INTRODUCTION
Decetaxel is a new taxoid structurally similar to paclitaxel, a semisynthetic product of a renewable resource, the needles of the European yew, Taxus baccata L. In comparison to paclitaxel, docetaxel is more potent as an inhibitor of microtubule depolymerization[1]. Docetaxel has shown an active effect against cancers[2-5]. Current studies have shown that docetaxel combined with other chemotherapeutic drugs has higher anticancer efficacy and reduced side effects in patients with breast, pancreatic, gastric, urothelial carcinomas[6-9]. But there is limited data about that in hepatocellular carcinoma and thus needs further investigation. Human hepatocellular carcinoma (HCC) is one of the major causes of death worldwide[10-12], and surgical resection is the only curative therapy[13]. However it has limitations for patients with multiple type or metastatic tumors. Searching for effective chemotherapeutic agents is important to improve the survival rate of patients with advanced or recurrent HCC after surgical treatment. HCC is usually insensitive to chemotherapeutic drugs currently used in clinical setting, and there is thus an urgent need for the evaluation of new active drugs against HCC. HCC is well known for its expression of multidrug resitance (MDR) gene and its poor response to chemotherapeutic agents, but it has been found that docetaxel is effective in treating mice bearing xenografts of MDR protein positive human tumors[14], also a low efflux of docetaxel from tumor cells being observed[15]. These results suggest that docetaxel may be effective in the treatment of HCC. But the related information is limited and the mechanism of docetaxel remains to be elucidated. This study is to investigate its growth inhibition and induction of apoptosis effect and the mechanism in human SMMC-7721 hepatocellular cell line, and to provide the theoretical basis for clinical application in patients with HCC.
MATERIALS AND METHODS
Reagents
Docetaxel (Rhone-Poulenc Rorer Pharmaceuticals Inc.) was stored as 100 µM stock solution in absolute ethanol at -80 °C. This solution was further diluted in the medium and used in the cell culture immediately before each experiment.
Cell cultures
Human hepatocellular carcinoma SMMC-7721 cell line was obtained from Liver Cancer Institute of Fudan University. The cells were cultured in flasks with RPMI1640 (Gibco BRL, Grand Island, NY) medium supplemented with 100 IU/mL penicillin, 100 µg/mL streptomycin and 10% new born bovine serum (Hyclone, Logan, UT) and maintained at 37 °C in humidified atmosphere containing 5% CO2.
Colony forming assay
Exponential growth phase SMMC-7721 cells were seeded in 60 mm culture plate (Corning, NY) at a density of 200 cells/plate, after 24hr, the media was discarded and replaced with equal volume of fresh media containing different doses of docetaxel (0, 10-11, 10-10, 10-9, 10-8, 10-7, 10-6 M). 24 hr later docetaxel containing media was again discarded and replaced by media without containing docetaxel. The cells were cultured continually for 9 d. The colonies were fixed with 95% ethanol and stained with Giemsa, and manually counted. Colonies ≥ 50 cells were considered survivors. All experiments were conducted in triplicate.
Flow cytometric analysis of DNA content after docetaxel treatment
After exponential growth phase SMMC-7721 cells were treated with different doses of docetaxel (0, 10-11, 10-10, 10-9, 10-8, 10-7, 10-6 M) for 24 hr and harvested with 0.25% trypsin and resuspended in RPMI1640 media. 1 × 106 cells was centrifuged at 300 g for 5 minutes and then washed once with PBS .The cell pellets were added with 100% precooled ethanol at 4 °C for 4 hr, centrifuged at 300 g for 5 minutes and then washed once, resuspended with PBS (1 × 106 cells/mL). The cells were added 500 u Rnase, incubated at 37 °C for 30 minutes, washed with PBS. 1 × 106 cells were stained with 50 µg/mL PI for 20 minutes in the dark. The DNA content of each cell was measured using a Becton Dickinson FACSCalibur flow cytometer and analyzed with ModFit LT software (San Jose, CA).
Morphological study with fluorescence microscope
Exponential growth phase SMMC-7721 cells were treated with 10 nM docetaxel for 24 hr and 48 hr and harvested with 0.25% trypsin and resuspended in RPMI1640 medium. 1 × 106 cells were washed once and resuspended with PBS. 25 µl of the cells suspension was mixed with 1 µl of dye mixture containing AO 100 µg/mL and EB 100 µg/mL in PBS[16]. The cells were visualized immediately under a fluorescence microscope, the peak excitation wave length was 490 nm.
Transmission electron microscopic observation
After exponential growth phase SMMC-7721 cells treated with 10 nM docetaxel for 24 hr and 48 hr, they were fixed in Karnovsky solution, followed by cacodylate buffer for ultrastructural examination. The cells were postfixed in 1% osmium tetroxide and dehydrated for staining with uranyl acetate and lead citrate. Thin sections were observed under electron microscope.
ROS measurement
After exponential growth phase SMMC-7721 cells were treated with different doses of docetaxel (0.25 nM, 0.5 nM, 10 nM) for 24 hr, the cells were incubated with 2’, 7’-dichlorofluorescin diacetate (DCF/DA; 5 µM; Sigma) at 37 °C for 50 minutes to estimate the ROS level. The cells were harvested and detected immediately for fluorescence intensity detection on Becton Dickinson FACSCalibur flow cytometer and data were acquired and analyzed using FACS/CELLQuest software (San Jose, CA) on a Power Macintosh 7600/120 computer (Apple Computers, Cupertino, CA)[17], three independent experiments were repeated in each drug treated group and the control group was not given docetaxel but incubated with DCF/DA. All experiments were conducted in triplicate.
Determination of intracellular GSH content
The determination of intracellular GSH content was conducted according to Shen et al[18] with modification. Briefly after logarithmic growth phase SMMC-7721 cells (≥ 106) were treated with different doses of docetaxel (0.25 nM, 0.5 nM, 10 nM) for 24 hr, harvested by trypsination, washed, resuspended in PBS and counted under phase contrast microscope. The cells suspension was centrifuged at 300 g for 5 minutes, the cell pellets were added 0.75 ml distilled water and 0.25 ml thiosalicylic acid to precipitate the protein. After centrifugation at 12000 g for 5 minutes at 4 °C, the supernatant was used for GSH assay. The control group was not given docetaxel. All experiments were conducted in triplicate.
Statistical analysis
The intracellular GSH content was expressed as mean ± SD. Their differences between drug treated groups and the control group were analyzed with Student’s t-test.
RESULTS
Effect of docetaxel on SMMC-7721 cells growth inhibition
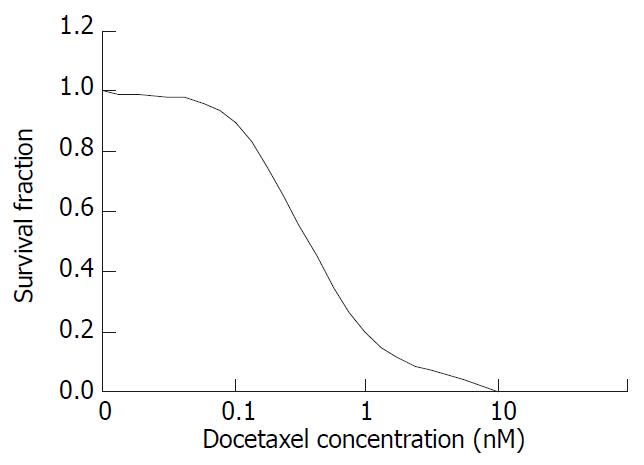
The colony forming ability decreased gradually with increasing dose of docetaxel treatment, when the docetaxel concentration was increased to 10-8 M, the colony forming inhibition rate attained 100% with IC50 5 × 10-10 M (0.5 nM) (Figure 1).
Figure 1 The survival fraction of SMMC-7721 cells treated with docetaxel for 24 hr.
Effect on of docetaxel cell cycle and apoptosis of SMMC-7721 cells
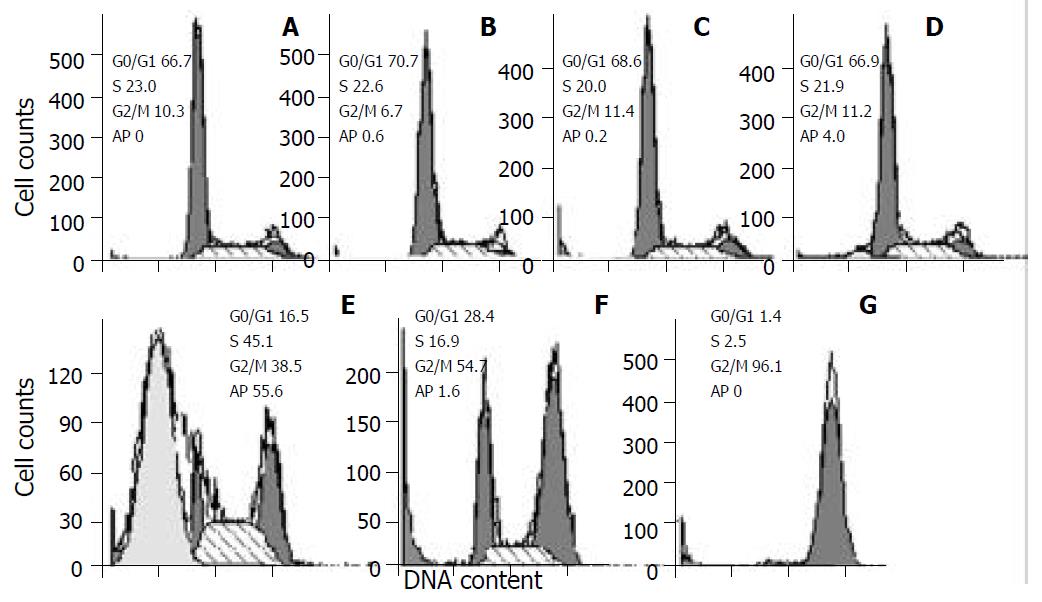
As demonstrated in Figure 2, docetaxel induced a marked apoptosis at 10-8 M for 24 hr, and caused cells G2/M phase arrest mainly at 10-7 M and 10-6 M, at 10-6 M the G2/M phase cells accounted for 96.1%.
Figure 2 Cell cycle changes and apoptosis induction in SMMC-7721 cells treated with docetaxel for 24hr.
Cells were treated with docetaxel at 0 M (A), 10-11 M (B), 10-10 M (C), 10-9 M (D), 10-8 M (E), 10-7 M (F), 10-6 M (G). A marked apoptosis was induced at 10-8 M, and a peaked G2/M phase accumulation was caused at 10-6 M. Ap: apoptosis.
Morphological observation with fluorescence microscope
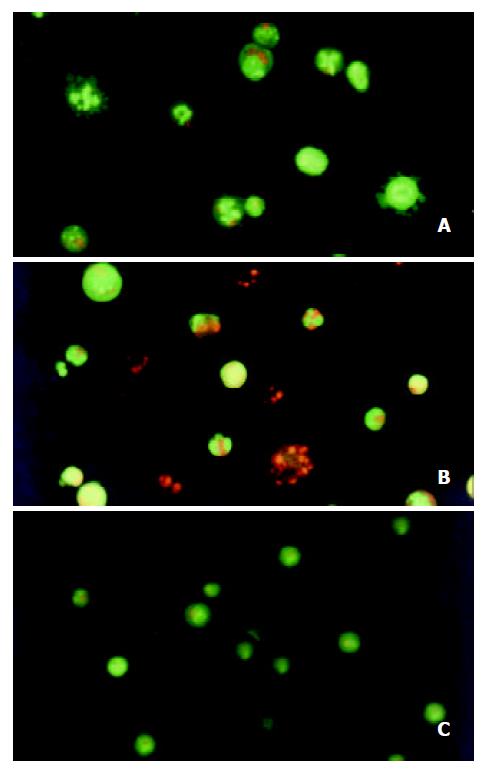
After the SMMC-7721 cells were given 10-8 M docetaxel for 24 hr, the early apoptotic cells could be observed: because of their cells membrane integrity, the cells were stained green with AO, the nuclei exhibited bright condensed chromatin or fragmented, some cells blebbed. After the cells being treated for 48hr, some late apoptotic cells could be observed: Since their cell membrane lost integrity, they were stained red with EB, the apoptotic bodies could be seen clearly. On the contrary, the untreated cells did not show these apoptotic characteristics (Figure 3).
Figure 3 Morphological study with fluorescence microscope.
After the SMMC-7721 cells were given 10-8 M docetaxel for 24 hr (A), the cells were stained green with AO, the nuclei exhibited bright condensed chromatin or fragmented, some cells blebbed. After being treated for 48 hr (B), cells were stained red with EB, the apoptotic bodies could be seen clearly. On the contrary, the untreated cells (C) did not show these apoptotic characteristics.
Transmission electron microscopic observation
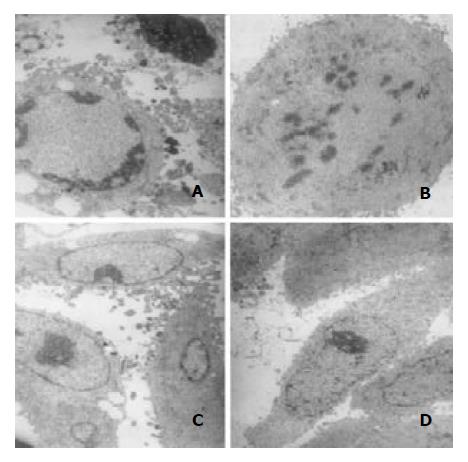
After treatment with 10-8 M docetaxel for 24 hr, the chromatin of some SMMC-7721 cells was located along the nuclear edges or formed irregularly shaped crescents at the nuclear edges, or became condensed or fragmented; Some cells nuclear membrane became irregular; The vacuole in some cytoplasma could be observed (Figure 4).
Figure 4 Transmission electron microscopic observation.
Af-ter treatment with 10-8 M docetaxel for 24 hr, the chromatin of some SMMC-7721 cells was located along the nuclear edges or formed irregularly shaped crescents at the nuclear edges (A, C),or became condensed or fragmented (A, B); The vacuole could be observed in some cytoplasm (A, C).
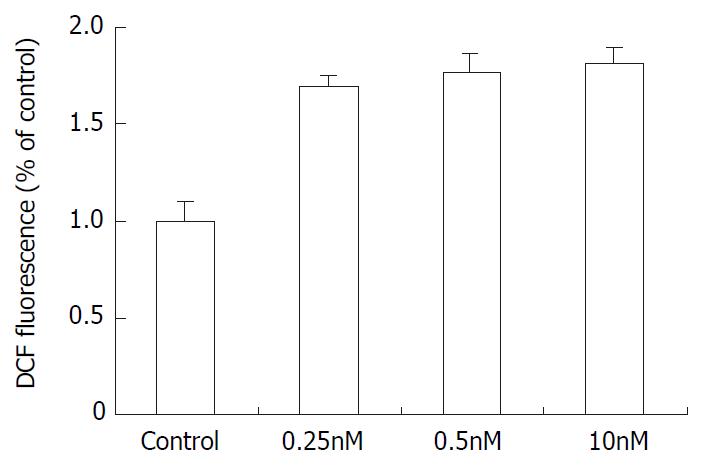
Effect of docetaxel on intracellular ROS
As demonstrated in Figure 5, The SMMC-7721 cells ROS level (corresponding to the fluorescence intensity) increased after treatment with 0.25 nM, 0.5 nM, 10 nM docetaxel. In comparison with the control group, the ROS level increased by 1.69, 1.78 and 1.80 times respectively.
Figure 5 The effect of docetaxel on the formation of ROS in SMMC-7721 cells.
Data are from three independent experiments.
Effect of docetaxel on intracellular GSH
The control group intracellular GSH content was 11.6 ± 1.1 fmol/cell, after treatment with docetaxel 0.25 nM, 0.5 nM, 10 nM for 24 hr, the SMMC-7721 cells GSH decreased to 4.7 ± 0.7 fmol/cell, 3.0 ± 0.5 fmol/cell, 1.0 ± 0.1 fmol/cell respectively, there was a significant difference between the drug treated groups and the control group (P < 0.05).
DISCUSSION
In the present study, we used colony forming assay to observe the effect of docetaxel on the growth of the human hepatocellular carcinoma SMMC-7721 cells in vitro, indicating that doctaxel can inhibit the SMMC-7721 cells growth and in concentration dependent manners, at 10-8 M the survival fraction decreased to 0 (Figure 1). A previous study demonstrated that docetaxel showed cytotoxic effect on other human hepatoma cell lines[17], together with this report, our result suggests that the growth-inhibiting effect of docetaxel may be a general phenomenon for human hepatocellular carcinoma cells. Also the flow cytometry showed that docetaxel causes SMMC-7721 cells apoptosis at a low dose (10-8 M), which was confirmed by fluorescence microscope, transmission electron microscope, and at 10-7 M, 10-6 M induced a marked G2/M phase arrest without apparent apoptosis, this phenomenon seems to suggest that docetaxel can induce apoptosis independent of G2/M phase arrest in SMMC-7721 cells.
In order to elucidate the mechanisms of the anti-hepatocellular carcinoma action of docetaxel, we examined its possible effect on cell cycle kinetics of SMMC-7721 cells. As demonstrated in Figure 2, following treatment with docetaxel for 24 hr, the G2/M phase cells increased with docetaxel doses increasing from 10-8 M, at 10-6 M the G2/M phase cells accounted for 96.1%. This result indicates that high dose of docetaxel blocks cell cycle at G2/M phase, disturbs mitosis and inhibits SMMC-7721 cells growth. In this clinical setting, such information may be useful for liver carcinoma therapy with docetaxel and other cytotoxic drugs which affect cell cycle progression.
According to Figure 1, docetaxel could inhibit SMMC-7721 cells at very low doses, the IC50 is 5 × 10-10 M. This dose of docetaxel had no significant effect on the cell apoptosis or cell cycle progression, which was confirmed by flow cytometry. In order to elucidate the mechanism underlying this phenomenon, the intracellular ROS and GSH were further investigated in this study. Our results indicated that docetaxel both at 1/2IC50, IC50 and apoptosis-inducing dose (10 nM) could cause significantly increased ROS levels (Figure 5) and significantly decreased GSH, compared with those of the control.
It has been reported that tumor cells have persistently higher levels of ROS[19]. The relationship between ROS and apoptosis, cell growth inhibition has been broadly investigated. ROS has been found to regulate translocation of NF-κB[20] as well as translocation of p53 and the p53-mediated apoptosis pathway[21,22]. Also it has been found that induction of apoptosis was accompanied with the generation of intracellular ROS prior to the activation of caspase-3[23] and down-regulation of Bcl-2[24]. Simizu et al[25] reported that some anticancer agents, including vinblastin and camptothecin, induced cells apoptosis with the generation of ROS. Recently it was reported that arsenic trioxide and l-S,R-buthionine sulfoximine (BSO) combination treatment enhanced hepatocellular carcinoma cells apoptosis through increased production of ROS[26], Also it was reported ROS played a mediatory role in the synergistic interaction between 1, 25-dihydroxyvitamin D (3) and anticancer cytokines[27], modulation of the ROS production intra and extracellularlly may influence the cell survival during oxidative insults[28].
Accordingly, we speculate that docetaxel-induced high level ROS may be involved in the growth inhibition and apoptosis of SMMC-7721 cells.
In order to confirm the SMMC-7721 cells redox status after docetaxel treatment, the intracellular GSH content was also investigated. Our result demonstrated that the intracellular GSH decreased significantly (P < 0.05) after both 1/2IC50, IC50 and apoptosis-inducing dose docetaxel treatment, compared with that of the control group. This indicates that the decrease of GSH may be involved in the SMMC-7721 cells growth inhibition and apoptosis caused by docetaxel. Liu et al[29] found Salvia miltiorrhiza inhibited human hepatoma HepG2 cells growth and induced apoptosis involving in intracellular GSH deletion, early studies have also demonstrated that the onset of apoptosis was associated with a fall of intracellular GSH in different cellular systems[30]. And GSH could significantly reduce apoptosis mediated by a monoclonal antibody directed to Fas antigen, and apoptosis could be nearly completely prevented by GSH[31]. GSH is an important cellular thiol which is regarded as the major determinant of the intracellular redox potential, on the other hand, apoptosis may be regulated by the reodx status within the cells[32]. Loss of GSH was shown to be tightly coupled with a number of down-stream events in apoptosis, in caspase activation and events in chromatin[33], and GSH depletion may act as a link between oxidative stress and ceramide-mediated apoptosis[34]. Also it was found that decreased cells proliferation associated with decreased levels of intracellular GSH, and blockade of GSH synthesis enhanced ROS production and suppressed cells proliferation[35], cells with compromised cellular GSH were susceptible to redox imbalance-induced inhibition of proliferation[36].
In summary, the present study demonstrates that docetaxel has profound effects on SMMC-7721 hepatocellular carcinoma cells in vitro. It reduces the proliferation of these cells, causes changes in their morphology, and induces cell death by apoptosis. It leads to ROS generation and GSH deletion, subsequently results in redox imbalance in SMMC-7721 cells, and it is speculated that the redox imbalance caused by docetaxel may play an important role in the growth inhibition and induction of apoptosis of SMMC-7721 cells. As far as we know, there is few report concerning the redox imbalance and docetaxel. Our results not only provide the basis for further in vivo and clinical research in hepatocellular carcinoma, but also contribute to understanding of the pharmacology of docetaxel further.