Copyright
©The Author(s) 2023.
World J Stem Cells. Sep 26, 2023; 15(9): 876-896
Published online Sep 26, 2023. doi: 10.4252/wjsc.v15.i9.876
Published online Sep 26, 2023. doi: 10.4252/wjsc.v15.i9.876
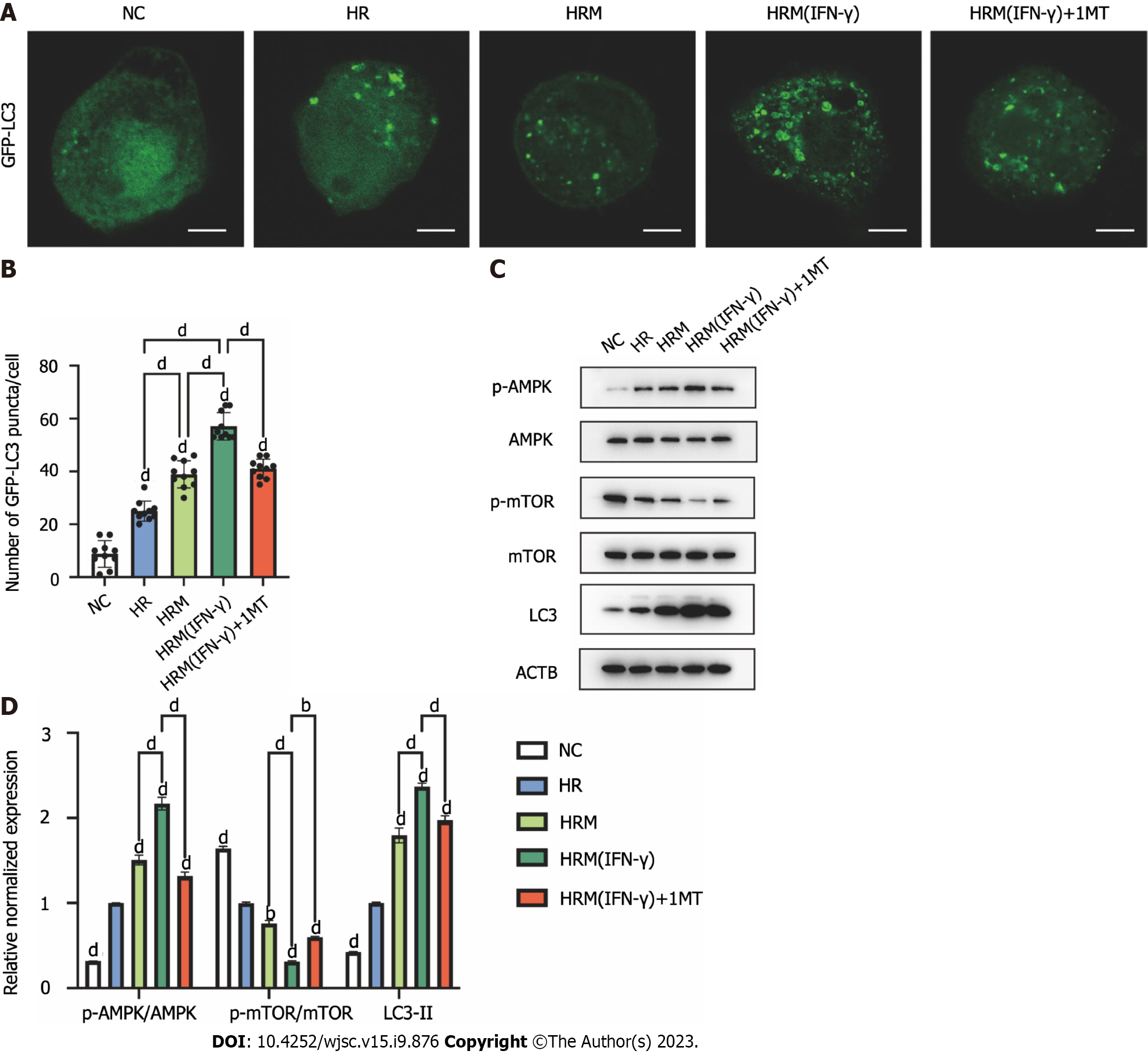
Figure 8 Indoleamine 2,3-dioxygenase secreted by interferon-γ-primed menstrual blood-derived stromal cells enhanced autophagy by inhibiting the mammalian target of rapamycin pathway and activating the AMPK pathway.
A: Immunofluorescence images of GFP-LC3. Scale bar 2 μm; B: The number of intense punctate GFP-LC3 aggregates in each cell was counted to determine the level of autophagy in each group. The data are expressed as the means ± SEMs (n = 10/group); C: The expression of p-AMPK, AMPK, p-mammalian target of rapamycin (p-mTOR), mTOR, LC3, and ACTB in L02 cells was determined by western blotting; D: The relative normalized protein expression was quantified based on the intensities of the western blot bands (n = 3/group). The data are presented as the means ± SEMs. aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001. NS represents not statistically significant. All P values were obtained by one-way ANOVA. NC: Negative control; 3MA: 3-methyladenine; 1MT: 1-methyl-D-tryptophan; MenSCs: Menstrual blood-derived stromal cells; IFN-γ: Interferon-γ; HR: Hypoxia/reoxygenation; HRM: Combination treated with hypoxia/reoxygenation and menstrual blood-derived stromal cells; HRM (IFN-γ): Combination treated with hypoxia/reoxygenation and I interferon-γ-primed menstrual blood-derived stromal cells; AMPK: AMP-activated protein kinase; mTOR: the Mammalian target of rapamycin signaling pathway.
- Citation: Zhang Q, Zhou SN, Fu JM, Chen LJ, Fang YX, Xu ZY, Xu HK, Yuan Y, Huang YQ, Zhang N, Li YF, Xiang C. Interferon-γ priming enhances the therapeutic effects of menstrual blood-derived stromal cells in a mouse liver ischemia-reperfusion model. World J Stem Cells 2023; 15(9): 876-896
- URL: https://www.wjgnet.com/1948-0210/full/v15/i9/876.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i9.876