Published online Nov 26, 2014. doi: 10.4252/wjsc.v6.i5.644
Revised: September 8, 2014
Accepted: September 16, 2014
Published online: November 26, 2014
Processing time: 61 Days and 16.8 Hours
Mesenchymal stem cells are currently considered as a promising tool for therapeutic application in acute kidney injury (AKI) management. AKI is characterized by acute tubular injury with rapid loss of renal function. After AKI, inflammation, oxidative stress and excessive deposition of extracellular matrix are the molecular events that ultimately cause the end-stage renal disease. Despite numerous improvement of supportive therapy, the mortality and morbidity among patients remain high. Therefore, exploring novel therapeutic options to treat AKI is mandatory. Numerous evidence in animal models has demonstrated the capability of mesenchymal stem cells (MSCs) to restore kidney function after induced kidney injury. After infusion, MSCs engraft in the injured tissue and release soluble factors and microvesicles that promote cell survival and tissue repairing. Indeed, the main mechanism of action of MSCs in tissue regeneration is the paracrine/endocrine secretion of bioactive molecules. MSCs can be isolated from several tissues, including bone marrow, adipose tissue, and blood cord; pre-treatment procedures to improve MSCs homing and their paracrine function have been also described. This review will focus on the application of cell therapy in AKI and it will summarize preclinical studies in animal models and clinical trials currently ongoing about the use of mesenchymal stem cells after AKI.
Core tip: Mesenchymal stem cells (MSCs) may have an important therapeutic potential in acute kidney injury management. A body of evidence has demonstrated that MSCs act through a paracrine/endocrine secretion of soluble factors and microvesicles. We summarize preclinical studies and ongoing clinical trials that evaluate the role of MSCs in restoring kidney function. We critically explain the current concerns about the use of MSCs and microvesicles that limit their applications in clinical trials. Then, we propose the future directions that could lead to extend MSCs use in humans.
- Citation: Bianchi F, Sala E, Donadei C, Capelli I, La Manna G. Potential advantages of acute kidney injury management by mesenchymal stem cells. World J Stem Cells 2014; 6(5): 644-650
- URL: https://www.wjgnet.com/1948-0210/full/v6/i5/644.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i5.644
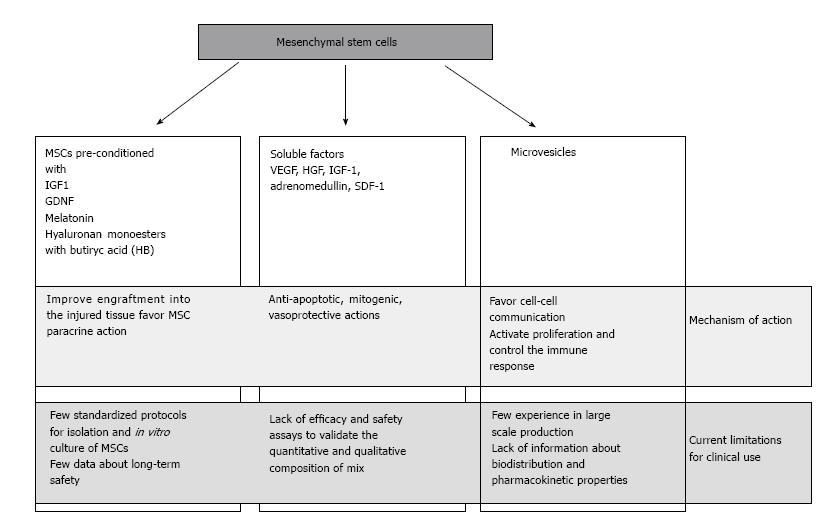
Acute kidney injury (AKI) is a complex clinical syndrome that affects up to 20% of hospitalized patients. Ischemia/reperfusion injury (IRI) is a major cause of AKI, and it is characterized by acute tubular injury and rapid renal dysfunction, generally caused by ischemic or toxic insults[1-3]. The kidney undergoing IRI presents an extensive and complex inflammatory/oxidative stress response, that may result in fibroblast proliferation and excessive deposition of extracellular matrix and has been recognized as a major contributor to end-stage kidney disease[4]. Although many efforts have been made to deal with this problem, such as new drugs and modern dialysis techniques, innovative interventions beyond supportive therapy are not available yet[5]; therefore, a potent therapeutic intervention for ischemia AKI is imperative. In recent years, a promising approach to manage renal IRI is the use of mesenchymal stem cells (MSCs). Their use in treating different kind of diseases as immunological, vascular, cardiac and renal diseases has been extensively explored[6,7]. MSCs can be isolated from various sources, such as bone marrow or adipose tissue, but other organs have their own niches of MSC-like cells, such as the kidney. Besides their broad distribution in the body and an easy isolation, the interest in MSC was originally raised by their capacity to differentiate into other cell types, suggesting that they could be a source of healthy cells to repair/replace injured tissue[8]. There is evidence from both in vitro studies and animal models of AKI that MSCs can promote regenerative responses in the injured kidney, leading to tissue repair and improvement of renal function[9-11]. These beneficial effects have been initially ascribed to the trans-differentiation of MSCs into organ specific cells. However, at least in the kidney, this is a very rare event and the kidney-protective effects of MSCs have been attributed mainly to paracrine mechanisms[12]. This review will focus on the application of cell therapy in AKI, and it will summarize the recent preclinical and clinical results about the use of MSCs in renal IRI (Figure 1).
Mesenchymal stem cells are undifferentiated adult stem cells derived from mesodermal embryonic layer that can differentiate into a broad range of different mesenchymal tissues, including cartilage, bone, muscle, stroma, fat, tendon, and other connective tissues[13]. These cells have been originally isolated from bone marrow where they regulate the self-renewal, maturation and recruitment of hematopoietic stem cells to vascular compartment[14], thanks to their peculiar property to adhere to tissue culture plastic[15]. MSCs are able to in vitro differentiate into cells of mesodermal lineages, such as adipocytes, chondrocytes and osteocytes by the exposure to appropriate conditioning media. A variety of protocols for isolation and expansion are currently used to prepare mesenchymal stem cells for preclinical and clinical use. However, the International Society for Cellular Therapy has identified some potential biomarkers useful to fully characterize MSCs, including the surface antigens CD105, CD73 and CD90, and the lack of the hematopoietic markers CD34, CD45, CD14 or CD11, CD79α or CD19, and HLS class II[16].
Other sources of MSCs are the blood cord and the adipose tissue. Blood cord MSCs have characteristics and immune phenotype similar to BM MSCs, but a differentiating potential limited to osteocytes and chondrocytes while MSCs from adipose tissue have more potent anti-inflammatory and immune-modulatory properties than BM MSCs[17]. MSCs from adipose tissue can be easily prepared after non-invasive liposuction according to the guidelines of International Federation of Adipose Therapeutics e International Society for Cellular Therapy[18] that has appositely proposed a document to standardize international parameters to use MSC from adipose tissue in preclinical and clinical use. This attempt to standardize the use of MSC in biomedical research is pivotal and should be extended to other sources of MSCs in order to promptly define functional and qualitative criteria for these cells. Indeed, the heterogeneity of protocols of isolation and expansion has the results that investigators have used MSCs with different properties without frequently being aware of these differences[19]. The use to record in process data during MSCs preparation and the availability of this information in the supplemental material could be useful to partially overcome the problem and optimize the comparison among different studies.
It has widely documented that extra-renal MSCs contribute to kidney repair after injury. Interestingly, renoprotection derives from a paracrine/endocrine secretion of bioactive factors and exosomes[20-22] and not from direct homing the injured tissue by MSCs. The infusion of MSCs in AKI animal models has demonstrated that few cells are able to engraft the damaged renal tissue and are preferentially localized into the peritubular and, less frequently, in the tubular epithelium[23,24]. Although the cellular scarcity, the regenerative outcomes in terms of functional restoring and animal survival are evident, thus supporting the notion that MSCs act through a trans-differentiation- independent mechanism[25]. The evidence of paracrine/endocrine secretion of bioactive factors to recover renal function has achieved in mice injected with cisplatin to generate tubular injury and apoptosis. When a conditioned medium from BM-SC culture was injected with intraperitoneal administration in these mice, tubular cell apoptosis diminished, survival increased, and renal injury improved, as well as when MSCs were directly injected[26]. Interestingly, similar results have been obtained in an in vitro model of AKI with a conditioned medium produced by genetically modified MSCs. MSCs were manipulated to over-express Lnc2; the conditioned medium produced from these cells was used to treat that cisplatin treated HEK 293 kidney cells in which it prevented apoptosis and increased the expression of growth factors, thus ameliorating and repairing injured cells[27].
MSCs secrete a number of factors, including VEGF, HGF, IGF-1, adrenomedullin, SDF-1, that exert anti-apoptotic, mitogenic, vasoprotective, and angiogenic actions in AKI. In particular, it seems that a pivotal role in kidney regeneration is played by VEGF and IGF1. VEGF knock out mice and IGF1 silencing models show limited renal function restoring and tubular repair after injury[28,29]. Chemotactic factors, including the SDF1-CRCX4 axis and CD44 interacting with hyaluronic acid, are important during MSC engraftment: BM-MSC isolated from CD44 KO mice lost the ability to migrate in the injured kidney and failed to improve the functional and morphological recovery of acute renal failure induced by glycerol treatment[30].
Several molecular strategies to improve MSC homing into the injured renal tissue have been exploited in order to maximize the paracrine action of MSC in the site of injury. Pre-treatment with growth factor and cytokines, or genetic modifications seem the most promising techniques. Retroviral transduction of MSCs to overexpress the homing receptors CRCX4 or serine protease kallikrein improves renal function recovery and enhances the protective anti-inflammatory action in ischemic injured kidney[31,32]. IGF1 preconditioning before infusion increases the expression of IGF1 and CRCX4 in BM-MSCs and improves cellular migration and renal functional restoring after AKI[11]. The glial derived-cell line neurotrophic factor (GDNF) favors the up-regulation of CD44/HA axis and CRCX4, and the release of IL6, VEGF, SDF1 in cultured human amniotic fluid stem cells. After infusion in AKI animal models, these preconditioned cells show enhanced paracrine activity and improved renoprotection capacity[33]. Pre-treatment with melatonin ameliorates survival, mitogenic and angiogenic properties of rat BM-MSCs, up-regulating the expression of HGF and bFGF and anti-oxidant enzymes[34]. The hyaluronan monoesters with butyric acid (HB) show significant properties to induce metanephric differentiation, formation of capillary-like structures, and secretion of angiogenic cytokines in vitro. In vivo infusion of human mesenchymal stem cells from fetal membranes (FMhMSCs) in AKI rat models after pre-treatment with HB reduces inflammation and accelerates renal function recovery[35]. In addition to MSCs treatment, other molecules, such as NGAL, should be used to regulate the immune response to inflammation and facilitate renal functioning[36]. The combined intravenous administration of bone marrow MSC and muscone in rat with gentamycin induced AKI induces the expression of CXCR7 and CRCX4 on cell surface, thus promoting migration and proliferation of MSCs[37].
All these preclinical murine models offer the proof of concept that the use of MSCs in the management of acute renal failure is rational and feasible. Before implementing clinical studies, it is important to validate the model and standardize some parameters to facilitate the comparison among different protocols of MSCs application. Firstly, it could be important to determine the better route of MSCs administration. The direct comparison of the administration through the tail vein, carotid artery or renal artery has demonstrated that an injection of 105 cells in the renal artery of rat results in a greater improvement of renal function and morphology than those obtained with the other administration routes[38]. Secondarily, the choice of MSC source is another issue that has to be solved. Bone marrow is the most common source of MSC in preclinical studies, but the use of stem cells from other tissues is also reported. KaSzuno et al[39] have compared the regenerative potential of human MSC derived from adipose tissue or bone marrow and cultured in vitro in presence of high serum or low serum. In rat AKI model, only MSCs derived from adipose tissue and cultured in low serum condition have ameliorated AKI via HGF- mediated paracrine effect[39]. CD133(+) renal progenitors from the human inner medulla has been compared with bone marrow derived cells in glycerol induced tubular damage model. CD133(+) progenitor cells promoted the recovery of renal function, preventing tubular cell necrosis and stimulating resident cell proliferation and survival, similarly to mesenchymal stem cells[40]. Therefore, the choice of MSCs or, more generally, stem cells is critical to implement the use of cell-based protocol in regenerative medicine. The feasibility of cell-based protocol is strictly dependent on the procedure to obtain and amplify stem cells. In this contest, the possibility to recover MSCs from adipose tissue after liposuction seems to be favorable because the procedure is inexpensive and non-invasive. Another point to fix is the use of autologous or allogeneic cells. Tögel et al[41] have compared the outcomes in terms of renoprotection after injecting in rat AKI model autologous or allogeneic bone marrow stromal cells. Identical doses of autologous MSCs were more effective than allogeneic, but both autologous and allogeneic cells were able to reduce late renal fibrosis and loss of renal function in surviving animals[41]. However, some factors, such as age or systemic disease, may influence and lessen the regenerative potential of autologous MSCs, therefore it is important to assess patient’s suitability for autologous transplantation. Bone marrow MSCs from remnant rat with chronic renal disease showed no benefit in healing glomerular lesions and exhibited cellular modifications and other deficit in vivo, likely due to cellular senescence[42].
Therefore, even if some preliminary evidence is available in terms of safety and protocol validation, further studies are required to meet the quality and safety criteria for the use of MSCs in humans.
Recently, several groups have demonstrated the potent therapeutic activity of microvesicles (MVs), termed as exosomes and shedding vesicles[43]. MVs are released from stem cells and are particularly enriched in certain molecules, including adhesion molecules, membrane trafficking molecules, cytoskeleton molecules, heat-shock proteins, cytoplasmic enzymes, signal transduction proteins and, importantly, functional mRNAs and microRNAs. Their role in vivo may be related to cell-to-cell communication and to proteins and RNAs exchange among cells both locally and at distance[44]. The discovery of mRNAs and miRNAs in exosomes foreshadows an important new direction for their application as delivery vehicles for therapeutics. In vitro and in vivo experiments have demonstrated that MVs released by BM MSC activate proliferation in tubular epithelial cells and restore renal function after glycerol-induced injury. This regenerative activity is related to the presence of specific mRNAs that encode proteins responsible for controlling proliferation, transcription and immune response[45]. MVs intravenously injected in rat immediately after inducing ischemic- reperfusion injury reduced apoptosis and increased cellular proliferation of tubular cells. Inactivation of MV cargos with RNAase determined the lack of protective effects[46]. Multiple injections of MVs in cisplatin- induced lethal model of AKI SCID mice reduced mortality and produced a normal histological phenotype with normal renal function in surviving animals[47]. The analysis of bio-distribution and renal localization of MVs has demonstrated that MVs accumulated specifically in the kidneys of the mice with AKI compared with the healthy controls. Two different protocols have been used to dye MVs: the near infra-red dye was added to cell culture medium or MVs were stained after purification. Interestingly, the signal generated by the labeled MVs produced by cells was more specific for the injured tissue than those from directly labeled MVs[48]. A therapeutic effect in renal ischemia-reperfusion injury has been shown also by MVs derived from human Wharton-Jelly MSCs. Indeed, a single administration of these MVs in rat immediately after inducing AKI reduced inflammation, and, as long-term outcome, improved renal function and decreased fibrosis[49]. The mechanism of action of Wharton-Jelly MSC derived MVs has not been completely elucidated, but it has been observed that these MVs mitigated the oxidative stress and declined NOX2 expression and reactive oxygen species generation[50]. A further source of MVs with renoprotective activity is the kidney mesenchymal stem cells (KMSC). These microparticles were isolated from the supernatants of KMSC cultured in anoxic conditions in serum-deprived media for 24 h; when injected in mice with acute renal ischemia, they significantly improved renal function, favoring endothelial cells proliferation and ameliorating peritubular microvascular rarefaction[51].
The interesting results obtained in preclinical studies prompt to the translation of MSC-based treatments into humans, although the clinical studies are still limited (Table 1). A phase I clinical trial (NCT00733876) has been designed to determine if the administration of allogeneic MSCs at defined doses is safe in patients who are at high risk of developing AKI after undergoing on-pump cardiac surgery. Preliminary data shown that kidney function is preserved up to 16 mo and that none of the patients required dialysis. Any therapy-related adverse events were noted in these patients[52]. The explorative study (phase I) on three patients who have developed acute renal failure after cisplatin treatment for solid cancer has demonstrated that intravenous infusion of autologous ex-vivo expanded MSCs improves renal function and the procedure is safe (NCT 01275612). Another phase II trial (NCT 01602328) to assess human MSC safety and efficacy in patients that develop AKI after cardiac surgery is ongoing with 156 patients enrolled. The results from these clinical studies will clarify the potential of mesenchymal stem cells in AKI management. An overall view of the preliminary results currently available confirm the safety of the treatment, but other data are required to assess clinical benefit and long term safety. Up to date, none clinical study on microvescicles and AKI is ongoing.
| Study | Phase | Aim | Enrolled patients | Status |
| NCT00733876 | Phase 1 | To determine the safety of the administration of allogeneic MSCs at defined doses in patients with high risk of developing AKI after undergoing on-pump cardiac surgery | 15 | Completed[52] |
| NCT01275612 | Phase 1 | To test the feasibility and safety of systemic infusion of donor ex-vivo expanded MSCs to repair kidney and improve function in patients with solid organ cancers who develop acute renal failure after chemotherapy with cisplatin | 3 (estimated enrollment 9 patients) | Ongoing and recruiting patients |
| NCT01602328 | Phase 2 | To evaluate kidney recovery after a single injection of allogeneic bone marrow derived MSCs in patients who experience kidney injury within 48 h of their cardiac surgery | 156 | Terminated |
The use of MSC for AKI therapy is encouraging and is generally considered as safe. The experience from the increasing use of mesenchymal stem cells before or after renal transplant will furnish important suggestions to implement other clinical protocols with MSC in acute kidney injury. However, some concerns about the use of living cells should keep in account. In progressive rat model of glomerulonephritis, intrarenal injection of MSCs initially ameliorated acute renal failure; however, long-term examination has demonstrates that approximately 20% of the glomeruli of MSC-treated rats contained single or clusters of large adipocytes with pronounced surrounding fibrosis, thus indicating an abnormal and detrimental adipogenic differentiation of MSC[53].
MVs should be evaluated as a possible alternative of living MSCs. The delivery and internalization of MVs are receptor- mediated and targeted within specific cells and MVs may contain biological macromolecules that can be protected from degradation enzymes of plasma and tissue. Before moving to clinical trials, some important issues should be addressed, especially in terms of safety. Large scale production of MVs should be validated and optimized before clinical use; bio-distribution and pharmacokinetic properties should be determined and also long-term safety in animal models has to be tested before implementation in humans. Finally, the use of soluble factors that are released from MSCs for renoprotection may be pursued. Efficacy and safety assays are required to validate the quantitative and qualitative composition of mix of soluble factors to achieve functional restoring after acute renal injury.
Based on promising preliminary results in animal models and in ongoing preclinical studies, mesenchymal stem cells and their derivatives represent a potential therapeutic intervention to treat AKI.
P- Reviewer: Camussi G, Duan SB, Jung JS S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1154] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 2. | Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 725] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 3. | Fang Y, Ding X, Zhong Y, Zou J, Teng J, Tang Y, Lin J, Lin P. Acute kidney injury in a Chinese hospitalized population. Blood Purif. 2010;30:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 841] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 5. | Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 692] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 6. | Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 632] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 7. | Doorn J, Moll G, Le Blanc K, van Blitterswijk C, de Boer J. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev. 2012;18:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7:e44092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 10. | Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 11. | Xinaris C, Morigi M, Benedetti V, Imberti B, Fabricio AS, Squarcina E, Benigni A, Gagliardini E, Remuzzi G. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013;22:423-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Wise AF, Ricardo SD. Mesenchymal stem cells in kidney inflammation and repair. Nephrology (Carlton). 2012;17:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2663] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 14. | Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099-1100. [PubMed] |
| 15. | Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74. [PubMed] |
| 16. | Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 17. | Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 18. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1344] [Article Influence: 112.0] [Reference Citation Analysis (2)] |
| 19. | Reger RL, Prockop DJ. Should publications on mesenchymal stem/progenitor cells include in-process data on the preparation of the cells? Stem Cells Transl Med. 2014;3:632-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1614] [Cited by in RCA: 1530] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 21. | Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626-F1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 463] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 22. | Humphreys BD, Duffield JD, Bonventre JV. Renal stem cells in recovery from acute kidney injury. Minerva Urol Nefrol. 2006;58:13-21. [PubMed] |
| 23. | Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794-1804. [PubMed] |
| 24. | Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 25. | Morigi M, De Coppi P. Cell therapy for kidney injury: different options and mechanisms--mesenchymal and amniotic fluid stem cells. Nephron Exp Nephrol. 2014;126:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 27. | Halabian R, Roudkenar MH, Jahanian-Najafabadi A, Hosseini KM, Tehrani HA. Co-culture of bone marrow-derived mesenchymal stem cells overexpressing lipocalin 2 with HK-2 and HEK293 cells protects the kidney cells against cisplatin-induced injury. Cell Biol Int. 2014;Jul 22; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921-2928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Tögel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med. 2009;13:2109-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 31. | Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 330] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 32. | Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008;19:807-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Rota C, Imberti B, Pozzobon M, Piccoli M, De Coppi P, Atala A, Gagliardini E, Xinaris C, Benedetti V, Fabricio AS. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012;21:1911-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L, Bianchi P, Calise D. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | La Manna G, Bianchi F, Cappuccilli M, Cenacchi G, Tarantino L, Pasquinelli G, Valente S, Della Bella E, Cantoni S, Claudia C. Mesenchymal stem cells in renal function recovery after acute kidney injury: use of a differentiating agent in a rat model. Cell Transplant. 2011;20:1193-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | La Manna G, Ghinatti G, Tazzari PL, Alviano F, Ricci F, Capelli I, Cuna V, Todeschini P, Brunocilla E, Pagliaro P. Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS One. 2014;9:e89497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Liu P, Feng Y, Dong C, Yang D, Li B, Chen X, Zhang Z, Wang Y, Zhou Y, Zhao L. Administration of BMSCs with muscone in rats with gentamicin-induced AKI improves their therapeutic efficacy. PLoS One. 2014;9:e97123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Cai J, Yu X, Xu R, Fang Y, Qian X, Liu S, Teng J, Ding X. Maximum efficacy of mesenchymal stem cells in rat model of renal ischemia-reperfusion injury: renal artery administration with optimal numbers. PLoS One. 2014;9:e92347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Katsuno T, Ozaki T, Saka Y, Furuhashi K, Kim H, Yasuda K, Yamamoto T, Sato W, Tsuboi N, Mizuno M. Low serum cultured adipose tissue-derived stromal cells ameliorate acute kidney injury in rats. Cell Transplant. 2013;22:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Grange C, Moggio A, Tapparo M, Porta S, Camussi G, Bussolati B. Protective effect and localization by optical imaging of human renal CD133+ progenitor cells in an acute kidney injury model. Physiol Rep. 2014;2:e12009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Tögel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Klinkhammer BM, Kramann R, Mallau M, Makowska A, van Roeyen CR, Rong S, Buecher EB, Boor P, Kovacova K, Zok S. Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PLoS One. 2014;9:e92115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A Systematic Review of Preclinical Studies on the Therapeutic Potential of Mesenchymal Stromal Cell-Derived Microvesicles. Stem Cell Rev. 2014;Aug 5; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 44. | Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 45. | Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 1013] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 46. | Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 621] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 47. | Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 48. | Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. 2014;33:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 49. | Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 50. | Zhang G, Zou X, Miao S, Chen J, Du T, Zhong L, Ju G, Liu G, Zhu Y. The anti-oxidative role of Micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS One. 2014;9:e92129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Choi HY, Moon SJ, Ratliff BB, Ahn SH, Jung A, Lee M, Lee S, Lim BJ, Kim BS, Plotkin MD. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One. 2014;9:e87853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 52. | Tögel FE, Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis. 2012;60:1012-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18:1754-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |