Published online Dec 7, 2018. doi: 10.3748/wjg.v24.i45.5109
Peer-review started: August 15, 2018
First decision: October 10, 2018
Revised: October 22, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 7, 2018
To establish a rotavirus (RV)-induced diarrhea model using RV SA11 in neonatal rhesus monkeys for the study of the pathogenic and immune mechanisms of RV infection and evaluation of candidate vaccines.
Neonatal rhesus monkeys with an average age of 15-20 d and an average weight of 500 g ± 150 g received intragastric administration of varying doses of SA11 RV ( 107 PFUs/mL, 106 PFUs/mL, or 105 PFUs/mL, 10 mL/animal) to determine whether the SA11 strain can effectively infect these animals by observing their clinical symptoms, fecal shedding of virus antigen by ELISA, distribution of RV antigen in the organs by immunofluorescence, variations of viral RNA load in the organs by qRT-PCR, histopathological changes in the small intestine by HE staining, and apoptosis of small intestinal epithelial cells by TUNEL assay.
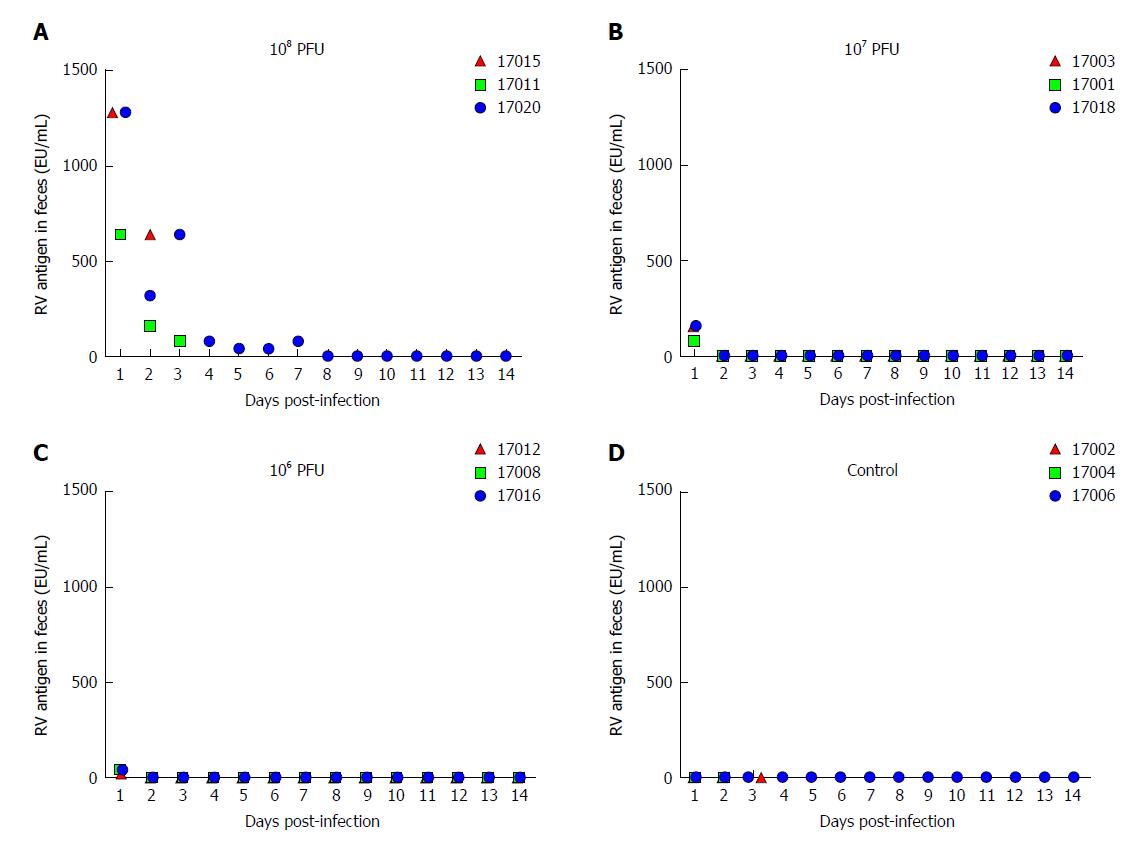
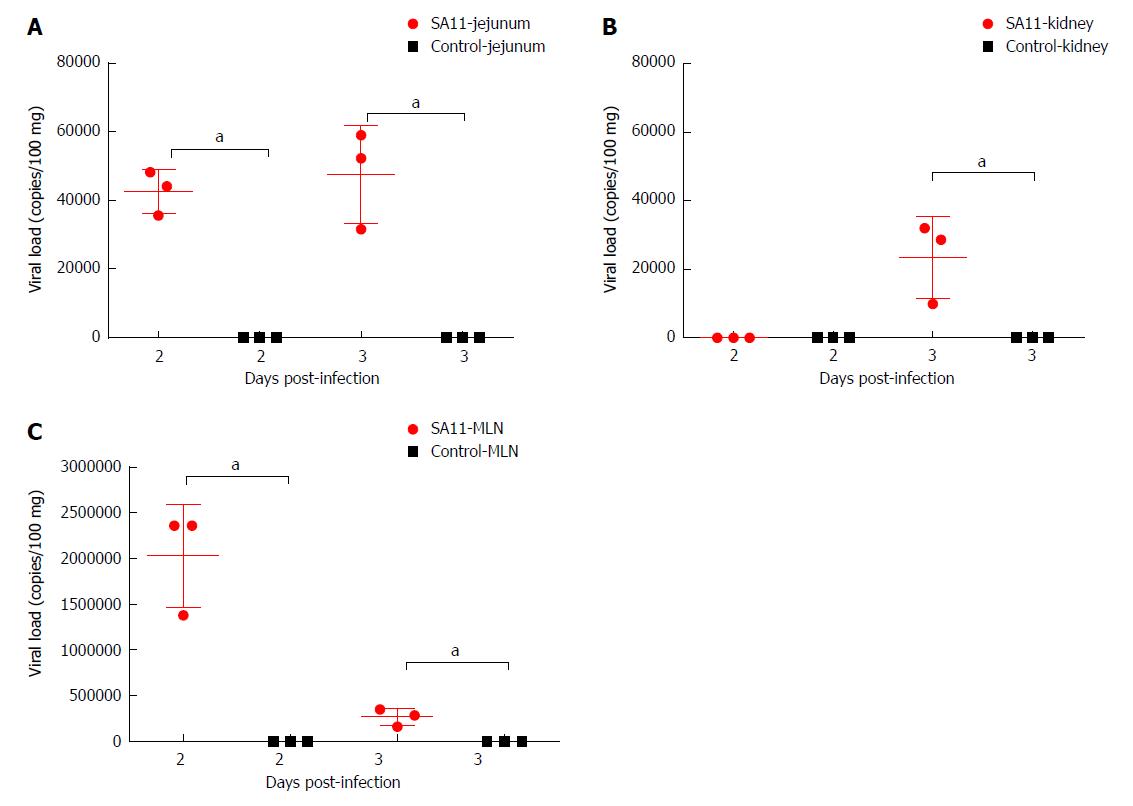
The RV monkey model showed typical clinical diarrhea symptoms in the 108 PFUs SA11 group, where we observed diarrhea 1-4 d post infection (dpi) and viral antigen shed in the feces from 1-7 dpi. RV was found in jejunal epithelial cells. We observed a viral load of approximately 5.85 × 103 copies per 100 mg in the jejunum at 2 dpi, which was increased to 1.09 × 105 copies per 100 mg at 3 dpi. A relatively high viral load was also seen in mesenteric lymph nodes at 2 dpi and 3 dpi. The following histopathological changes were observed in the small intestine following intragastric administration of SA11 RV: vacuolization, edema, and atrophy. Apoptosis in the jejunal villus epithelium was also detectable at 3 dpi.
Our results indicate that we have successfully established a RV SA11 strain diarrhea model in neonatal rhesus monkeys. Future studies will elucidate the mechanisms underlying the pathogenesis of RV infection, and we will use the model to evaluate the protective effect of candidate vaccines.
Core tip: Rotavirus (RV) is one of the main pathogens responsible for severe diarrhea in children under 5 years of age. Vaccine-induced immunity is an effective way to block RV disease. Nonhuman primates are the animals most closely related to humans and have advantages over non-primates as an animal model of RV diarrhea, so development of a nonhuman primate animal model of RV infection is needed to ensure the effectiveness and safety of these vaccines. Our current study has indicated that RV SA11 can lead to obvious diarrhea and pathological changes in the intestine of neonatal rhesus monkeys. The RV infection model we established is useful for us to further investigate the RV infection mechanism and the associated immune mechanisms in human infants and evaluate the cross protection of potential HRV vaccine candidates.
- Citation: Yin N, Yang FM, Qiao HT, Zhou Y, Duan SQ, Lin XC, Wu JY, Xie YP, He ZL, Sun MS, Li HJ. Neonatal rhesus monkeys as an animal model for rotavirus infection. World J Gastroenterol 2018; 24(45): 5109-5119
- URL: https://www.wjgnet.com/1007-9327/full/v24/i45/5109.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i45.5109
As the primary cause of severe acute gastroenteritis in infants and young children, rotavirus (RV) is one of the most important causes of pathogenicity worldwide[1]. RV infection can result in vomiting, fever, severe dehydration, diarrhea, and even death. Over 200000 infants and young children die each year due to RV infection worldwide, and low-income countries are disproportionately affected[2].
There are currently no specific drugs for the treatment of diarrhea caused by RV infection[3]; therefore, the development of safe and effective vaccines to control RV infection is particularly important[4-7]. Furthermore, an effective animal model of RV infection is needed to ensure the effectiveness and safety of these vaccines. Nonetheless, some progress has been made in the development of animal RV models, and have included gnotobiotic piglets, calves, lambs, suckling mice, and rabbits. While RV infection in calves and lambs results in mild clinical disease[8-10], gnotobiotic piglets are more susceptible to RV infection, since their immune system more closely resembles that of a human infant, and the period of susceptibility is very long[11-13]. However, breeding conditions for gnotobiotic piglets are quite strict and their cost is prohibitively high, restricting the study of RV infection in these animals. Suckling mice and rabbits have also been used to study RV infection[14-18] and they have strong reproductive abilities and are easy to maintain; however, these animal models have a distant evolutionary relationship with humans, which can limit the ability of data obtained from these animal models to improve the understanding of the pathogenesis of RV infection in humans. Therefore, developing models using non-human primates, the species more closely related to humans, is necessary[19-23].
The SA11 strain is a simian RV strain, obtained from an asymptomatic vervet monkey in vitro[24]. As described before, the SA11 strain can infect not only non-human primates, such as chimpanzee, macaque (cynomolgus monkey and rhesus monkey)[20,21], but also other non-primates, such as mice[14]. Petschow et al[25] inoculated five newborn cynomolgus monkeys with the simian RV strain SA11, and detected SA11 in feces of three monkeys for up to 2 d after inoculation. In this study, we infected the neonatal rhesus monkeys with RV SA11 through oral gavage to establish an RV diarrhea model. Our data indicated that 108 plaque forming units (PFUs) of SA11 can infect intestinal villous epithelial cells in neonatal rhesus monkeys, and result in obvious pathological changes in the small intestine as well as clinical symptoms including diarrhea. Together, these findings indicate that the neonatal rhesus monkey could be used as an animal model for RV infection, providing a powerful tool for further study of the pathogenesis of RV and the associated immune mechanisms in human infants and evaluation of RV vaccines.
The experimental animals in this study were provided by the Primate Experimental Center of the Institute of Medical Biology, Chinese Academy of Medical Sciences. A total of 12 healthy neonatal rhesus monkeys with an average age of 15-20 d and an average weight of 500 g ± 150 g were randomly divided into three experimental groups and a control group, each with three monkeys. The experimental animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Institute of Medical Biology, Chinese Academy of Medical Sciences. A neutralizing antibody test was conducted to confirm that the monkeys did not have antibodies against RV SA11 prior to the study. All of the animals were housed in a separate incubator one week before the initiation of the experiment.
The activated RVs were adjusted to 1000 PFUs/100 μL in serum-free MEM. The serum samples were diluted from 1:10 to 1:1280 in 100 μL serum-free MEM. The diluted RVs and serum samples were mixed with each and incubated at 37 °C for 1 h. The mixtures were transferred to the 96-well plates covered with a confluent monolayer of MA104 cells and were cultured at 37 °C for 5 d. The cultures were completely transferred to the wells of the ELISA plate coated with a goat anti-RV polyclonal antibody (Millipore, AB1129) and blocked with 3% (w/v) BSA (Biosharp, BS043D). The cultures were inoculated at 37 °C for 1 h. A rabbit anti-RV polyclonal antibody (prepared by the Department of Molecular Biology, Institute of Medical Biology, Chinese Academy of Medical Science and Peking Union Medical College) conjugated with horseradish peroxidase (HRP) was used to detect RV antigen at a dilution of 1:2000 (v/v) in PBS at 37 °C for 1 h. All ELISA plates were developed using TMB (TIANGEN, PA107-01) to generate a colorimetric reaction and terminated with 2 mol/L H2SO4. The absorbance was read on a universal microplate reader (El × 800, Bio-Tek, United States) at 450 nm and 630 nm. A serum specimen was determined to be positive if the OD value was less than or equal to two times the average OD value of the negative control. The neutralization titers were defined as the highest dilution.
MA104 cells were maintained in MEM supplemented with 10% fetal bovine serum (Gibco, 16000-044) and grown to a confluent monolayer in roller bottles (850 cm2) (CORNING, 430849). The RV strain used in this study was standard strain SA11 (G3P[2]) that was originally isolated from a monkey. Prior to infection, SA11 was activated with 20 μg/mL trypsin (Gibco, 15090-046) at 37 °C for 45 min. Infected cultures were harvested by freezing at -20 °C and thawing at room temperature, followed by centrifugation at 8873 g for 20 min. The harvested virus titer reached 107 PFUs/mL[26].
The neonatal rhesus monkeys were inoculated with varying doses of RV SA11 (108/107/106 PFUs/monkey) or 10 mL medium without serum via oral gavage (Supplementary Table 1). After the infection, each neonatal rhesus monkey was housed alone in the incubator.
| Dose (PFUs) | Monkey ID | Diarrhea score1 | Mean diarrhea score2 | Percentage with diarrhea (%)3 | |||||||||
| 1 dpi | 2 dpi | 3 dpi | 4 dpi | 1 dpi | 2 dpi | 3 dpi | 4 dpi | 1 dpi | 2 dpi | 3 dpi | 4 dpi | ||
| 108 | 17020 | 4 | 4 | 3 | 2 | ||||||||
| 17011 | 3 | 4 | 4 | - | 3.67 | 4 | 3.5 | - | 100 | 100 | 100 | - | |
| 17015 | 4 | 4 | - | - | |||||||||
| 107 | 17018 | 2 | 0 | 0 | 1 | ||||||||
| 17003 | 2 | 0 | 0 | 1 | 2.67 | 0.67 | 0.33 | 1 | 100 | 33.3 | 0 | 0 | |
| 17001 | 4 | 2 | 1 | 1 | |||||||||
| 106 | 17008 | 0 | 2 | 0 | 1 | ||||||||
| 17012 | 0 | 1 | 2 | 0 | 0 | 1.33 | 1 | 0.33 | 0 | 33.3 | 33.3 | 0 | |
| 17016 | 0 | 1 | 1 | 0 | |||||||||
| 0 | 17006 | 0 | 0 | 0 | 0 | ||||||||
| 17002 | 0 | 0 | 0 | - | 0.33 | 0 | 0 | - | 0 | 0 | 0 | - | |
| 17004 | 1 | 0 | - | - | |||||||||
From 0 to 14 d post infection (dpi), the infected neonatal rhesus monkeys were monitored once daily for clinical signs, such as mental status, weight, body temperature, and hair, and diarrhea situation. The fecal samples were collected in a fecal collector by gently pressing the abdomen of the neonatal rhesus monkeys every day. According to the color, hardness, and quantity of the feces, diarrhea was scored from 1 to 4 points[11,15], and > 2 points were considered to be indicative of diarrhea. The fecal sample was suspended in 10% (s/v) cold PBS, followed by centrifugation at 8873 g for 20 min, after which the supernatant was collected and stored at -80 °C for the subsequent study. At 2 and 3 dpi, one monkey that was infected with 108 PFUs of SA11 or medium without serum was killed by electric shock with anesthesia. Two aliquots of each tissue sample, including the heart, liver, spleen, lung, kidney, intestine (duodenum, jejunum, and ileum), and brain, were harvested. One sample was stored in liquid nitrogen and the other was fixed in 10% formalin.
The wells of the ELISA plate were coated with a goat anti-RV polyclonal antibody (Millipore, AB1129) diluted at 1:1000 (v/v) in carbonate-bicarbonate buffer overnight at 4 °C. Then, the plates were blocked with 3% (w/v) BSA (Biosharp, BS043D) in PBS at 37 °C for 2 h. The fecal samples (100 μL/well) were serially two-fold diluted (dilution range from 1:2 to 1:4096) and incubated at 37 °C for 1 h. After washing, a rabbit anti-RV polyclonal antibody conjugated with HRP was used to detect RV antigen at a dilution of 1:2000 (v/v) in PBS at 37 °C for 1 h. All of the ELISA plates were developed using TMB (TIANGEN, PA107-01) to generate a colorimetric reaction and terminated with 2 mol/L H2SO4. A sample was determined to be positive if the OD value was greater than or equal to two times the average OD value of the negative control.
The small intestine was fixed in 10% (v/v) formalin in PBS, dehydrated in 70% (v/v) graded ethanol series, and embedded in paraffin before being sectioned at the 4.0 μm thickness for further hematoxylin and eosin staining. Histopathological observation of the small intestine was performed by light microscopy. For immunofluorescence microscopy, tissue sections were deparaffinized in xylene and hydrated through graded ethanol, followed by adding 0.03% (v/v) hydrogen peroxide solution. Antigen retrieval was performed with 0.01 mol/L sodium citrate (pH 6.0). The glass slides were blocked with normal goat serum (BOSTER, SA1006) at a 1:10 dilution for 20 min. Next, the glass slides were incubated with a goat anti-RV polyclonal antibody (prepared by the Department of Molecular Biology, Institute of Medical Biology, Chinese Academy of Medical Science and Peking Union Medical College) at a 1:500 dilution at 37 °C for 90 min. The glass slides were incubated with an FITC-labeled rabbit anti-goat IgG antibody (Jackson ImmunoResearch, 705-095-147) at a 1:1000 dilution at 37 °C for 60 min. After incubation, the glass slides detected under a fluorescence microscope.
Viral RNA was isolated from fresh tissue of experimental animals with Trizol (Ambion, 15596026). Trizol (1 mL) was added to 50-100 mg of fresh tissue and the sample was grinded on the ice with an electric grinder. The homogenized sample was incubated for 5-10 min at room temperature. Chloroform (0.2 mL) was added to the homogenized samples. The tube was shaken vigorously by hands for 15 s and incubated for 15 min at room temperature. Next, the tube was centrifuged at 12000 rpm for 15 min at 4 °C and the aqueous phase was transferred to a clean tube. Isopropyl alcohol (0.5 mL) was added to the tubes. Then, the tube was incubated for 10 min at room temperature and centrifuged at 12000 rpm for 10 min at 4 °C. The supernatant was removed and the RNA was washed with 1 mL of 75% ethanol. The tube was centrifuged at 12000 rpm for 5 min at 4 °C, and RNA was washed again and dried for 5-10 min at room temperature. Finally, RNA was completely dissolved in 30 μL of RNase-free water and stored at -70 °C.
At the same time, viral RNA was isolated from venous blood samples using the QIAamp Viral RNA Mini Kit, according to the manufacture’s protocol (Qiagen, 52904).
RT-PCR assays were performed using the TransScript II Probe One-Step qRT-PCR SuperMix (TRANS, AQ321-01) in the Real-Time System (CFX96, BIO-RAD, United States). The reaction system included 5 μL of RNA template, forward primer at 20 nm, reverse primer at 20 nm, FAM-labeled probe at 20 nm, and E-mix in a total reaction volume of 20 μL. The sequences for the primers were as follows: forward primer, 5’-GTTGTCATCTATGCATAACCCTC-3’; reverse primer, 5’-ACATAACGCCCCTATAGCCA-3’; FAM-labeled probe, 5’-ATGAGCACAATAGTTAAAAGCTAACACTGTCAA-3’. The following protocol was used for all of the RT-PCR: 5 min at 50 °C; 30 s at 94 °C; followed by 40 cycles of 94 °C for 5 s and 60 °C for 30 s. A standard reference curve was obtained by measurement of standard virus RNA. According to the standard reference curve, the viral load was quantified in each sample.
We detected cell apoptosis in the small intestine of the infected neonatal rhesus monkeys using the TUNEL Bright Green Apoptosis Detection Kit (Vazyme, A112-03), according to the manufacturer’s protocol. The analysis of apoptosis was performed by fluorescence microscopy.
Neonatal rhesus monkeys received intragastric administration of 108/107/106 PFUs of SA11 or medium without serum. Only the 108 PFUs group showed significantly characteristic symptoms, and all of the neonatal rhesus monkeys in that group showed obvious clinical symptoms including depression, dull hair color, lethargy, weakened activity, and diarrhea between 1 and 3 dpi. On day 1, monkeys infected with 108 PFUs of SA11 developed diarrhea that was flocculent or watery (Table 1). On day 2 and 3, significant fecal pollution was observed around the anus, and diarrhea symptoms were the most serious. On day 4, obvious symptom relief was observed, physiological characteristics began to improve, diarrhea stopped gradually, and animals recovered to normal excreta. In the 107 PFUs group, only one monkey developed severe diarrhea at 1 dpi. None of the neonatal rhesus monkeys infected with 106 PFUs of RV or medium without serum developed obvious clinical symptoms of RV infection, and other physiological characteristics, including body temperature and body weight, did not change significantly from 0-4 dpi in the experimental group compared to the control group (Supplementary Figure 1).
RV is transmitted through the fecal-oral route[27]. RV primarily infects human or animal small intestinal villous epithelial cells[28], and is expulsed through feces in vivo. The viral shedding observed in the feces reflects the replication and infection level of the virus in the body. We evaluated fecal viral shedding by ELISA in neonatal rhesus monkeys receiving either SA11 or medium without serum. Neonatal rhesus monkeys infected with 108 PFUs of SA11 virus shed antigen beginning at 1 dpi and it lasted for 7 days (Figure 1). A small amount of virus antigen was shed in the feces of neonatal rhesus monkeys infected with 107 PFUs or 106 PFUs of SA11 strain at 1 dpi, and no virus antigen was detected in the control group (Figure 1).
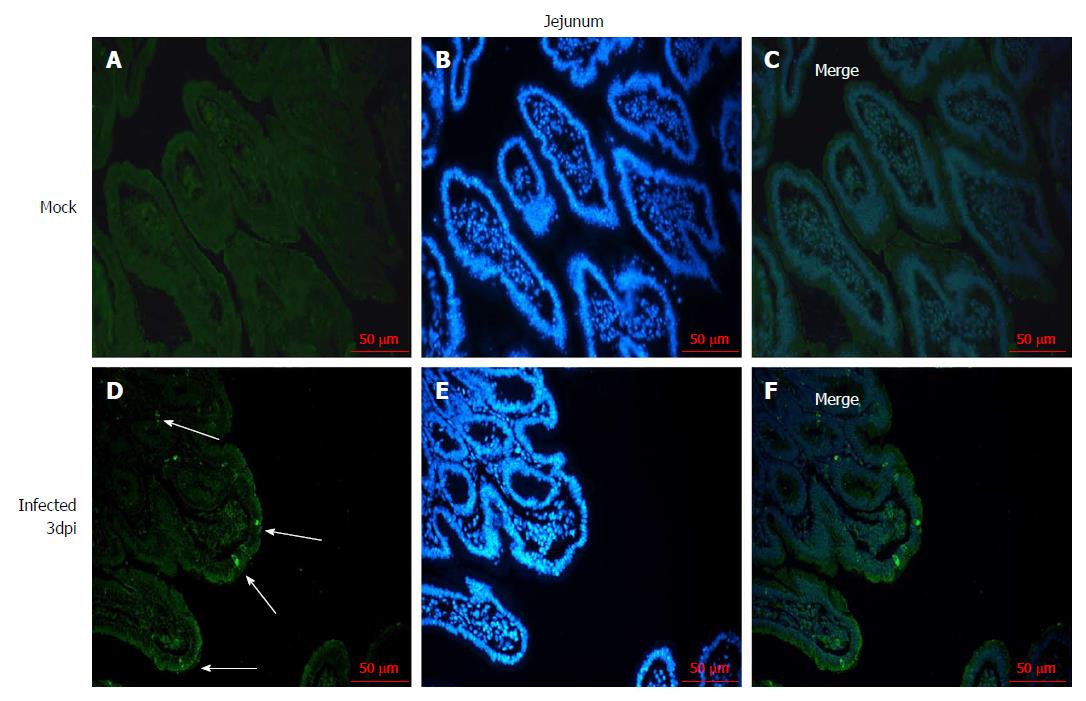
The distribution of RV in the jejunum was detected by immunofluorescence to confirm that the RV infects villus epithelial cells in the small intestine. RV antigen was detected in the jejunal epithelial cells in the 108 PFUs of SA11-infected neonatal rhesus monkeys at 3 dpi. No RV antigen was detected in the jejunal epithelial cells in the control group (Figure 2).
The viral load variations in various organs of the monkeys infected by 108 PFUs of SA11 were detected by qRT-PCR at 2 and 3 dpi to understand the transmission and distribution of RV across the various organs. We observed a viral load of approximately 5.85 × 103 copies per 100 mg in the jejunum at 2 dpi, which was increased to 1.09 × 105 copies per 100 mg at 3 dpi (Figure 3). A relatively high viral load of 9.9 × 106 copies per 100 mg was seen in the mesenteric lymph nodes at 2 dpi, but was decreased to 2.42 × 106 copies per 100 mg at 3 dpi (Figure 3). A viral load of 1.02 × 105 copies per 100 mg in the kidney was detected at 2 dpi, but no viral load was detected in the kidney at 3 dpi (Figure 3). No virus was detected in the heart, liver, spleen, lung, or brain (Supplementary Table 2). We also collected blood samples at 12 h post infection (hpi), 24 hpi, 48 hpi, and 72 hpi, and detected no viral load.
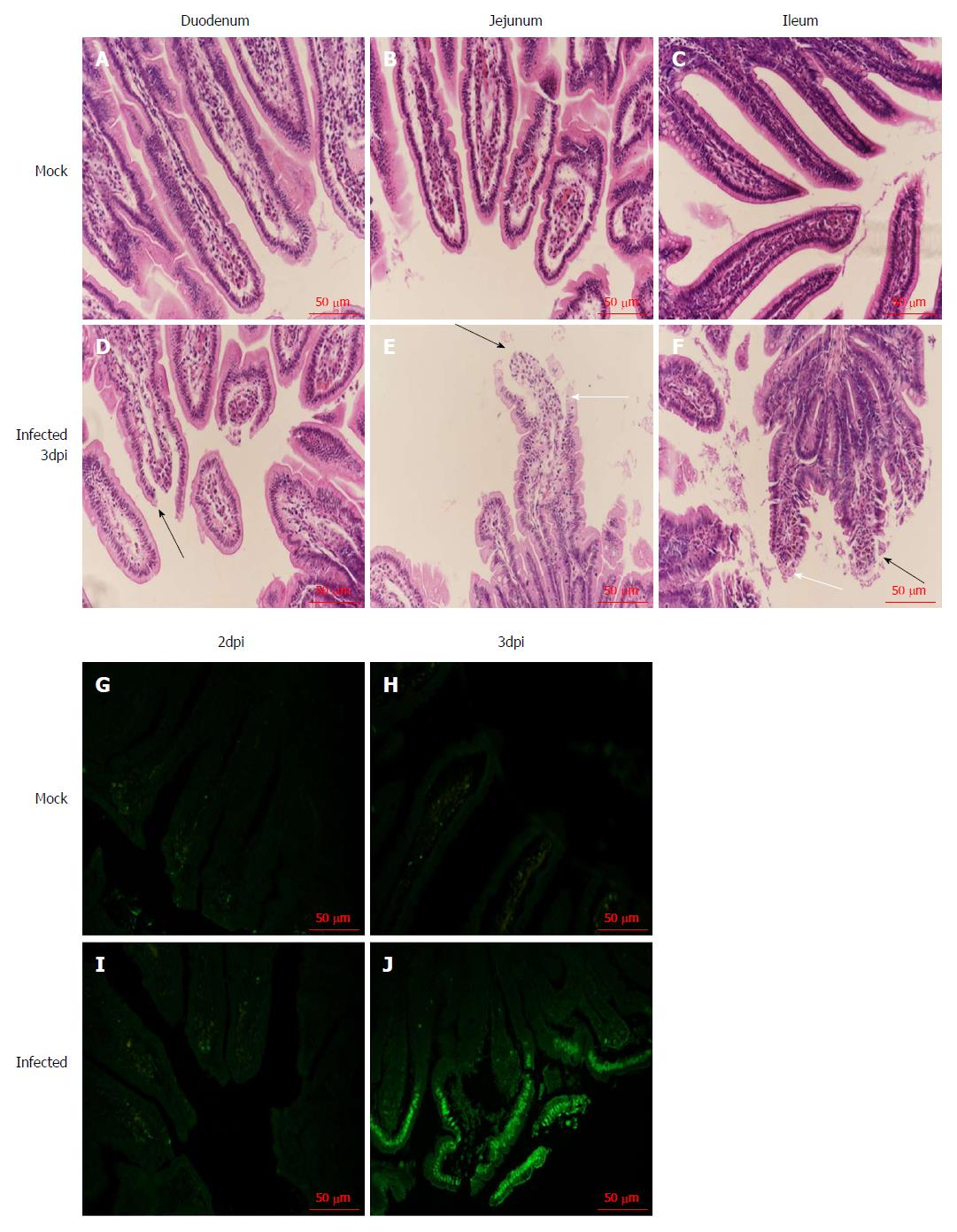
We examined the small intestine of the infected neonatal rhesus monkeys at 2 dpi and 3 dpi to confirm whether RV infection causes histopathological changes, and found inflation and swelling in the small intestine of some infected animals. We collected the tissues (duodenum, jejunum, and ileum) and examined the small intestine by HE staining. We found obvious pathological changes in the small intestinal tissues in the RV SA11 group compared to negative controls, including vacuolization, edema, atrophy, and breakage of the small intestinal villus cells, as well as an absence of obvious inflammatory cell infiltration (Figure 4).
We used the TUNEL method to detect apoptosis of jejunal epithelial cells after SA11 infection. Apoptotic cells were not observed in the mock infection group (Figure 4). In infected animals, apoptosis of jejunal epithelial cells was not increased at 2 dpi (Figure 4), but at 3 dpi, apoptosis of the upper jejunal villus epithelial cells increased significantly, with apoptotic cells arranged in clusters along the villus top and some apoptotic cells detached from the jejunal villi (Figure 4).
A RV diarrhea model is crucial for the study of the pathogenic and immune protection mechanisms of RV infection and vaccine development. Various models have been used to study RV infection; however, these animal models are evolutionarily distant from humans and cannot simulate the process of RV infection in human infants. Therefore, a nonhuman primate RV diarrhea model is needed for the study of human RV infection.
RV infection is age-restricted[17]. We inoculated rhesus monkeys of various ages (15-20 d, 60 d, 120 d, and 1 year) with the same dose of SA11 strain in previous studies. Monkeys with an average age of 15-20 d were more sensitive to the SA11 strain and presented obvious clinical symptoms.
The SA11 is one of classic RVs that several study have used to model infection of newborn animals. They showed that 5 × 106 PFUs infection of newborn mice with 5 × 106 PFUs of RV and infection of newborn rats with 108 PFUs could induce obvious diarrhea[29,30]. In our present study, we used different doses of SA11 virus to infect neonatal rhesus monkeys based on our calculation of its median diarrhea dose (DD50; 107.47 PFUs/kg), and observed the most obvious symptoms in animals receiving 108 PFUs (3.38DD50) of the virus. This dose was consistent with that used in newborn mice, after equal conversion of body weight/metering. The clinical symptoms of RV-infected human infants include fever, vomiting, and diarrhea, with the clinical symptoms lasting for 7-14 d[31]. We observed a similar time course in the neonatal rhesus monkeys infected with SA11. Compared to the clinical symptoms observed in RV-infected infants, neonatal rhesus monkeys infected with SA11 suffered from diarrhea only. This symptom developed at 1 dpi, and the most serious diarrhea was observed from 2-3 dpi, which was alleviated and improved from 4 dpi. Viral shedding in the feces occurred from 1-7 dpi, and was the highest at 1 to 3 dpi. RV can cause weight loss in suckling mice during the early stages of infection[32]; however, the weight of the neonatal rhesus monkeys infected with SA11 was not significantly lower than that of the negative control group in this study. RV infection in human infants can also cause serious dehydration and even death in the absence of aggressive hydration. However, no monkeys died during these experiments, which may be related to the sensitivity of the virus strain or the immune state of the monkey. RV infection can cause viremia and results in lesions to the liver, gallbladder, respiratory system, nervous system, and urinary system[33]. The occurrence of viremia depends on the virus strain and the immune state of the host[34,35]. RV escapes the gastrointestinal tract through the blood and lymphatic system. Previous reports have detected RV in the blood of some children infected with RV in the clinic[36-38], but no RV was found in the blood following infection in the current study. Furthermore, we determined the viral load of RV in the organs of the infected monkeys at 2 and 3 dpi. Our data showed that a viral RNA load of 1.02 × 105 copies per 100 mg in the kidney was detected at 2 dpi and a relatively high viral load was seen in the mesenteric lymph nodes at 2 dpi. Therefore, we speculate that RV SA11 is capable of escaping from the intestine and transmitted to the kidney via the mesenteric lymph nodes. A mechanism of the extra-intestinal spread of RV has been discussed in a report of a neonatal mouse model of RV[39]. Rhesus rotavirus (RRV) and reassortant R7 rotavirus (R7 RV) can spread from the intestine to the terminal ileum, mesenteric lymph nodes, and peripheral tissues. Previous studies suggested that the transmission capacity of RV in the neonatal mouse was related to the NSP3 and VP6 regions[40], and whether they facilitate RV transmission in monkeys remains to be examined.
Diarrhea is one of the most typical symptoms of RV infection. Early studies have shown that RV infection leads to shortened intestinal villi and loss of epithelial cells at the top of the villus[28]. In the current study, a large amount of vacuolization, cell edema, and intestinal villus atrophy, and various degrees of breakage occurred in the villus cells of neonatal rhesus monkeys infected with SA11. Apoptosis of small intestinal epithelial cells is also a cause of diarrhea[41], and apoptosis has been observed in RV-infected HT-29 cells[42-45]. We analyzed the apoptosis of small intestinal epithelial cells after infection, and observed apoptosis in the apical layer of the intestinal villus epithelial cells.
In conclusion, our results indicate that we have successfully established a RV SA11 strain diarrhea model in neonatal rhesus monkeys. The RV infection model we established was useful for us to further investigate the RV infection mechanism and evaluate the cross protection of potential HRV vaccine candidates[29,46].
Rotavirus (RV) is one of the main pathogens responsible for severe diarrhea in children under 5 years of age. There are currently no specific drugs for the treatment of diarrhea caused by RV infection. Therefore, the development of safe and effective vaccines to control RV infection is particularly important. An effective animal model of RV infection is needed to ensure the effectiveness and safety of these vaccines.
Nonhuman primates are the animals most closely related to humans and have advantages over non-primates as an animal model of RV diarrhea, so development of a nonhuman primate animal model of RV infection is needed to ensure the effectiveness and safety of RV candidate vaccines.
To establish a monkey model of RV infection.
Neonatal rhesus monkeys with an average age of 15-20 d and an average weight of 500 g ± 150 g received intragastric administration of varying doses of SA11 RV to determine whether the SA11 strain can effectively infect these animals by observing their clinical symptoms, fecal shedding of virus antigen by ELISA, distribution of RV antigen in the organs by immunofluorescence, variations of viral RNA load in the organs by qRT-PCR, histopathological changes in the small intestine by HE staining, and apoptosis of small intestinal epithelial cells by TUNEL assay.
The RV monkey model showed typical clinical diarrhea symptoms in the 108 PFUs SA11 group, where we observed diarrhea 1-4 d post infection (dpi) and viral antigen shed in the feces from 1-7 dpi. RV was found in jejunal epithelial cells. We observed a viral load of approximately 5.85 × 103 copies per 100 mg in the jejunum at 2 dpi, which was increased to 1.09 × 105 copies per 100 mg at 3 dpi. A relatively high viral load was also seen in the mesenteric lymph nodes at 2 dpi and 3 dpi. The following histopathological changes were observed in the small intestine following intragastric administration of SA11 RV: vacuolization, edema, and atrophy. Apoptosis of the jejunal villus epithelium was also detectable at 3 dpi.
We successfully established a RV SA11 strain diarrhea model in neonatal rhesus monkeys.
The monkey model of RV infection is useful for us to further investigate the RV infection mechanism and evaluate the protection of potential HRV vaccine candidates.
We thank Jia-Hong Gao for making paraffin sections.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Krishnan T, Mesquita J S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O’Ryan M, Kang G. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 324] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 2. | Tate JE, Burton AH, Boschi-Pinto C, Parashar UD; World Health Organization–Coordinated Global Rotavirus Surveillance Network. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000-2013. Clin Infect Dis. 2016;62 Suppl 2:S96-S105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 693] [Cited by in F6Publishing: 760] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 3. | Leung AK, Robson WL. Acute gastroenteritis in children: role of anti-emetic medication for gastroenteritis-related vomiting. Paediatr Drugs. 2007;9:175-184. [PubMed] [Cited in This Article: ] |
| 4. | Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality From Diarrhea. J Infect Dis. 2017;215:1666-1672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 5. | Lepage P, Vergison A. Impact of rotavirus vaccines on rotavirus disease. Expert Rev Anti Infect Ther. 2012;10:547-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB. Effect of human rotavirus vaccine on severe diarrhea in African infants. Malawi Med J. 2016;28:108-114. [PubMed] [Cited in This Article: ] |
| 7. | Wang CM, Chen SC, Chen KT. Current status of rotavirus vaccines. World J Pediatr. 2015;11:300-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Reynolds DJ, Hall GA, Debney TG, Parsons KR. Pathology of natural rotavirus infection in clinically normal calves. Res Vet Sci. 1985;38:264-269. [PubMed] [Cited in This Article: ] |
| 9. | Archambault D, Morin G, Elazhary Y, Roy RS. Study of virus excretion in feces of diarrheic and asymptomatic calves infected with rotavirus. Zentralbl Veterinarmed B. 1990;37:73-76. [PubMed] [Cited in This Article: ] |
| 10. | Snodgrass DR, Ferguson A, Allan F, Angus KW, Mitchell B. Small intestinal morphology and epithelial cell kinetics in lamb rotavirus infections. Gastroenterology. 1979;76:477-481. [PubMed] [Cited in This Article: ] |
| 11. | Li JT, Wei J, Guo HX, Han JB, Ye N, He HY, Yu TT, Wu YZ. Development of a human rotavirus induced diarrhea model in Chinese mini-pigs. World J Gastroenterol. 2016;22:7135-7145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis. 2011;203:188-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl. 1996;12:153-161. [PubMed] [Cited in This Article: ] |
| 14. | Ciarlet M, Conner ME, Finegold MJ, Estes MK. Group A rotavirus infection and age-dependent diarrheal disease in rats: a new animal model to study the pathophysiology of rotavirus infection. J Virol. 2002;76:41-57. [PubMed] [Cited in This Article: ] |
| 15. | Boshuizen JA, Reimerink JH, Korteland-van Male AM, van Ham VJ, Koopmans MP, Büller HA, Dekker J, Einerhand AW. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J Virol. 2003;77:13005-13016. [PubMed] [Cited in This Article: ] |
| 16. | Du J, Lan Z, Liu Y, Liu Y, Li Y, Li X, Guo T. Detailed analysis of BALB/c mice challenged with wild type rotavirus EDIM provide an alternative for infection model of rotavirus. Virus Res. 2017;228:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Ciarlet M, Gilger MA, Barone C, McArthur M, Estes MK, Conner ME. Rotavirus disease, but not infection and development of intestinal histopathological lesions, is age restricted in rabbits. Virology. 1998;251:343-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Ciarlet M, Estes MK, Conner ME. Simian rhesus rotavirus is a unique heterologous (non-lapine) rotavirus strain capable of productive replication and horizontal transmission in rabbits. J Gen Virol. 2000;81:1237-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | McNeal MM, Sestak K, Choi AH, Basu M, Cole MJ, Aye PP, Bohm RP, Ward RL. Development of a rotavirus-shedding model in rhesus macaques, using a homologous wild-type rotavirus of a new P genotype. J Virol. 2005;79:944-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Kalter SS, Heberling RL, Rodriguez AR, Lester TL. Infection of baboons (Papio cynocephalus) with rotavirus (SA11). Dev Biol Stand. 1983;53:257-261. [PubMed] [Cited in This Article: ] |
| 21. | Soike KF, Gary GW, Gibson S. Susceptibility of nonhuman primate species to infection by simian rotavirus SA-11. Am J Vet Res. 1980;41:1098-1103. [PubMed] [Cited in This Article: ] |
| 22. | Majer M, Behrens F, Weinmann E, Mauler R, Maass G, Baumeister HG, Luthardt T. Diarrhea in newborn cynomologus monkeys infected with human rotavirus. Infection. 1978;6:71-72. [PubMed] [Cited in This Article: ] |
| 23. | Wyatt RG, Sly DL, London WT, Palmer AE, Kalica AR, Van Kirk DH, Chanock RM, Kapikian AZ. Induction of diarrhea in colostrum-deprived newborn rhesus monkeys with the human reovirus-like agent of infantile gastroenteritis. Arch Virol. 1976;50:17-27. [PubMed] [Cited in This Article: ] |
| 24. | MALHERBE H, HARWIN R. The cytopathic effects of vervet monkey viruses. S Afr Med J. 1963;37:407-411. [PubMed] [Cited in This Article: ] |
| 25. | Petschow BW, Litov RE, Young LJ, McGraw TP. Response of colostrum-deprived cynomolgus monkeys to intragastric challenge exposure with simian rotavirus strain SA11. Am J Vet Res. 1992;53:674-678. [PubMed] [Cited in This Article: ] |
| 26. | Smith EM, Estes MK, Graham DY, Gerba CP. A plaque assay for the simian rotavirus SAII. J Gen Virol. 1979;43:513-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Mayanskiy NA, Mayanskiy AN, Kulichenko TV. [Rotavirus infection: epidemiology, pathology, vaccination]. Vestn Ross Akad Med Nauk. 2015;47-55. [PubMed] [Cited in This Article: ] |
| 28. | Lundgren O, Svensson L. Pathogenesis of rotavirus diarrhea. Microbes Infect. 2001;3:1145-1156. [PubMed] [Cited in This Article: ] |
| 29. | Offit PA, Clark HF, Kornstein MJ, Plotkin SA. A murine model for oral infection with a primate rotavirus (simian SA11). J Virol. 1984;51:233-236. [PubMed] [Cited in This Article: ] |
| 30. | Guerin-Danan C, Meslin JC, Lambre F, Charpilienne A, Serezat M, Bouley C, Cohen J, Andrieux C. Development of a heterologous model in germfree suckling rats for studies of rotavirus diarrhea. J Virol. 1998;72:9298-9302. [PubMed] [Cited in This Article: ] |
| 31. | Karampatsas K, Osborne L, Seah ML, Tong CYW, Prendergast AJ. Clinical characteristics and complications of rotavirus gastroenteritis in children in east London: A retrospective case-control study. PLoS One. 2018;13:e0194009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Salim AF, Phillips AD, Walker-Smith JA, Farthing MJ. Sequential changes in small intestinal structure and function during rotavirus infection in neonatal rats. Gut. 1995;36:231-238. [PubMed] [Cited in This Article: ] |
| 33. | Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213-10220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Fenaux M, Cuadras MA, Feng N, Jaimes M, Greenberg HB. Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice. J Virol. 2006;80:5219-5232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Crawford SE, Patel DG, Cheng E, Berkova Z, Hyser JM, Ciarlet M, Finegold MJ, Conner ME, Estes MK. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J Virol. 2006;80:4820-4832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Chitambar SD, Tatte VS, Dhongde R, Kalrao V. High frequency of rotavirus viremia in children with acute gastroenteritis: discordance of strains detected in stool and sera. J Med Virol. 2008;80:2169-2176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Chiappini E, Azzari C, Moriondo M, Galli L, de Martino M. Viraemia is a common finding in immunocompetent children with rotavirus infection. J Med Virol. 2005;76:265-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Huang XL, Chen J, Yu YP, Chen LQ, Li ZY, Zhao ZY. [Viraemia and extraintestinal involvement after rotavirus infection]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2006;35:69-75. [PubMed] [Cited in This Article: ] |
| 39. | Mossel EC, Ramig RF. A lymphatic mechanism of rotavirus extraintestinal spread in the neonatal mouse. J Virol. 2003;77:12352-12356. [PubMed] [Cited in This Article: ] |
| 40. | Mossel EC, Ramig RF. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J Virol. 2002;76:6502-6509. [PubMed] [Cited in This Article: ] |
| 41. | Bhowmick R, Halder UC, Chattopadhyay S, Chanda S, Nandi S, Bagchi P, Nayak MK, Chakrabarti O, Kobayashi N, Chawla-Sarkar M. Rotaviral enterotoxin nonstructural protein 4 targets mitochondria for activation of apoptosis during infection. J Biol Chem. 2012;287:35004-35020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Goodarzi Z, Soleimanjahi H, Arefian E, Saberfar E. The effect of bovine rotavirus and its nonstructural protein 4 on ER stress-mediated apoptosis in HeLa and HT-29 cells. Tumour Biol. 2016;37:3155-3161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Superti F, Ammendolia MG, Tinari A, Bucci B, Giammarioli AM, Rainaldi G, Rivabene R, Donelli G. Induction of apoptosis in HT-29 cells infected with SA-11 rotavirus. J Med Virol. 1996;50:325-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 44. | Frias AH, Jones RM, Fifadara NH, Vijay-Kumar M, Gewirtz AT. Rotavirus-induced IFN-β promotes anti-viral signaling and apoptosis that modulate viral replication in intestinal epithelial cells. Innate Immun. 2012;18:294-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Chaïbi C, Cotte-Laffitte J, Sandré C, Esclatine A, Servin AL, Quéro AM, Géniteau-Legendre M. Rotavirus induces apoptosis in fully differentiated human intestinal Caco-2 cells. Virology. 2005;332:480-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |