Copyright
©The Author(s) 2023.
World J Stem Cells. May 26, 2023; 15(5): 400-420
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.400
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.400
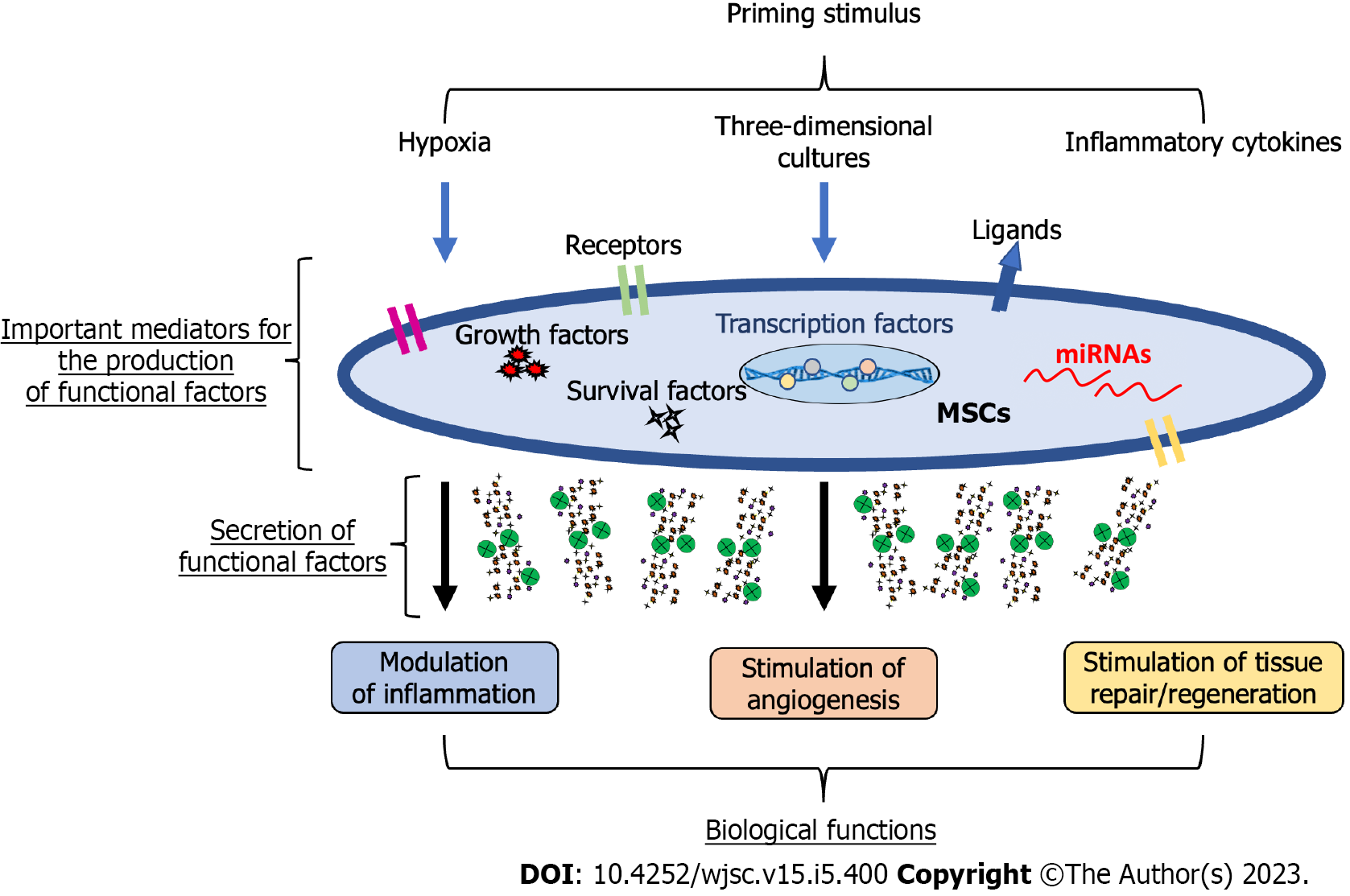
Figure 1 Potential mechanisms mediating mesenchymal stromal/stem cell-primed therapeutic properties.
Mesenchymal stromal/stem cells (MSCs) can be primed through different signals, including hypoxia, three-dimensional cultures, and inflammatory cytokines to obtain a therapeutic phenotype. The potential mediators of this new phenotype comprise a plethora of regulatory molecules within MSCs, including surface receptors and ligands, signalling molecules inducing survival/growth, regulatory molecules such as microRNAs, and transcription factors regulating several pathways. Thus, primed MSCs can modulate inflammation, stimulate angiogenesis, and promote tissue repair/regeneration. MSCs: Mesenchymal stromal/stem cells; miRNAs: MicroRNAs.
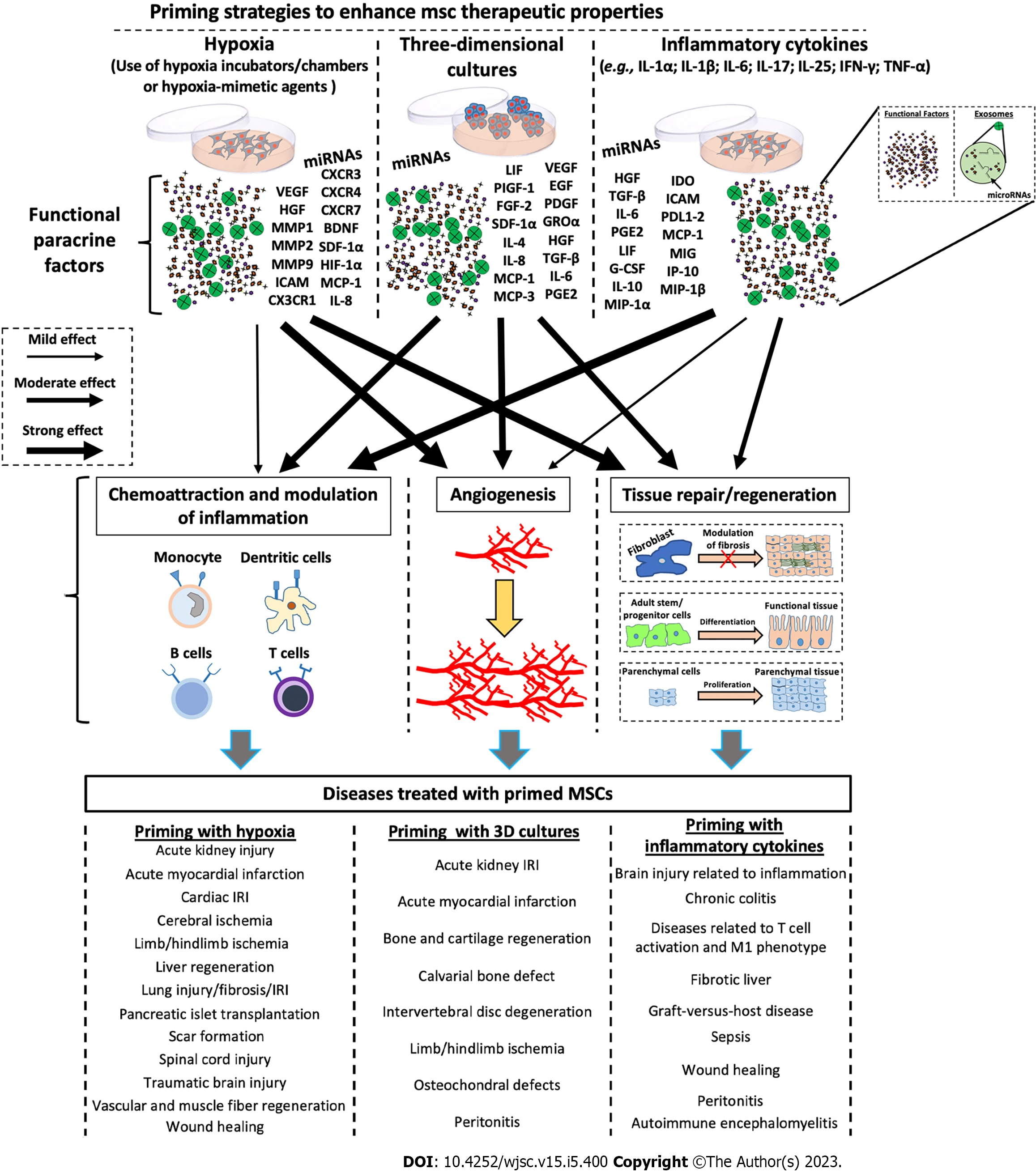
Figure 2 Schematic representation of the molecular effects after priming of mesenchymal stromal/stem cells.
Mesenchymal stromal/stem cells (MSCs) can be primed through various stimuli, including hypoxia, three-dimensional cultures, and pro-inflammatory cytokines to enhance their therapeutic potential. Each priming method induces the production of specific factors (e.g., trophic factors, angiogenetic factors, chemokines, cytokines, and exosomes containing both proteins and microRNAs), which induce the activation of biological processes such as angiogenesis, tissue repair/regeneration, chemoattraction, and modulation of inflammation. Each priming strategy seems to stimulate the production of functional factors in a different way, thus eliciting different responses. miRNA: MicroRNA; VEGF: Vascular endothelial-derived growth factor; CXCR: Chemokine receptor; HGF: Hepatocyte growth factor; MMP: Matrix metallopeptidase; BDNF: Brain-derived neurotrophic factor; SDF: Stromal cell-derived factor; HIF: Hypoxia-inducible factor; ICAM: Intercellular adhesion molecules; MCP: Monocyte chemoattractant protein; IL: Interleukin; LIF: Leukemia inhibitory factor; PIGF: Placental growth factor; EGF: Epidermal growth factor; FGF: Basic fibroblast growth factor; PDGF: Platelet-derived growth factor; GRO: Growth-related oncogene; TGF: Transforming growth factor; PGE2: Prostaglandin E2; IDO: Indoleamine 2,3-dioxygenase; PDL1-2: Programmed death ligand 1-2; MIG: Monokine induced by interferon-gamma; G-CSF: Granulocyte colony-stimulating factor; IP-10: Induced protein 10; MIP: Macrophage inflammatory protein; IRI: Ischemia/reperfusion injury; MSCs: Mesenchymal stromal/stem cells; 3D: Three-dimensional.
Figure 3 Schematic illustration of the physiological role and biological action of mesenchymal stromal/stem cells primed in vivo in a model of tissue injury and repair.
During tissue injury and repair, mesenchymal stromal/stem cells (MSCs) are differently activated by various microenvironment stimuli to orchestrate tissue repair and functional recovery. First, naïve MSC activation (hypoxic activation) leads to the release of both angiogenic factors and chemokines, which stimulate the formation of new blood vessels, the recruitment of neutrophils, and the expression of adhesion molecules. Neutrophil action is followed by macrophage activity, which ensures sustained release of pro-inflammatory cytokines, and potentiation of the fibroproliferative response. If this process is not adequately regulated, a state of chronic inflammation occurs; the MSC phenotype is switched into an anti-inflammatory phenotype. MSCs: Mesenchymal stromal/stem cells; 3D: Three-dimensional.
- Citation: Miceli V, Zito G, Bulati M, Gallo A, Busà R, Iannolo G, Conaldi PG. Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use. World J Stem Cells 2023; 15(5): 400-420
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/400.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.400