymal stem cells and fermented soybean extracts, Natto. J Biomed Sci. 2009;16:75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Cheng FC, Tai MH, Sheu ML, Chen CJ, Yang DY, Su HL, Ho SP, Lai SZ, Pan HC. Enhancement of regeneration with glia cell line-derived neurotrophic factor-transduced human amniotic fluid mesenchymal stem cells after sciatic nerve crush injury. J Neurosurg. 2010;112:868-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 114. | Yang DY, Sheu ML, Su HL, Cheng FC, Chen YJ, Chen CJ, Chiu WT, Yiin JJ, Sheehan J, Pan HC. Dual regeneration of muscle and nerve by intravenous administration of human amniotic fluid-derived mesenchymal stem cells regulated by stromal cell-derived factor-1α in a sciatic nerve injury model. J Neurosurg. 2012;116:1357-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 115. | Biernaskie JA, McKenzie IA, Toma JG, Miller FD. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1:2803-2812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 116. | Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1189] [Cited by in F6Publishing: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 117. | Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 461] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 118. | Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci. 1997;17:1642-1659. [PubMed] [Cited in This Article: ] |
| 119. | Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 587] [Cited by in F6Publishing: 564] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 120. | McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26:6651-6660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 121. | Walsh SK, Gordon T, Addas BM, Kemp SW, Midha R. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp Neurol. 2010;223:221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Khuong HT, Kumar R, Senjaya F, Grochmal J, Ivanovic A, Shakhbazau A, Forden J, Webb A, Biernaskie J, Midha R. Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp Neurol. 2014;254:168-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 123. | Marchesi C, Pluderi M, Colleoni F, Belicchi M, Meregalli M, Farini A, Parolini D, Draghi L, Fruguglietti ME, Gavina M. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia. 2007;55:425-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 124. | Walsh S, Biernaskie J, Kemp SW, Midha R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164:1097-1107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 125. | Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 126. | Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:13495-13500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 127. | Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 278] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 128. | Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol. 2010;130:1227-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 129. | Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231:258-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 130. | Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102:5530-5534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 131. | Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1000] [Cited by in F6Publishing: 977] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 132. | Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA. 2004;101:13291-13295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 133. | Amoh Y, Aki R, Hamada Y, Niiyama S, Eshima K, Kawahara K, Sato Y, Tani Y, Hoffman RM, Katsuoka K. Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. J Dermatol. 2012;39:33-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 134. | Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, Hoffman RM, Katsuoka K. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: an advantageous alternative to ES and iPS cells. J Cell Biochem. 2009;107:1016-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 135. | Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA. 2005;102:17734-17738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 241] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 136. | Lin H, Liu F, Zhang C, Zhang Z, Guo J, Ren C, Kong Z. Pluripotent hair follicle neural crest stem-cell-derived neurons and schwann cells functionally repair sciatic nerves in rats. Mol Neurobiol. 2009;40:216-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 137. | Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009;27:2229-2237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 138. | Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28:1634-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 139. | Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 140. | Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004;19:2388-2398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 141. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17989] [Cited by in F6Publishing: 17055] [Article Influence: 947.5] [Reference Citation Analysis (0)] |
| 142. | Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 825] [Cited by in F6Publishing: 765] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 143. | Denham M, Dottori M. Neural differentiation of induced pluripotent stem cells. Methods Mol Biol. 2011;793:99-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 144. | Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 669] [Cited by in F6Publishing: 701] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 145. | Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335-4340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 735] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 146. | Ikeda M, Uemura T, Takamatsu K, Okada M, Kazuki K, Tabata Y, Ikada Y, Nakamura H. Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system. J Biomed Mater Res A. 2014;102:1370-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 147. | Satarian L, Javan M, Kiani S, Hajikaram M, Mirnajafi-Zadeh J, Baharvand H. Engrafted human induced pluripotent stem cell-derived anterior specified neural progenitors protect the rat crushed optic nerve. PLoS One. 2013;8:e71855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 148. | Uemura T, Takamatsu K, Ikeda M, Okada M, Kazuki K, Ikada Y, Nakamura H. Transplantation of induced pluripotent stem cell-derived neurospheres for peripheral nerve repair. Biochem Biophys Res Commun. 2012;419:130-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 149. | Wang A, Tang Z, Park IH, Zhu Y, Patel S, Daley GQ, Li S. Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011;32:5023-5032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 150. | Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 653] [Cited by in F6Publishing: 613] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 151. | Sukarto A, Yu C, Flynn LE, Amsden BG. Co-delivery of adipose-derived stem cells and growth factor-loaded microspheres in RGD-grafted N-methacrylate glycol chitosan gels for focal chondral repair. Biomacromolecules. 2012;13:2490-2502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 152. | Galateanu B, Dimonie D, Vasile E, Nae S, Cimpean A, Costache M. Layer-shaped alginate hydrogels enhance the biological performance of human adipose-derived stem cells. BMC Biotechnol. 2012;12:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 153. | Espandar L, Bunnell B, Wang GY, Gregory P, McBride C, Moshirfar M. Adipose-derived stem cells on hyaluronic acid-derived scaffold: a new horizon in bioengineered cornea. Arch Ophthalmol. 2012;130:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 154. | Brandl FP, Seitz AK, Tessmar JK, Blunk T, Göpferich AM. Enzymatically degradable poly(ethylene glycol) based hydrogels for adipose tissue engineering. Biomaterials. 2010;31:3957-3966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 155. | Natesan S, Zhang G, Baer DG, Walters TJ, Christy RJ, Suggs LJ. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng Part A. 2011;17:941-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 156. | Cho SW, Kim I, Kim SH, Rhie JW, Choi CY, Kim BS. Enhancement of adipose tissue formation by implantation of adipogenic-differentiated preadipocytes. Biochem Biophys Res Commun. 2006;345:588-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 157. | Stillaert F, Findlay M, Palmer J, Idrizi R, Cheang S, Messina A, Abberton K, Morrison W, Thompson EW. Host rather than graft origin of Matrigel-induced adipose tissue in the murine tissue-engineering chamber. Tissue Eng. 2007;13:2291-2300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 158. | Pang CJ, Tong L, Ji LL, Wang ZY, Zhang X, Gao H, Jia H, Zhang LX, Tong XJ. Synergistic effects of ultrashort wave and bone marrow stromal cells on nerve regeneration with acellular nerve allografts. Synapse. 2013;67:637-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 159. | Wang Y, Zhao Z, Ren Z, Zhao B, Zhang L, Chen J, Xu W, Lu S, Zhao Q, Peng J. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Lett. 2012;514:96-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 160. | Zhao Z, Wang Y, Peng J, Ren Z, Zhang L, Guo Q, Xu W, Lu S. Improvement in nerve regeneration through a decellularized nerve graft by supplementation with bone marrow stromal cells in fibrin. Cell Transplant. 2014;23:97-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 161. | Barcelos AS, Rodrigues AC, Silva MD, Padovani CR. Inside-out vein graft and inside-out artery graft in rat sciatic nerve repair. Microsurgery. 2003;23:66-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 162. | Berenholz L, Segal S, Gilad VH, Klein C, Yehezkeli E, Eviatar E, Kessler A, Gilad GM. Agmatine treatment and vein graft reconstruction enhance recovery after experimental facial nerve injury. J Peripher Nerv Syst. 2005;10:319-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 163. | Sun F, Zhou K, Mi WJ, Qiu JH. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials. 2011;32:8118-8128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 164. | Hou SY, Zhang HY, Quan DP, Liu XL, Zhu JK. Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience. 2006;140:101-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 165. | Hu N, Wu H, Xue C, Gong Y, Wu J, Xiao Z, Yang Y, Ding F, Gu X. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials. 2013;34:100-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 166. | Bell JH, Haycock JW. Next generation nerve guides: materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B Rev. 2012;18:116-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 167. | Dadon-Nachum M, Sadan O, Srugo I, Melamed E, Offen D. Differentiated mesenchymal stem cells for sciatic nerve injury. Stem Cell Rev. 2011;7:664-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 168. | Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, Burrone J, Greensmith L, Lieberam I. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science. 2014;344:94-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |