Published online Aug 26, 2010. doi: 10.4252/wjsc.v2.i4.81
Revised: August 11, 2010
Accepted: August 16, 2010
Published online: August 26, 2010
Cell-based regenerative medicine is of growing interest in biomedical research. The role of stem cells in this context is under intense scrutiny and may help to define principles of organ regeneration and develop innovative therapeutics for organ failure. Utilizing stem and progenitor cells for organ replacement has been conducted for many years when performing hematopoietic stem cell transplantation. Since the first successful transplantation of umbilical cord blood to treat hematological malignancies, non-hematopoietic stem and progenitor cell populations have recently been identified within umbilical cord blood and other perinatal and fetal tissues. A cell population entitled mesenchymal stromal cells (MSCs) emerged as one of the most intensely studied as it subsumes a variety of capacities: MSCs can differentiate into various subtypes of the mesodermal lineage, they secrete a large array of trophic factors suitable of recruiting endogenous repair processes and they are immunomodulatory.
Focusing on perinatal tissues to isolate MSCs, we will discuss some of the challenges associated with these cell types concentrating on concepts of isolation and expansion, the comparison with cells derived from other tissue sources, regarding phenotype and differentiation capacity and finally their therapeutic potential.
- Citation: Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells 2010; 2(4): 81-92
- URL: https://www.wjgnet.com/1948-0210/full/v2/i4/81.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v2.i4.81
Regenerative medicine is of growing interest in biomedical research. The role of stem cells to regenerate, repair and replace tissues or organs is intensely studied. In general, organ injuries or local defects induce a mobilisation of endogenous immature progenitor cells either locally or systemically. Then mediated by the milieu, regulated by factors of the extracellular matrix, cellular components or soluble mediators, the precursors differentiate along a hierarchy of committed to mature cells to functionally regenerate the cellular compartment of the organ[1]. Hematopoietic stem cell (HSC) transplantation conducted in a routine scale is mimicking these processes[2]. In HSC transplantation, umbilical cord or placental blood (CB) came into focus as a third source after the first successful transplantation performed by Gluckman et al[3] in 1989. Despite this approach, cells from fetal tissues have become increasingly interesting also for tissue engineering approaches to regenerate solid organs. This is based on reports of solid organ engraftment after experimental or clinical whole CB transplantation[4].
Multipotent cells have been observed in the fetal circulation but there seems to be an inverse correlation in frequency of cells with endothelial and mesodermal differentiation potential[5]. Besides mesenchymal stromal cells (MSCs), a plethora of different stem and progenitor cell populations have been described in perinatal tissues with potential ranging from embryonal-like[6-8] to lineage-committed progenitor cells[5,9]. Whether or not these differentially named cells refer to similar cell populations obtained by different isolation or culture methods, whether these cells relate to a common ancestor[10], are in constant (epi)genetical transition enabling them to shift to different phenotypes[11], emerge upon in vitro culture or by dedifferentiation effects[12] remains unidentified and represents a major challenge for the future.
Human embryonic stem cells (hESCs) are derived from the inner cell mass exhibiting a tremendous proliferative potential and in addition pluripotent differentiation potential into cells of all three germ layers. Currently however, three major factors limit their clinical application[13]. Firstly, the ethical debate: in some countries it is not allowed to generate or even work with hESC lines. Secondly, pluripotent hESCs induce teratoma formation after transplantation. Still methods are inferior to ascertain that no residual pluripotent hESCs are present in a transplant of differentiated cells. Thus the risk of teratoma formation cannot be fully excluded. Thirdly, hESCs may elicit immune reactions after transplantation. In the future, probably autologous induced pluripotent stem cells from adult somatic cells may help to overcome at least issues one and three[14,15]. By then, adult tissues will have to be regarded as an ethically sound option.
Adult stem cells have been identified in a variety of tissues; MSCs for example in every tissue tested so far[16]. Here the problem arises on how to isolate these very rare cells and from which sources. The most often analysed tissue is bone marrow (BM). But cell procurement is highly invasive and cell numbers are low and necessitate further ex vivo expansion. Furthermore, cell numbers, at least in BM, have been shown to decline with age[17].
Thus, postnatal gestational tissues inherit numerous advantages starting with the young chronological age that minimizes the feasibility for incorporated mutations and ending with the non-invasive procurement. hESC cells from Wharton’s Jelly (WJ) and the amnion arise from the epiblast but are not ethically controversial or tumorigenic[18]. Early focus on perinatal tissues harbouring stem cells arose from HSCs identified in CB[19]. Subsequently, besides cord blood, fetal liver, lung, brain, villous placenta, fetal membranes as well as amniotic fluid were identified to host MSCs[20-22]. Apart from abortal tissues, in the majority of cases perinatal tissues are discarded at birth and thus cells are harvestable without any risk for the baby or its mother. Accordingly, there is an unlimited supply, easy access and minimal ethical/legal issues associated with perinatal tissues. Tissues may be stored for autologous use or also allogeneic settings as fetal cells have been demonstrated to be immuno-privileged[21]. Hence CB storage is one strategy increasingly followed in numerous countries, not only for allogeneic but also for potential autologous applications[23].
Antenatal stem cells may be derived from diagnostic samples during amniocentesis[24]. Due to their extensive proliferation potential, a low amount of cells may expand to similar numbers as cells obtained at high numbers from adult tissues. In addition to enhanced expansion capacities, stem cell properties might be enriched in fetal/perinatal stromal cells (Table 1). Frequencies of cells exerting telomerase activity and expressing pluripotency markers are significantly higher compared to stem cells derived from the adult[21,25,26]. Thus, perinatal tissues feature a promising source for MSCs for cell therapy due to their multipotency, immuno-privilege and not-tumorigenicity.
| Source of MSCs | Isolation | Origin | Isolation efficacy | Frequency | Expansion potential | Immunephenotype | Differentiationa | Immuno-suppression | Senescence/genetic stability/safety | Ref. |
| Cord blood | Ficoll gradient Lineage depletion | Fetal | At best 60% | Very rare, 1/108 MNC Inverse correlation with gestational age | Dependent on seeding density | SSEA-3, SSEA-4, TRA 1-60, TRA 1-82, Nanog | Osteogenic Chondrogenic Adipogenic-significantly reduced compared to adult MSC endothelial | Inhibition of T cell proliferation | Cells senescence Stable Karyotype Non tumorigenic | [20,32] and own unpublished data[49,61,93,95,96] |
| Umbilical Cord (Wharton´s jelly) Including UC matrix stem cells, UC perivascular cells, UC stroma cells | Removal of vein and arteries Mechanical dissection, Collagenase + hyaluronidase digestion | Fetal | 100% | 10-50 × 103 /cm UC | High Expansion potential | 20% no HLA class I, nor class II SSEA-4, TRA 1-60 | Osteogenic Chondrogenic Adipogenic-significantly reduced compared to adult MSC Neurogenic Myogenic, cardiac endothelial | Inhibition of T cell proliferation | Late senescence Telomerase Stable karyotype Non-tumorigenic | Reviewed in [59,95,97] |
| Amniotic Fluid | Centrifugation | Fetal | 21/23 samples | Frequency 3 CFU-f/mL AF range 1-6 0.9%-1.5% | Expansion significantly higher than for BM-MSCs doubling time 18 h | Lower expression of CD44 and CD105 compared to BM-MSCs 90% positive for Oct-4 | Osteogenic Adipogenic Chondrogenic Hepatogenic Neurogenic Myogenic, cardio | Inhibition of T cell proliferation | Longer telomeres than BM-MSCs Stable karyotype Non tumorigenic | [95,98] |
| Placenta (Amnion, chorion and decidua basalis) | dissection digestion with collagenase and DNase, Percoll gradient | Fetal/maternal | 62.5%-100% | Expansion significantly higher than for BM-MSC | SSEA-4, TRA 1-60, TRA 1-81 | Osteogenic Chondrogenic Adipogenic-significantly reduced compared to adult MSC | Inhibition of T cell proliferation | Cells senescence Rarely chromosomal changes no transformation No toxicity when injecting 1x107MSC/kg | [26,50,99,100] | |
| Amnion (contains besides MSCs amniotic epithelial cells) | Mechanical peeling, mincing Trypsin treatment, digestion with collagenase or/and DNase | Fetal | 4 × 106/100 cm2 starting material Or 1 × 106 /g of tissue | First trimester proliferate better than third trimester | SSEA-3, SSEA-4, Nanog | Adipogenic Chondrogenic Osteogenic Myogenic, skeletal Myogenic, cardio Angiogenic Neurogenic Pancreatic | Inhibition of T cell proliferation | Cells senescence Stable Karyotype Non tumorigenic | Reviewed in [21] [95,101,102] | |
| Chorion (contains besides MSCs chorionic trophoblastic cells) | Mechanical removal, dispase and collagenase digestion | Fetal | Expansion significantly higher than for BM-MSC | Adipogenic Chondrogenic Osteogenic Myogenic, skeletal Neurogenic | Inhibition of T cell proliferation | Cells senescence Stable Karyotype Non tumorigenic | Reviewed in [21] |
The acronym MSCs is used to abbreviate marrow stromal cells, mesenchymal stem cells and mesenchymal stromal cells. Classically, MSCs have been isolated from the BM. However, alternative tissues have been identified, including adipose tissue, cutaneous tissues, fetal tissues, dental pulp, hair follicle, synovium, blood, etc[16]. MSCs exhibit an in vitro expansion potential and more importantly a broad differentiation potential into not only mesodermal (including osteoblasts, adipocytes and chondrocytes) but also endodermal (hepatocyte-like cells) and ectodermal cells (neuronal, neuroglial cells) (For a comprehensive overview, we recommend the special issues 35/3 and 35/4 2008 in Transfusion Medicine and Hemotherapy).
Admittedly, the common definition of a stem cell has not been fulfilled with MSCs so far[27]: it has not been demonstrated that a single implanted MSC can regenerate and maintain a whole tissue compartment as has been shown for HSCs. In addition, MSCs grown in culture do not exert an unlimited self-renewal capacity associated by a lack of telomerase activity and telomere length shortening upon proliferation[28]. This is in contrast to hESCs which display no replicative senescence in culture. Hence, there is a trend towards redefining these cells as “mesenchymal stromal cells” (MSCs)[29].
Nevertheless, MSCs emerged as central candidates in the entire field of cellular therapies. Besides the secretion of trophic factors, MSCs exert fundamental immunomodulatory functions[30]. In vitro analyses provide a multiplicity of data demonstrating that MSCs, despite the constitutive expression of HLA class I and the interferon γ (IFN γ)-inducible expression of HLA class II, can suppress allogeneic T, NK and B cell responses but also can affect dendritic cell functions and tumor cell growth[31].
Although MSCs seem to be present in any tissue analyzed so far, the presence of circulating MSC has been controversially discussed[32]. Indeed MSCs circulate in peripheral blood and CB but at much lower frequencies than their hematopoietic counterparts. Thus they are difficult to isolate and culture. Furthermore the frequency of MSCs within fetal blood decreases sharply with advanced gestational age[5,33]. But, interesting for future use, MSCs have also been isolated from cryopreserved term CB[34].
The first reports dealing with issues of MSCs in CB claimed that either stromal feeder cells[35,36] or osteoprogenitors can be cultured[37]. Later on, Goodwin et al[38] and Erices et al[39] demonstrated cells capable of differentiating into bone, cartilage and adipose tissue. This encouraging data were neglected by a number of authors who tried but failed to isolate MSCs from CB but succeeded with BM[40,41]. Analyzing factors which might influence the unpredictable isolation success in full-term CB ranging between 20%-40% of utilized CB units, we demonstrated that by selecting units with decreased storage time and a high volume of cell-rich CB, the isolation success can be enhanced towards 60%[20]. The infrequent isolation suggests that either MSCs circulate in extremely low frequencies in CB or that reliable culture conditions equivalent to those for BM have yet to be defined. Anyhow, currently the chance of using CB-MSCs in autologous therapeutic settings is considerably hampered[42] (Table 1).
At present no unique phenotype comparable to CD34 for HSC[43] has been identified which allows a standardised prospective isolation of MSCs with predictable differentiative potential[44]. Recent data indicate a perivascular origin of MSCs[16,45]. MSCs could be prospectively isolated using markers for pericytes, but it is doubted that all pericytes inhabit MSC characteristics[46]. With the markers to prospectively isolate pure MSCs still under debate, the expression profile of culture-expanded MSCs consists of a variety of markers typical for other cell lineages. Thus a combination of expressed and not-expressed (most importantly all hematopoietic lineage) markers is currently used to define MSCs. Classically, MSCs express CD44, CD73 (SH-3, SH-4), CD90 (Thy-1), CD105 (SH-2, Endoglin), CD106 (VCAM-1) and HLA class I but lack the expression of CD14, CD34, CD45 (Leucocyte Common Antigen) and HLA class II[47] (Table 1).
Since currently the immunophenotype is insufficient to define MSCs, the demanded assay is to analyze their differentiative capacity at least towards the mesodermal lineage. MSCs respond to osteogenic stimuli with the upregulation of osteogenic markers, assessed either by PCR, immuno- or histochemical staining. When assessed quantitatively, CB-MSCs seem to have a stronger osteogenic potential
in vitro compared to BM-MSCs[48,49]. The differentiation into the chondrogenic lineage can be induced by using a micromass culture and various growth factors. CB-MSCs form cartilage as well as BM-MSCs but the type of cartilage may differ dependent on the tissues source used[50].
Differing observations have been published regarding the adipogenic differentiation capacity of CB-MSCs. In some studies CB-MSCs demonstrate no to only low level adipogenic differentiation after culture in adipogenic media[48,50-52] (Table 1). However, other authors reported no limited adipogenic potential[38,39,53].
A variety of data support the idea that there is no mesodermal germ-layer restricted differentiation potential in CB-MSCs. Depending on the in vitro stimulus, CB-MSCs can also differentiate into neural cells, neuroglial and hepatocyte-like cells, endothelial cells, skeletal myoblasts, respiratory epithelial cells and cardiomyogenic cells[32] (Table 1). Goodwin et al[38] observed a variety of markers indicative for osteoblastic and neural lineages already expressed on BM-MSCs but induced in CB-MSCs upon induction, suggesting a commitment of BM-MSCs in contrast to CB-MSCs. The inducible expression as well as differing responsiveness to differentiative stimuli may therefore relate to a more primitive cell population contained in CB.
Comparative analysis of genomic and proteomic expression profiles in comparison to mature lineages as well as hESCs revealed shared patterns but also marked differences which might result from differing isolation and culture conditions and may have to be re-evaluated after adjusting to common and standardised protocols[32].
The presence of fibroblast-like cells with myogenic properties within the umbilical cord (UC) matrix was described years ago[54]. Colony-forming units fibroblast (CFU-F) can be obtained at higher frequencies and in contrast to CB from every UC[55] (Table1). Cells derived from WJ, the gelatinous part of the UC, but also cells in the perivascular region have been associated with multilineage differentiation potential[25,56,57]. Recently, Ishige et al[58] compared MSCs derived from the arterial, venous or gelatinous part of the cord. They observed different frequencies, slightly different osteogenic potential but similar phenotype in these populations. In contrast to circulating MSCs, UC-derived cells express pan-cytokeratin markers. Expression however may vary when perivascular cells are compared to WJ cells[55,59].
Similar to cells derived from CB, UC-derived cells exert higher proliferative capacities compared to BM but also improved properties of in vitro osteogenesis and neurogenesis at the expense of adipogenesis[55]. Comparing MSCs from the same donor derived from either the cord blood or cord matrix revealed significant differences in the gene expression profiles[60]. In CB-MSCs genes related to development, osteogenesis and immune system were expressed whereas UC-MSCs express genes associated with cell adhesion, morphogenesis, secretion, angiogenesis and neurogenesis.
The composition and origin of cells within amniotic fluid change throughout gestation[61,62]. Human amniotic fluid-derived MSCs (AF-MSCs) are abundant and can be isolated by plastic adherence in minimal medium. The proliferative capacities exceed that of BM-MSCs but markers and properties are shared with MSCs from adult tissues. One exception is observed regarding the expression of HLA class I and II which seems to be reduced, suggesting even more pronounced immunological inertness compared to adult MSCs.
The human placenta, besides supporting fetal development, may also represent a reservoir of stem/progenitor cells. Four regions can be distinguished harbouring different cell types: the amniotic epithelial (AEC), amniotic mesenchymal (AMSC), chorionic mesenchymal (CMSC) and finally the chorionic trophoblastic cells[21]. As these cell preparations might be contaminated with maternal cells, the fetal origin has to be demonstrated with methods sensitive enough to detect less than 1% of maternal cells (Table 1).
The two fetal membranes, amnion and chorion, emerge from the basal surface of the placenta to encase the fetus within the amniotic fluid. Amnion is composed of a mono-epithelial layer and a fibroblast layer beneath the basal membrane. A layer of collagen fibres separates the amniotic and chorionic mesoderm. After delivery, to separate stem cells from the fetal membranes, both membranes are peeled apart and enzymatically digested. AMSCs are gained after removing the epithelial layer by trypsin followed by collagenase treatment. For chorion, the maternal parts are mechanically removed and then the trophoblast layers digested using dispase and the chorion stromal cells released by collagenase. Primary yields are high with approximately 6 × 106 AECs and 2 × 106 AMSCs per gram amnion[63].
Like BM-MSCs, AMSCs and CMSCs display a fibroblastoid phenotype upon adherence to plastic, can form typical colonies, display differentiation potential into mesodermal lineages and express the range of markers used to characterise MSCs. Furthermore these cells express some pluripotency markers, SSEA-4, TRA-1-61, and TRA-1-80[26] (Table 1). Regarding the differentiation potential, slight differences have been described. AMSCs seem to be more directed to an adipogenic potential whereas CMSCs more to chrondo-, osteo-, myo- and neurogenic lineages[24]. Portmann-Lanz et al[64] demonstrated no obvious effects by the gestational age but the excess of adipo- and neurogenic potential was detected to be higher in ASCs compared to CSCs. ASCs seem to inherit also some vasculogenic and hepatic potential[21,65].
MSCs are promising candidates for use in regenerative medicine. Most clinical studies have been conducted using BM-MSCs (http://www.clinicaltrials.gov). Current studies listed there and case reports published focusing on perinatal MSC sources are summarized in Table 2.
| UCB-MSCs | Safety and efficacy study of umbilical cord blood-derived mesenchymal stem cells to promote engraftment of unrelated hematopoietic stem cell transplantation |
| UCB-MSCs | Safety and efficacy study of umbilical cord/placenta-derived mesenchymal stem cells to treat myelodysplastic syndromes |
| UCB-MSCs | Stem cell therapy for type 1 diabetes mellitus |
| UCB-MSCs | Study to compare the efficacy and safety of cartistem® and microfracture in patients with knee articular cartilage injury or defect |
| UC-MSCs | A research study looking at specific tissue of the umbilical cord |
| UC-MSCs | Allogeneic mesenchymal stem cell for Graft-versus-host disease treatment |
| UC-MSCs/Placenta-MSCs | Safety and efficacy study of umbilical cord/placenta-derived mesenchymal stem cells to treat myelodysplastic syndromes |
| Placenta-MSCs | Safety of intramuscular injection of Allogeneic PLX-PAD cells for the treatment of critical limb ischemia |
| UCB-MSCs | Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model[103] |
| UCB-MSCs | A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study[104] |
Studies in general focus on either local or systemic administration of MSCs. Preclinical models indicated that the site-directed administration appears to result in engraftment and integration of the MSCs mediated by extent of tissue injury. But owing to problems in quantifying engraftment, published results vary enormously for MSC biodistribution and assessing the therapeutic outcome[66].
The systemic administration, in contrast, seems to result in general in even less persistence of tissue-localized MSCs. After infusion, MSCs remain in the circulation for no more than 1 h[67]. Thereafter MSCs are detectable primarily in the lungs and then secondarily in the injured organs, albeit at low frequencies. Specific homing to and survival in the BM has been shown not only for BM-MSCs but also for CB-MSCs after transplantation into immunodeficient nude mice without conditioning pretreatment[68].
Basically, clinical expectations are associated with three functional aspects of MSC: (1) Tissue repair by either reparative cells directly or by secretion of paracrine effectors; (2) Stromal capacities to support engraftment, of especially HSCs; and (3) Immune modulation.
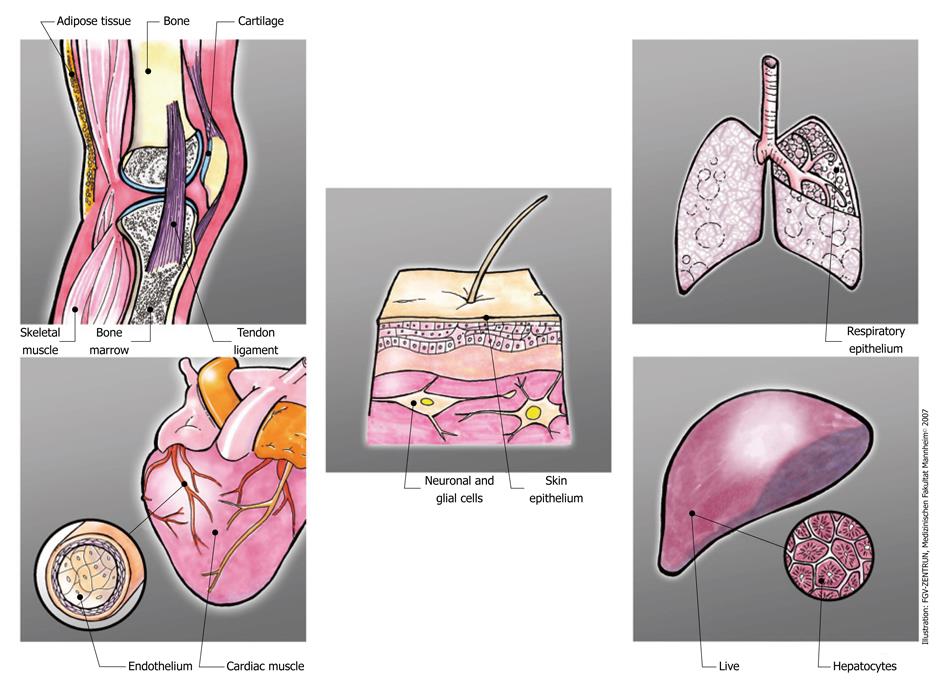
Figure 1 depicts a range of those tissues which have been the object of pre-clinical investigation to induce tissue repair or tissue regeneration with perinatal MSCs. Interestingly, in preclinical models, tissue targets of all three germ layers have been investigated and proved some success of perinatal stem cell transplantation (Table 3). Whether this success resulted from direct engraftment and cellular differentiation is doubted more and more because overall engraftment levels are extremely low and lack long-term persistence. Rather, in a variety of settings, the secretion of regenerative or immune-suppressive factors ameliorated clinical signs.
| Mesoderm | Endoderm | Ectoderm | |||
| Bone | [74,75,105-109] | Skin, skin epithelium | [110] | Lung, respiratory epithelium | [111,112] |
| Cartilage | [76,113,114] | Nerve, neuronal, glial | [64,115-124] | Liver, hepatocytes | [125-128] |
| Adipose tissue | [48,49,51,129] | Islets, beta cells | [130,131] | ||
| Skeletal muscle | [54,132-136] | ||||
| Cardiac muscle | [77,137-142] | ||||
| Vessels, Endothelium | [116,129,143-145] | ||||
| Stromal support | [79,81,82,146,147] | ||||
| Immune regulation | [83,148-151] | ||||
Preclinical models are difficult to design and interpret because a multitude of parameters like animal and disease model, mode of application, cellular source (species and tissue source), cell number, ex vivo culture and differentiation, post transplantation analysis have been demonstrated to affect the experimental outcome. This is exemplified in the following: Zhao et al[69] and Satore et al[70] transplanted AF-MSCs to treat acute ischemic myocardium in a porcine or rat model. In the study of Zhao et al[69], the transplanted cells survived for at least 2 mo and exhibited myocardial commitment with finally myocardiocyte in situ differentiation. However, Sartore et al[70] could not detect significant myocardial engraftment. These contrary results may be due to the mixed cell population used because Zhao et al[69] excluded AEC cells before transplantation. Furthermore, ex vivo culture conditioning and species sources differed. Sartore et al[70] ex vivo cultivated the cells before autotransplantation into porcine ischemic hearts. Zhao et al[69] cocultivated human MSCs with neonatal rat heart explants before xenotransplating in rat ischemic hearts. Also, cell passaging and cell dose may affect the capacity of engraftment. This example highlights that preclinical data have to be extremely carefully compared and evaluated to extrapolate therapeutic efficacy.
The concept of using fetal stromal cells for fetal tissue engineering is the one most intensely studied to treat congenital abnormalities[71]. Obtaining fetal biopsies for autologous purposes is amendable but associated with a not negligible risk and thus currently considered for life-threatening diseases. By means of tissue engineering, a few studies indicate that a variety of defects may be treatable. In one study, diaphragm reconstruction was achieved using hAMSCs[72]. The Hoerstrup group is focussing on fetal MSCs in cardiovascular engineering, e.g. to generate living heart valves[73]. Apart from treating congenital abnormalities, the tendency of improved osteogenic differentiation potential, prompted investigators to study bone and cartilage formation with fetal stromal cells[22,74-76]. All data indicate that fetal tissue derived MSCs might be candidates for hard tissue engineering.
Further publications suggest that the search for the optimal tissue to derive MSCs might be advisable. For example Iop et al[77] indicate dissimilar cardiovascular properties of fetal compared to adult MSCs. This is also suggested by a variety of authors with regard to neuroregenerative protocols[64].
In CB transplantation, the cell dose as major determinant of rate and incidence of hematopoietic recovery is limited to the volume which can be collected from one placenta. Strategies to improve engraftment are under investigation and include ex vivo expansion of HSCs and co-infusion of MSCs. Ex vivo expansion of CB-HSCs has been achieved on monolayers of MSCs derived from various tissues. In fact CB-MSCs constitutively secrete a variety of growth factors affecting HSCs[78]. Thus, stromal layers derived from CB-MSCs as well as from BM-MSCs were capable of maintaining and amplifying colony-forming cells over a prolonged period of time[79,80]. Furthermore the cotransplantation of CB-MSCs can lead to an enhanced and accelerated engraftment of CB-HSCs within the murine NOD/SCID (nonobese diabetic/severe combined immune deficiency) transplantation model[81,82].
Several studies based on initial reports by Le Blanc et al[31] report that MSCs not only evade immune recognition but furthermore play a role in modulating immune and inflammatory responses. They are currently clinically exploited as a tool for managing tolerance in clinical transplantation, including graft versus host disease. MSCs interact with a variety of immune cells to affect both the innate and adaptive immune system modulating various effector functions to induce immunosuppression and an anti-inflammatory milieu in various injury models. Immunological responses elicited by fetal and adult MSCs seem to be comparable and fetal MSCs are also anti-proliferative to T cells[83]. Comparing MSCs from BM, adipose tissue, CB and WJ indicated similar immunomodulatory capacities of all cell types, demonstrating that this is a broad stromal capacity not restricted to a developmental or tissue origin[84]. Analyzing specific aspects of immune regulations in more detail, however, indicate that cells from different tissues or developmental age may behave slightly differently. Unrestricted somatic stem cells from CB can induce a balance between T cell effector responses and dendritic cell maturation depending on the cytokine milieu. Here especially, interferon-γ and tumor necrosis factor-alpha play a role[85,86].
MSCs are considered to be non-immunogenic. The fetal-maternal interface seems to be immunologically special to enable maternal acceptance of the fetal allograft. Thus fetal or perinatal MSCs might be specifically interesting for allogeneic settings[21]. This idea is challenged by findings by Cho et al[87]. They demonstrated in a swine model that the first injection of WJ derived MSCs was non-immunogenic. However, repetitive administrations as well as injection into inflamed skin or interferon activation mediated allo-reactive immune responses.
The employment of adult stem cell types in clinical studies, in general, necessitates manufacturing, processing and testing of cellular products according to the current national regulations, including current good tissue practice (GTP) and good manufacturing practice (GMP)[17].
As indicated, fetal MSCs have proven valuable in fetal tissue engineering. Having diagnosed a structural birth defect and performed diagnostic amniocentesis to procure AF-MSCs, the months until birth allow for cell isolation, expansion, cryopreservation, thawing, secondary expansion and tissue engineering of the graft. A protocol validating a three stage procedure for manufacturing AF-MSCs has been introduced by Steigman et al[88] and Brooke et al[89] provide a protocol for placental MSCs.
The authors also discuss the aspect of using fetal bovine serum (FBS). FBS contains xenogeneic proteins which are internalized by MSCs. Consequently, a host of potential problems can arise such as viral and prion transmission or immunological reactions. These risks have initiated the search for alternative substitutes: recently serum, plasma or platelet derived supplements have been introduced enabling the FBS-free propagation of MSCs[90-92]. Obviously the same holds true for CB-MSCs: very recently human platelet lysate has been established as supplement to expand CB-MSCs for clinical applications, paving its way to the clinic[93,94].
The potential application of autologous stem cells collected at birth requires immense technical and financial resources for storing the frozen cell samples throughout the period of life. Although such a procedure seems possible from a technical point of view, it is highly debatable whether the eventual use and possible benefits justify these efforts.
For allogeneic use, the presence of stem cell populations collectable at birth provides a readily accessible and currently probably under-utilized stem cell source with little ethical conflict and numerous advantages. For allogeneic applications however, efficient and reproducible methods to isolate, expand and differentiate and quality control these perinatal progenitor cells are required. If one can extrapolate from the lessons learned in HSC transplantation, stem cell populations harvestable at the time of birth promise to develop as adequate alternatives to other adult tissues.
Peer reviewer: Najimi Mustapha, PhD, Laboratory of Pediatric Hepatology and Cell Therapy, Avenue Hippocrate 10/1301, 1200 Brussels, Belgium
S- Editor Wang JL L- Editor Roemmele A E- Editor Yang C
| 1. | Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833-840. [Cited in This Article: ] |
| 2. | Storb R. Allogeneic hematopoietic stem cell transplantation--yesterday, today, and tomorrow. Exp Hematol. 2003;31:1-10. [Cited in This Article: ] |
| 3. | Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174-1178. [Cited in This Article: ] |
| 4. | Körbling M, Robinson S, Estrov Z, Champlin R, Shpall E. Umbilical cord blood-derived cells for tissue repair. Cytotherapy. 2005;7:258-261. [Cited in This Article: ] |
| 5. | Javed MJ, Mead LE, Prater D, Bessler WK, Foster D, Case J, Goebel WS, Yoder MC, Haneline LS, Ingram DA. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res. 2008;64:68-73. [Cited in This Article: ] |
| 6. | Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21:297-303. [Cited in This Article: ] |
| 7. | McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, Tilton R, Denner L. Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 2005;38:245-255. [Cited in This Article: ] |
| 8. | Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123-135. [Cited in This Article: ] |
| 9. | Buzańska L, Machaj EK, Zabłocka B, Pojda Z, Domańska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci. 2002;115:2131-2138. [Cited in This Article: ] |
| 10. | Ebihara Y, Masuya M, Larue AC, Fleming PA, Visconti RP, Minamiguchi H, Drake CJ, Ogawa M. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp Hematol. 2006;34:219-229. [Cited in This Article: ] |
| 11. | Zipori D. The nature of stem cells: state rather than entity. Nat Rev Genet. 2004;5:873-878. [Cited in This Article: ] |
| 12. | Prindull GA, Fibach E. Are postnatal hemangioblasts generated by dedifferentiation from committed hematopoietic stem cells? Exp Hematol. 2007;35:691-701. [Cited in This Article: ] |
| 13. | Jung KW. Perspectives on human stem cell research. J Cell Physiol. 2009;220:535-537. [Cited in This Article: ] |
| 14. | Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877-886. [Cited in This Article: ] |
| 15. | Cai J, Li W, Su H, Qin D, Yang J, Zhu F, Xu J, He W, Guo X, Labuda K. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J Biol Chem. 2010;285:11227-11234. [Cited in This Article: ] |
| 16. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [Cited in This Article: ] |
| 17. | Bieback K, Schallmoser K, Klüter H, Strunk D. Clinical Protocols for the Isolation and Expansion of Mesenchymal Stromal Cells. Transfus Med Hemother. 2008;35:286-294. [Cited in This Article: ] |
| 18. | Bongso A, Fong CY, Gauthaman K. Taking stem cells to the clinic: Major challenges. J Cell Biochem. 2008;105:1352-1360. [Cited in This Article: ] |
| 19. | Prindull G, Prindull B, Meulen N. Haematopoietic stem cells (CFUc) in human cord blood. Acta Paediatr Scand. 1978;67:413-416. [Cited in This Article: ] |
| 20. | Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625-634. [Cited in This Article: ] |
| 21. | Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300-311. [Cited in This Article: ] |
| 22. | Guillot PV, De Bari C, Dell'Accio F, Kurata H, Polak J, Fisk NM. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation. 2008;76:946-957. [Cited in This Article: ] |
| 23. | Rubinstein P. Cord blood banking for clinical transplantation. Bone Marrow Transplant. 2009;44:635-642. [Cited in This Article: ] |
| 24. | Gucciardo L, Lories R, Ochsenbein-Kölble N, Done' E, Zwijsen A, Deprest J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG. 2009;116:166-172. [Cited in This Article: ] |
| 25. | Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50-60. [Cited in This Article: ] |
| 26. | Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3-9. [Cited in This Article: ] |
| 27. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [Cited in This Article: ] |
| 28. | Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675-682. [Cited in This Article: ] |
| 29. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [Cited in This Article: ] |
| 30. | Sensebé L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87:S49-S53. [Cited in This Article: ] |
| 31. | Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K. Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther. 2007;9:301. [Cited in This Article: ] |
| 32. | Bieback K, Klüter H. Mesenchymal stromal cells from umbilical cord blood. Curr Stem Cell Res Ther. 2007;2:310-323. [Cited in This Article: ] |
| 33. | Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396-2402. [Cited in This Article: ] |
| 34. | Lee MW, Choi J, Yang MS, Moon YJ, Park JS, Kim HC, Kim YJ. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem Biophys Res Commun. 2004;320:273-278. [Cited in This Article: ] |
| 35. | Prindull G, Ben-Ishay Z, Ebell W, Bergholz M, Dirk T, Prindull B. CFU-F circulating in cord blood. Blut. 1987;54:351-359. [Cited in This Article: ] |
| 36. | Ye ZQ, Burkholder JK, Qiu P, Schultz JC, Shahidi NT, Yang NS. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci USA. 1994;91:12140-12144. [Cited in This Article: ] |
| 37. | Hutson EL, Boyer S, Genever PG. Rapid isolation, expansion, and differentiation of osteoprogenitors from full-term umbilical cord blood. Tissue Eng. 2005;11:1407-1420. [Cited in This Article: ] |
| 38. | Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7:581-588. [Cited in This Article: ] |
| 39. | Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242. [Cited in This Article: ] |
| 40. | Yu M, Xiao Z, Shen L, Li L. Mid-trimester fetal blood-derived adherent cells share characteristics similar to mesenchymal stem cells but full-term umbilical cord blood does not. Br J Haematol. 2004;124:666-675. [Cited in This Article: ] |
| 41. | Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099-1100. [Cited in This Article: ] |
| 42. | Pojda Z, Machaj EK, Ołdak T, Gajkowska A, Jastrzewska M. Nonhematopoietic stem cells of fetal origin--how much of today's enthusiasm will pass the time test? Folia Histochem Cytobiol. 2005;43:209-212. [Cited in This Article: ] |
| 43. | Flores AI, McKenna DH, Montalbán MA, De la Cruz J, Wagner JE, Bornstein R. Consistency of the initial cell acquisition procedure is critical to the standardization of CD34+ cell enumeration by flow cytometry: results of a pairwise analysis of umbilical cord blood units and cryopreserved aliquots. Transfusion. 2009;49:636-647. [Cited in This Article: ] |
| 44. | Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109-116. [Cited in This Article: ] |
| 45. | Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martín-Vasallo P, Díaz-Flores L Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909-969. [Cited in This Article: ] |
| 46. | Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229-230. [Cited in This Article: ] |
| 47. | Rojewski MT, Weber BM, Schrezenmeier H. Phenotypic Characterization of Mesenchymal Stem Cells from Various Tissues. Transfus Med Hemother. 2008;35:168-184. [Cited in This Article: ] |
| 48. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [Cited in This Article: ] |
| 49. | Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006;24:679-685. [Cited in This Article: ] |
| 50. | Montesinos JJ, Flores-Figueroa E, Castillo-Medina S, Flores-Guzmán P, Hernández-Estévez E, Fajardo-Orduña G, Orozco S, Mayani H. Human mesenchymal stromal cells from adult and neonatal sources: comparative analysis of their morphology, immunophenotype, differentiation patterns and neural protein expression. Cytotherapy. 2009;11:163-176. [Cited in This Article: ] |
| 51. | Chang YJ, Tseng CP, Hsu LF, Hsieh TB, Hwang SM. Characterization of two populations of mesenchymal progenitor cells in umbilical cord blood. Cell Biol Int. 2006;30:495-499. [Cited in This Article: ] |
| 52. | Markov V, Kusumi K, Tadesse MG, William DA, Hall DM, Lounev V, Carlton A, Leonard J, Cohen RI, Rappaport EF. Identification of cord blood-derived mesenchymal stem/stromal cell populations with distinct growth kinetics, differentiation potentials, and gene expression profiles. Stem Cells Dev. 2007;16:53-73. [Cited in This Article: ] |
| 53. | Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669-1675. [Cited in This Article: ] |
| 54. | Kobayashi K, Kubota T, Aso T. Study on myofibroblast differentiation in the stromal cells of Wharton's jelly: expression and localization of alpha-smooth muscle actin. Early Hum Dev. 1998;51:223-233. [Cited in This Article: ] |
| 55. | Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319-331. [Cited in This Article: ] |
| 56. | Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105-110. [Cited in This Article: ] |
| 57. | Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220-229. [Cited in This Article: ] |
| 58. | Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, Sugimoto M, Nakauchi H, Tojo A. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton's jelly explants of human umbilical cord. Int J Hematol. 2009;90:261-269. [Cited in This Article: ] |
| 59. | Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591-599. [Cited in This Article: ] |
| 60. | Secco M, Moreira YB, Zucconi E, Vieira NM, Jazedje T, Muotri AR, Okamoto OK, Verjovski-Almeida S, Zatz M. Gene expression profile of mesenchymal stem cells from paired umbilical cord units: cord is different from blood. Stem Cell Rev. 2009;5:387-401. [Cited in This Article: ] |
| 61. | Guillot PV, O'Donoghue K, Kurata H, Fisk NM. Fetal stem cells: betwixt and between. Semin Reprod Med. 2006;24:340-347. [Cited in This Article: ] |
| 62. | Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler MM, Fauza DO. The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg. 2001;36:1662-1665. [Cited in This Article: ] |
| 63. | Bilic G, Zeisberger SM, Mallik AS, Zimmermann R, Zisch AH. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant. 2008;17:955-968. [Cited in This Article: ] |
| 64. | Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, Surbek DV. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194:664-673. [Cited in This Article: ] |
| 65. | Chen CP, Liu SH, Huang JP, Aplin JD, Wu YH, Chen PC, Hu CS, Ko CC, Lee MY, Chen CY. Engraftment potential of human placenta-derived mesenchymal stem cells after in utero transplantation in rats. Hum Reprod. 2009;24:154-165. [Cited in This Article: ] |
| 66. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [Cited in This Article: ] |
| 67. | Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12-20. [Cited in This Article: ] |
| 68. | Erices AA, Allers CI, Conget PA, Rojas CV, Minguell JJ. Human cord blood-derived mesenchymal stem cells home and survive in the marrow of immunodeficient mice after systemic infusion. Cell Transplant. 2003;12:555-561. [Cited in This Article: ] |
| 69. | Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79:528-535. [Cited in This Article: ] |
| 70. | Sartore S, Lenzi M, Angelini A, Chiavegato A, Gasparotto L, De Coppi P, Bianco R, Gerosa G. Amniotic mesenchymal cells autotransplanted in a porcine model of cardiac ischemia do not differentiate to cardiogenic phenotypes. Eur J Cardiothorac Surg. 2005;28:677-684. [Cited in This Article: ] |
| 71. | Turner CG, Fauza DO. Fetal tissue engineering. Clin Perinatol. 2009;36:473-488, xii. [Cited in This Article: ] |
| 72. | Kunisaki SM, Fuchs JR, Kaviani A, Oh JT, LaVan DA, Vacanti JP, Wilson JM, Fauza DO. Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte- and myoblast-based constructs. J Pediatr Surg. 2006;41:34-39; discussion 34-39. [Cited in This Article: ] |
| 73. | Schmidt D, Achermann J, Odermatt B, Genoni M, Zund G, Hoerstrup SP. Cryopreserved amniotic fluid-derived cells: a lifelong autologous fetal stem cell source for heart valve tissue engineering. J Heart Valve Dis. 2008;17:446-455; discussion 455. [Cited in This Article: ] |
| 74. | Zhang ZY, Teoh SH, Chong MS, Lee ES, Tan LG, Mattar CN, Fisk NM, Choolani M, Chan J. Neo-vascularization and bone formation mediated by fetal mesenchymal stem cell tissue-engineered bone grafts in critical-size femoral defects. Biomaterials. 2010;31:608-620. [Cited in This Article: ] |
| 75. | Zhang ZY, Teoh SH, Chong MS, Schantz JT, Fisk NM, Choolani MA, Chan J. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126-137. [Cited in This Article: ] |
| 76. | Wang L, Tran I, Seshareddy K, Weiss ML, Detamore MS. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:2259-2266. [Cited in This Article: ] |
| 77. | Iop L, Chiavegato A, Callegari A, Bollini S, Piccoli M, Pozzobon M, Rossi CA, Calamelli S, Chiavegato D, Gerosa G. Different cardiovascular potential of adult- and fetal-type mesenchymal stem cells in a rat model of heart cryoinjury. Cell Transplant. 2008;17:679-694. [Cited in This Article: ] |
| 78. | Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005;32:270-279. [Cited in This Article: ] |
| 79. | Bakhshi T, Zabriskie RC, Bodie S, Kidd S, Ramin S, Paganessi LA, Gregory SA, Fung HC, Christopherson KW 2nd. Mesenchymal stem cells from the Wharton's jelly of umbilical cord segments provide stromal support for the maintenance of cord blood hematopoietic stem cells during long-term ex vivo culture. Transfusion. 2008;48:2638-2644. [Cited in This Article: ] |
| 80. | Hayashi N, Takahashi K, Abe Y, Kashiwakura I. Placental/umbilical cord blood-derived mesenchymal stem cell-like stromal cells support hematopoietic recovery of X-irradiated human CD34+ cells. Life Sci. 2009;84:598-605. [Cited in This Article: ] |
| 81. | Chan SL, Choi M, Wnendt S, Kraus M, Teng E, Leong HF, Merchav S. Enhanced in vivo homing of uncultured and selectively amplified cord blood CD34+ cells by cotransplantation with cord blood-derived unrestricted somatic stem cells. Stem Cells. 2007;25:529-536. [Cited in This Article: ] |
| 82. | Hiwase SD, Dyson PG, To LB, Lewis ID. Cotransplantation of placental mesenchymal stromal cells enhances single and double cord blood engraftment in nonobese diabetic/severe combined immune deficient mice. Stem Cells. 2009;27:2293-2300. [Cited in This Article: ] |
| 83. | Magatti M, De Munari S, Vertua E, Gibelli L, Wengler GS, Parolini O. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells. 2008;26:182-192. [Cited in This Article: ] |
| 84. | Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150-156. [Cited in This Article: ] |
| 85. | Winter M, Wang XN, Däubener W, Eyking A, Rae M, Dickinson AM, Wernet P, Kögler G, Sorg RV. Suppression of cellular immunity by cord blood-derived unrestricted somatic stem cells is cytokine-dependent. J Cell Mol Med. 2009;13:2465-2475. [Cited in This Article: ] |
| 86. | van den Berk LC, Jansen BJ, Siebers-Vermeulen KG, Netea MG, Latuhihin T, Bergevoet S, Raymakers RA, Kögler G, Figdor CC, Adema GJ. Toll-like receptor triggering in cord blood mesenchymal stem cells. J Cell Mol Med. 2009;[Epub ahead of print]. [Cited in This Article: ] |
| 87. | Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, Lo DP, Harris IR, Popma SH, Sachs DH, Huang CA. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111:430-438. [Cited in This Article: ] |
| 88. | Steigman SA, Armant M, Bayer-Zwirello L, Kao GS, Silberstein L, Ritz J, Fauza DO. Preclinical regulatory validation of a 3-stage amniotic mesenchymal stem cell manufacturing protocol. J Pediatr Surg. 2008;43:1164-1169. [Cited in This Article: ] |
| 89. | Brooke G, Rossetti T, Pelekanos R, Ilic N, Murray P, Hancock S, Antonenas V, Huang G, Gottlieb D, Bradstock K. Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. Br J Haematol. 2009;144:571-579. [Cited in This Article: ] |
| 90. | Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, Klüter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331-2341. [Cited in This Article: ] |
| 91. | Kocaoemer A, Kern S, Klüter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25:1270-1278. [Cited in This Article: ] |
| 92. | Bieback K, Ha VA, Hecker A, Grassl M, Kinzebach S, Solz H, Sticht C, Klüter H, Bugert P. Altered Gene Expression in Human Adipose Stem Cells Cultured with Fetal Bovine Serum Compared to Human Supplements. Tissue Eng Part A. 2010;[Epub ahead of print]. [Cited in This Article: ] |
| 93. | Reinisch A, Bartmann C, Rohde E, Schallmoser K, Bjelic-Radisic V, Lanzer G, Linkesch W, Strunk D. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen Med. 2007;2:371-382. [Cited in This Article: ] |
| 94. | Avanzini MA, Bernardo ME, Cometa AM, Perotti C, Zaffaroni N, Novara F, Visai L, Moretta A, Del Fante C, Villa R. Generation of mesenchymal stromal cells in the presence of platelet lysate: a phenotypic and functional comparison of umbilical cord blood- and bone marrow-derived progenitors. Haematologica. 2009;94:1649-1660. [Cited in This Article: ] |
| 95. | Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med. 2009;4:423-433. [Cited in This Article: ] |
| 96. | Terai M, Uyama T, Sugiki T, Li XK, Umezawa A, Kiyono T. Immortalization of human fetal cells: the life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol Biol Cell. 2005;16:1491-1499. [Cited in This Article: ] |
| 97. | Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886-2895. [Cited in This Article: ] |
| 98. | Sessarego N, Parodi A, Podestà M, Benvenuto F, Mogni M, Raviolo V, Lituania M, Kunkl A, Ferlazzo G, Bricarelli FD. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica. 2008;93:339-346. [Cited in This Article: ] |
| 99. | Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, Doody M, Venter D, Pain S, Gilshenan K. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095-1107. [Cited in This Article: ] |
| 100. | In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [Cited in This Article: ] |
| 101. | De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100-106. [Cited in This Article: ] |
| 102. | Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, Redl H, Gabriel C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173-1183. [Cited in This Article: ] |
| 103. | Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK, Kang KS. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620-1626. [Cited in This Article: ] |
| 104. | Kang KS, Kim SW, Oh YH, Yu JW, Kim KY, Park HK, Song CH, Han H. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy. 2005;7:368-373. [Cited in This Article: ] |
| 105. | Liu G, Li Y, Sun J, Zhou H, Zhang W, Cui L, Cao Y. In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood-derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng Part A. 2010;16:971-982. [Cited in This Article: ] |
| 106. | Kang JM, Kang SW, La WG, Yang YS, Kim BS. Enhancement of in vivo bone regeneration efficacy of osteogenically undifferentiated human cord blood mesenchymal stem cells. J Biomed Mater Res A. 2010;93:666-672. [Cited in This Article: ] |
| 107. | Jang BJ, Byeon YE, Lim JH, Ryu HH, Kim WH, Koyama Y, Kikuchi M, Kang KS, Kweon OK. Implantation of canine umbilical cord blood-derived mesenchymal stem cells mixed with beta-tricalcium phosphate enhances osteogenesis in bone defect model dogs. J Vet Sci. 2008;9:387-393. [Cited in This Article: ] |
| 108. | Jäger M, Degistirici O, Knipper A, Fischer J, Sager M, Krauspe R. Bone healing and migration of cord blood-derived stem cells into a critical size femoral defect after xenotransplantation. J Bone Miner Res. 2007;22:1224-1233. [Cited in This Article: ] |
| 109. | Guillot PV, Abass O, Bassett JH, Shefelbine SJ, Bou-Gharios G, Chan J, Kurata H, Williams GR, Polak J, Fisk NM. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood. 2008;111:1717-1725. [Cited in This Article: ] |
| 110. | Dai Y, Li J, Li J, Dai G, Mu H, Wu Q, Hu K, Cao Q. Skin epithelial cells in mice from umbilical cord blood mesenchymal stem cells. Burns. 2007;33:418-428. [Cited in This Article: ] |
| 111. | Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, Kang S, Jin HJ, Yang YS, Park WS. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869-886. [Cited in This Article: ] |
| 112. | Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701-711. [Cited in This Article: ] |
| 113. | Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178-187. [Cited in This Article: ] |
| 114. | Hildner F, Wolbank S, Redl H, van Griensven M, Peterbauer A. How chondrogenic are human umbilical cord matrix cells? A comparison to adipose-derived stem cells. J Tissue Eng Regen Med. 2010;4:242-245. [Cited in This Article: ] |
| 115. | Zwart I, Hill AJ, Al-Allaf F, Shah M, Girdlestone J, Sanusi AB, Mehmet H, Navarrete R, Navarrete C, Jen LS. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol. 2009;216:439-448. [Cited in This Article: ] |
| 116. | Chen MY, Lie PC, Li ZL, Wei X. Endothelial differentiation of Wharton's jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37:629-640. [Cited in This Article: ] |
| 117. | Mareschi K, Rustichelli D, Comunanza V, De Fazio R, Cravero C, Morterra G, Martinoglio B, Medico E, Carbone E, Benedetto C. Multipotent mesenchymal stem cells from amniotic fluid originate neural precursors with functional voltage-gated sodium channels. Cytotherapy. 2009;11:534-547. [Cited in This Article: ] |
| 118. | Zhang L, Zhang HT, Hong SQ, Ma X, Jiang XD, Xu RX. Cografted Wharton's jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem Res. 2009;34:2030-2039. [Cited in This Article: ] |
| 119. | Xia G, Hong X, Chen X, Lan F, Zhang G, Liao L. Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J Perinat Med. 2010;38:215-221. [Cited in This Article: ] |
| 120. | Lee HJ, Lee JK, Lee H, Shin JW, Carter JE, Sakamoto T, Jin HK, Bae JS. The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer's disease. Neurosci Lett. 2010;481:30-35. [Cited in This Article: ] |
| 121. | El-Badri NS, Hakki A, Saporta S, Liang X, Madhusodanan S, Willing AE, Sanberg CD, Sanberg PR. Cord blood mesenchymal stem cells: Potential use in neurological disorders. Stem Cells Dev. 2006;15:497-506. [Cited in This Article: ] |
| 122. | Lee H, Bae JS, Jin HK. Human umbilical cord blood-derived mesenchymal stem cells improve neurological abnormalities of Niemann-Pick type C mouse by modulation of neuroinflammatory condition. J Vet Med Sci. 2010;72:709-717. [Cited in This Article: ] |
| 123. | Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781-792. [Cited in This Article: ] |
| 124. | Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115-124. [Cited in This Article: ] |
| 125. | Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, Hung SC, Fu YS. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484-495. [Cited in This Article: ] |
| 126. | Kakinuma S, Asahina K, Okamura K, Teramoto K, Tateno C, Yoshizato K, Tanaka Y, Yasumizu T, Sakamoto N, Watanabe M. Human cord blood cells transplanted into chronically damaged liver exhibit similar characteristics to functional hepatocytes. Transplant Proc. 2007;39:240-243. [Cited in This Article: ] |
| 127. | Ren H, Zhao Q, Cheng T, Lu S, Chen Z, Meng L, Zhu X, Yang S, Xing W, Xiao Y. No contribution of umbilical cord mesenchymal stromal cells to capillarization and venularization of hepatic sinusoids accompanied by hepatic differentiation in carbon tetrachloride-induced mouse liver fibrosis. Cytotherapy. 2010;12:371-383. [Cited in This Article: ] |
| 128. | Yan Y, Xu W, Qian H, Si Y, Zhu W, Cao H, Zhou H, Mao F. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int. 2009;29:356-365. [Cited in This Article: ] |
| 129. | Cetrulo CL Jr. Cord-blood mesenchymal stem cells and tissue engineering. Stem Cell Rev. 2006;2:163-168. [Cited in This Article: ] |
| 130. | Yoshida S, Ishikawa F, Kawano N, Shimoda K, Nagafuchi S, Shimoda S, Yasukawa M, Kanemaru T, Ishibashi H, Shultz LD. Human cord blood--derived cells generate insulin-producing cells in vivo. Stem Cells. 2005;23:1409-1416. [Cited in This Article: ] |
| 131. | Ersek A, Pixley JS, Goodrich AD, Porada CD, Almeida-Porada G, Thain DS, Zanjani ED. Persistent circulating human insulin in sheep transplanted in utero with human mesenchymal stem cells. Exp Hematol. 2010;38:311-320. [Cited in This Article: ] |
| 132. | Chan J, O'Donoghue K, Gavina M, Torrente Y, Kennea N, Mehmet H, Stewart H, Watt DJ, Morgan JE, Fisk NM. Galectin-1 induces skeletal muscle differentiation in human fetal mesenchymal stem cells and increases muscle regeneration. Stem Cells. 2006;24:1879-1891. [Cited in This Article: ] |
| 133. | Chan J, Waddington SN, O'Donoghue K, Kurata H, Guillot PV, Gotherstrom C, Themis M, Morgan JE, Fisk NM. Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells. 2007;25:875-884. [Cited in This Article: ] |
| 134. | Koponen JK, Kekarainen T, E Heinonen S, Laitinen A, Nystedt J, Laine J, Ylä-Herttuala S. Umbilical cord blood-derived progenitor cells enhance muscle regeneration in mouse hindlimb ischemia model. Mol Ther. 2007;15:2172-2177. [Cited in This Article: ] |
| 135. | Abousleiman RI, Reyes Y, McFetridge P, Sikavitsas V. The human umbilical vein: a novel scaffold for musculoskeletal soft tissue regeneration. Artif Organs. 2008;32:735-742. [Cited in This Article: ] |
| 136. | De Coppi P, Callegari A, Chiavegato A, Gasparotto L, Piccoli M, Taiani J, Pozzobon M, Boldrin L, Okabe M, Cozzi E. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol. 2007;177:369-376. [Cited in This Article: ] |
| 137. | Ichim TE, Solano F, Brenes R, Glenn E, Chang J, Chan K, Riordan NH. Placental mesenchymal and cord blood stem cell therapy for dilated cardiomyopathy. Reprod Biomed Online. 2008;16:898-905. [Cited in This Article: ] |
| 138. | Martin-Rendon E, Sweeney D, Lu F, Girdlestone J, Navarrete C, Watt SM. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008;95:137-148. [Cited in This Article: ] |
| 139. | Walther G, Gekas J, Bertrand OF. Amniotic stem cells for cellular cardiomyoplasty: promises and premises. Catheter Cardiovasc Interv. 2009;73:917-924. [Cited in This Article: ] |
| 140. | Chang SA, Lee EJ, Kang HJ, Zhang SY, Kim JH, Li L, Youn SW, Lee CS, Kim KH, Won JY. Impact of myocardial infarct proteins and oscillating pressure on the differentiation of mesenchymal stem cells: effect of acute myocardial infarction on stem cell differentiation. Stem Cells. 2008;26:1901-1912. [Cited in This Article: ] |
| 141. | Lai RC, Arslan F, Tan SS, Tan B, Choo A, Lee MM, Chen TS, Teh BJ, Eng JK, Sidik H. Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles. J Mol Cell Cardiol. 2010;48:1215-1224. [Cited in This Article: ] |
| 142. | Ventura C, Cantoni S, Bianchi F, Lionetti V, Cavallini C, Scarlata I, Foroni L, Maioli M, Bonsi L, Alviano F. Hyaluronan mixed esters of butyric and retinoic Acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J Biol Chem. 2007;282:14243-14252. [Cited in This Article: ] |
| 143. | Zhang P, Baxter J, Vinod K, Tulenko TN, Di Muzio PJ. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev. 2009;18:1299-1308. [Cited in This Article: ] |
| 144. | Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194-202. [Cited in This Article: ] |
| 145. | Chung DJ, Choi CB, Lee SH, Kang EH, Lee JH, Hwang SH, Han H, Lee JH, Choe BY, Lee SY. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J Neurosci Res. 2009;87:3554-3567. [Cited in This Article: ] |
| 146. | Boissel L, Tuncer HH, Betancur M, Wolfberg A, Klingemann H. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008;14:1031-1038. [Cited in This Article: ] |
| 147. | Mizokami T, Hisha H, Okazaki S, Takaki T, Wang XL, Song CY, Li Q, Kato J, Hosaka N, Inaba M. Preferential expansion of human umbilical cord blood-derived CD34-positive cells on major histocompatibility complex-matched amnion-derived mesenchymal stem cells. Haematologica. 2009;94:618-628. [Cited in This Article: ] |
| 148. | Magatti M, De Munari S, Vertua E, Nassauto C, Albertini A, Wengler GS, Parolini O. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 2009;18:899-914. [Cited in This Article: ] |
| 149. | Jones BJ, Brooke G, Atkinson K, McTaggart SJ. Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta. 2007;28:1174-1181. [Cited in This Article: ] |
| 150. | Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008;36:1370-1376. [Cited in This Article: ] |