Published online Aug 14, 2018. doi: 10.3748/wjg.v24.i30.3462
Peer-review started: May 26, 2018
First decision: July 6, 2018
Revised: July 9, 2018
Accepted: July 16, 2018
Article in press: July 16, 2018
Published online: August 14, 2018
Heterotopic gastric mucosa (HGM) in the rectum is an extremely rare clinical entity which may be missed or misdiagnosed due to a lack of knowledge. In the present study, a 14-year-old girl visited our hospital due to a 5-year history of repeated hematochezia. Colonoscopy showed a solitary superficial depressed lesion approximately 5 cm in size and a concomitant 1.5 cm deep diverticulum in the rectum. Histological examination of the endoscopic biopsy showed typical ectopic gastric mucosa in the depressed lesion and inside the diverticulum. Narrow band imaging further confirmed the histological results. Endoscopic ultrasound indicated that the lesion originated from the mucosal layer, and partially involved the submucosal layer. Endoscopic submucosal dissection was performed in this patient due to the large size and shape of the lesion. No bleeding, perforation or other adverse events were observed. The presence of HGM in the diverticular cavity greatly increased the surgical difficulty. A literature review was also carried out in our study.
Core tip: Heterotopic gastric mucosa (HGM) in the rectum is an extremely rare clinical entity with unclear pathogenesis and no standard guidelines regarding optimal treatment. We present a patient with HGM and a concomitant diverticulum in the rectum, indicated its endoscopic ultrasound and narrow band imaging characteristics. Endoscopic submucosal dissection (ESD) was performed in this patient due to the large size and shape of the lesion. This is the first report of the resection of HGM and a concomitant diverticulum by ESD. A literature review was also carried out to investigate the clinical characteristics, diagnosis and management of HGM in the rectum.
- Citation: Chen WG, Zhu HT, Yang M, Xu GQ, Chen LH, Chen HT. Large heterotopic gastric mucosa and a concomitant diverticulum in the rectum: Clinical experience and endoscopic management. World J Gastroenterol 2018; 24(30): 3462-3468
- URL: https://www.wjgnet.com/1007-9327/full/v24/i30/3462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i30.3462
Heterotopic gastric mucosa (HGM) is the most commonly reported epithelial heterotopia and has been described as heteroplasia in organogenesis or metaplasia during the repair of damaged epithelium[1]. Although HGM may be congenital or acquired, the true pathogenesis of this condition remains unclear. HGM has been reported to be ubiquitous throughout the gastrointestinal (GI) tract from the oral cavity to the anorectum and can also involve the pancreas, biliary tract, gallbladder, scrotum, and mediastinum[2]. In the GI tract, HGM is predominantly observed in the esophagus, duodenum, Meckel’s diverticulum, and in gastrointestinal duplication[3]. HGM in the rectum is an extremely rare clinical entity, which may be missed or misdiagnosed due to a lack of knowledge. To the best of our knowledge, only 50-100 cases of HGM in the rectum have been reported in the literature since the first description by Ewell and Jackson in 1939[4]. The majority of these patients were from Europe and North America.
In this report, we present the case of a young girl with a large 5 cm HGM in the rectum. Interestingly, a diverticular cavity 1.5 cm deep was found which was also lined with HGM. We report our clinical experience of this patient followed by a review of the recent literature on this entity. The clinical characteristics, endoscopic findings, diagnosis, treatment, and the relationship with Helicobacter pylori (H. pylori) infection and neoplastic transformation were analyzed to enhance physician awareness. In addition, our therapeutic approach using endoscopic submucosal dissection (ESD) which, to our knowledge, has been rarely reported.
A 14-year-old girl visited our hospital due to a 5-year history of repeated hematochezia. The bleeding episodes occurred intermittently without abdominal pain, mucous, pus or other symptoms. Her medical and family history was unremarkable. Carbohydrate antigen (CA) 19-9 was 61.2 U/mL (normal range 0-37 U/mL), hemoglobin was 116 g/L (normal range 113-151 g/L) and stool occult blood examination was weakly positive. Other laboratory examinations were normal. Abdominal computed tomography (CT), gastroscopy, and capsule endoscopy (CE) revealed no obvious GI lesions. A technetium-99m (Tc-99m) pertechnetate scan for Meckel’s diverticulum and other sites of ectopic gastric mucosa was normal. On rectal examination, the patient had no anal fissures, hemorrhoids or fistulae.
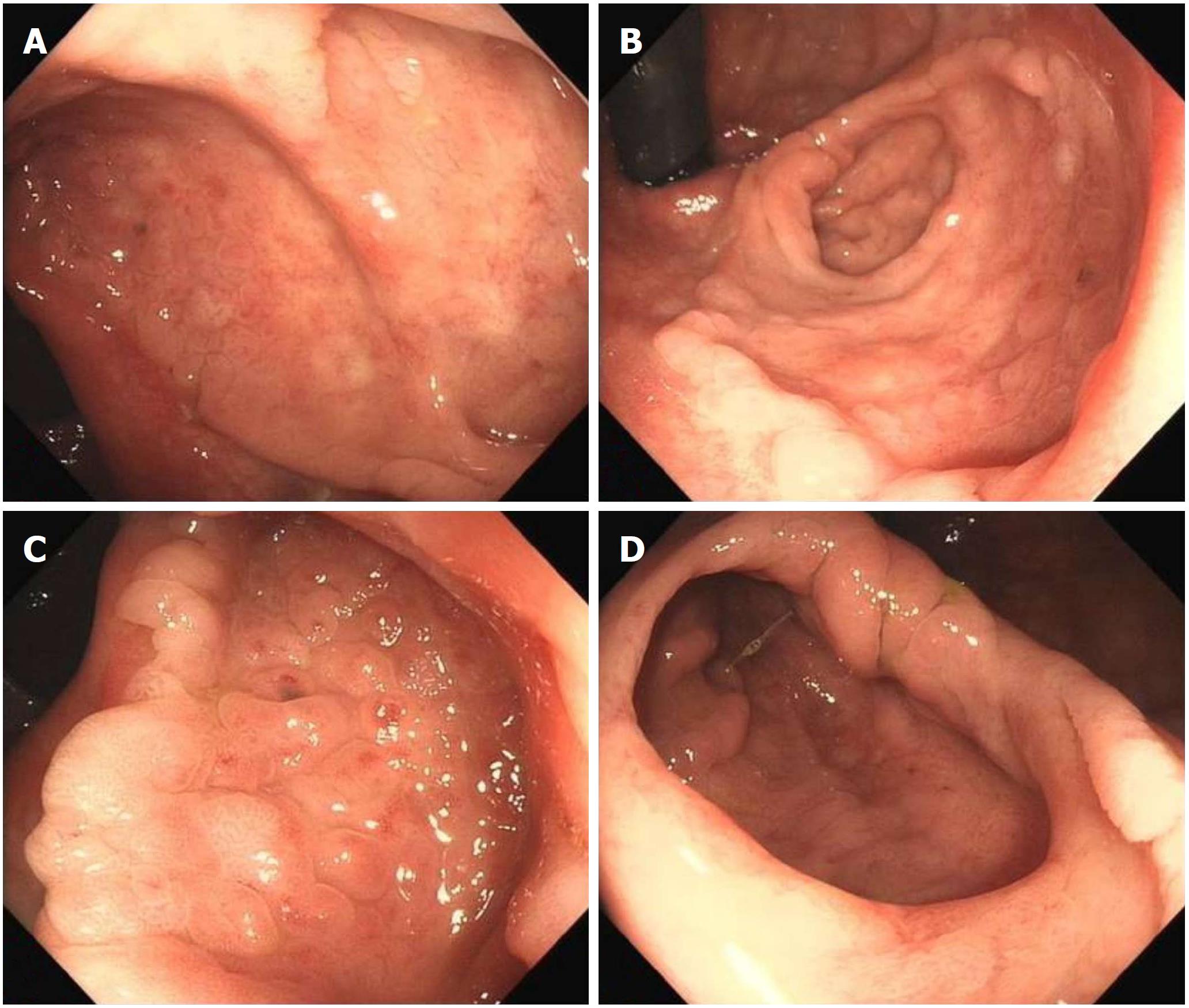
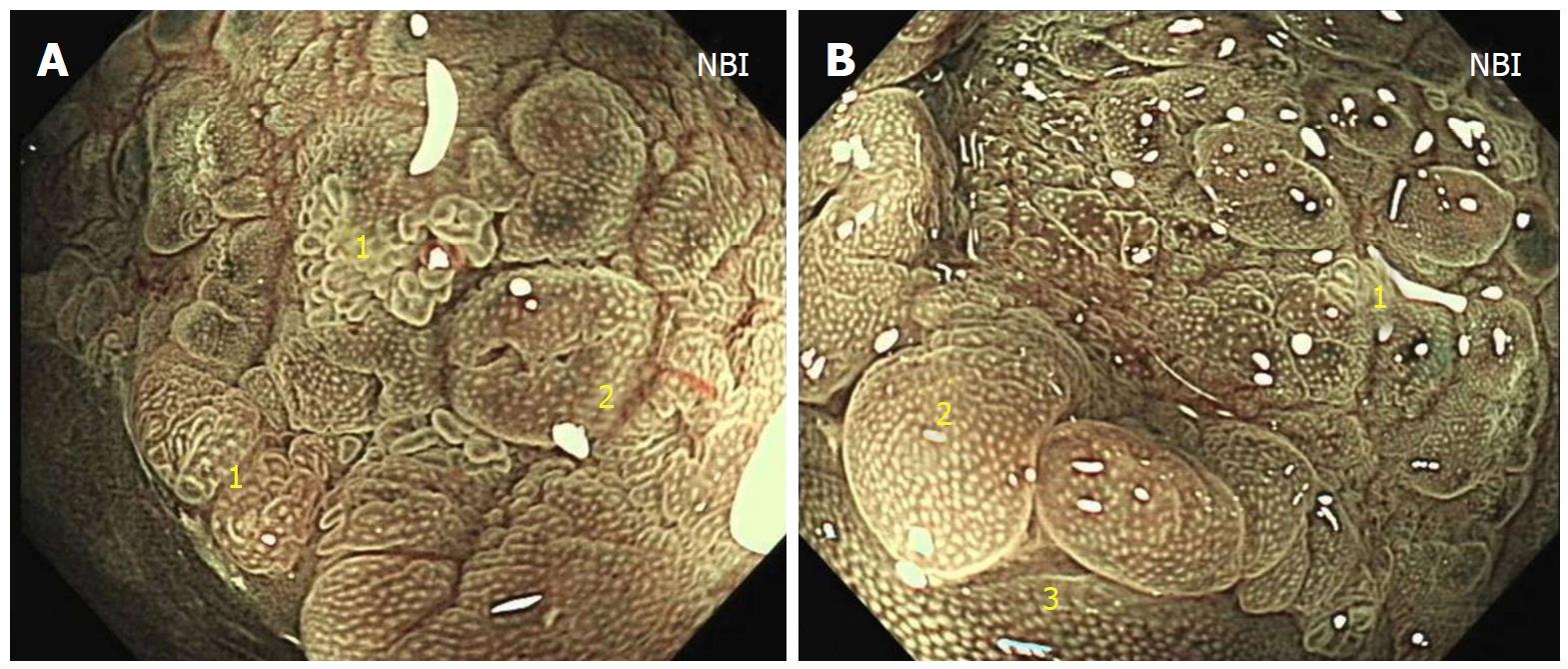
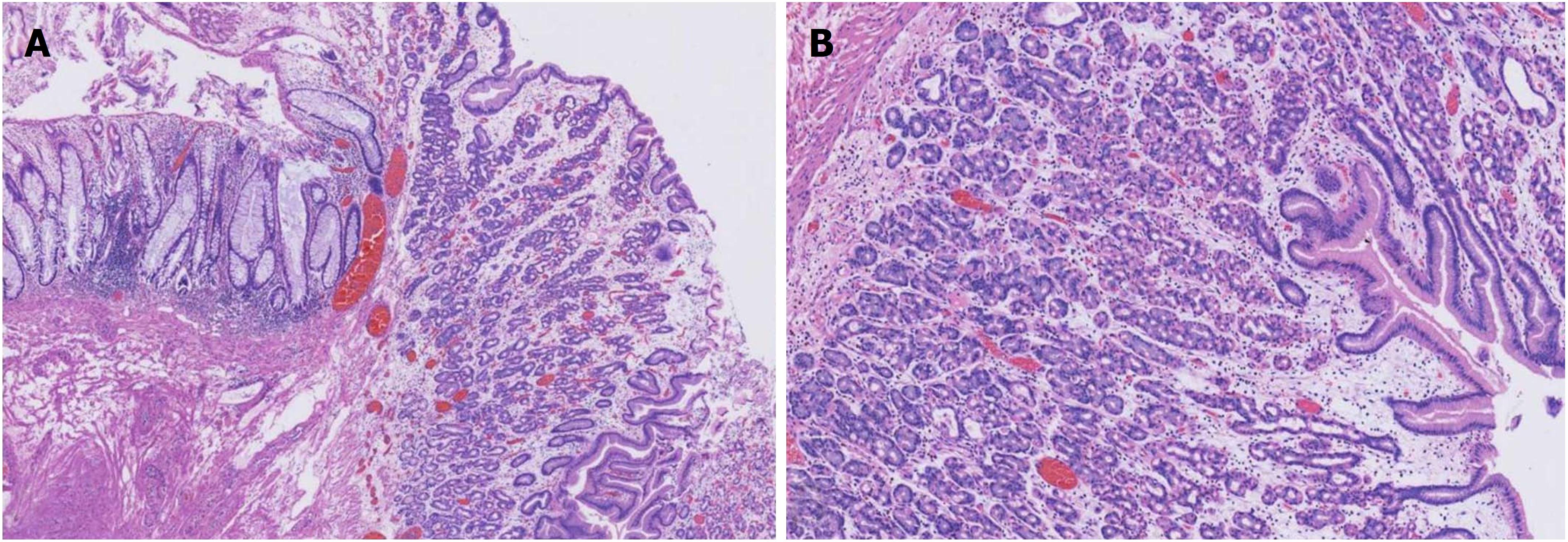
At colonoscopy, a solitary superficial depressed lesion, approximately 5 cm in size, with a diverticular cavity was identified in the posterior wall of the lower part of the rectum proximal to the dentate line of the anal canal. The lesion was well-circumscribed, covering 1/3 of the circumference of the enteric cavity. Mucosa in the lesion appeared hyperemia and erythematous, with nodule changes. The tissue in the border of the lesion was slightly raised. The diverticulum was located in the anal side of the lesion, and was 1.5 cm deep and 2 cm in diameter. The mucosa was similar to the surrounding tissue. Histological examination of the endoscopic biopsy showed typical ectopic gastric mucosa in the depressed lesion and inside the diverticulum. Intestinal metaplasia, dysplasia, malignancy and colonization by H. pylori were not observed. Thus, the diagnosis of this rare entity was made on the basis of histological findings. Narrow band imaging (NBI) examination further confirmed the histological results. By means of NBI-magnifying endoscopy examination, it could visualize the microsurface and microvascular architecture immediately.
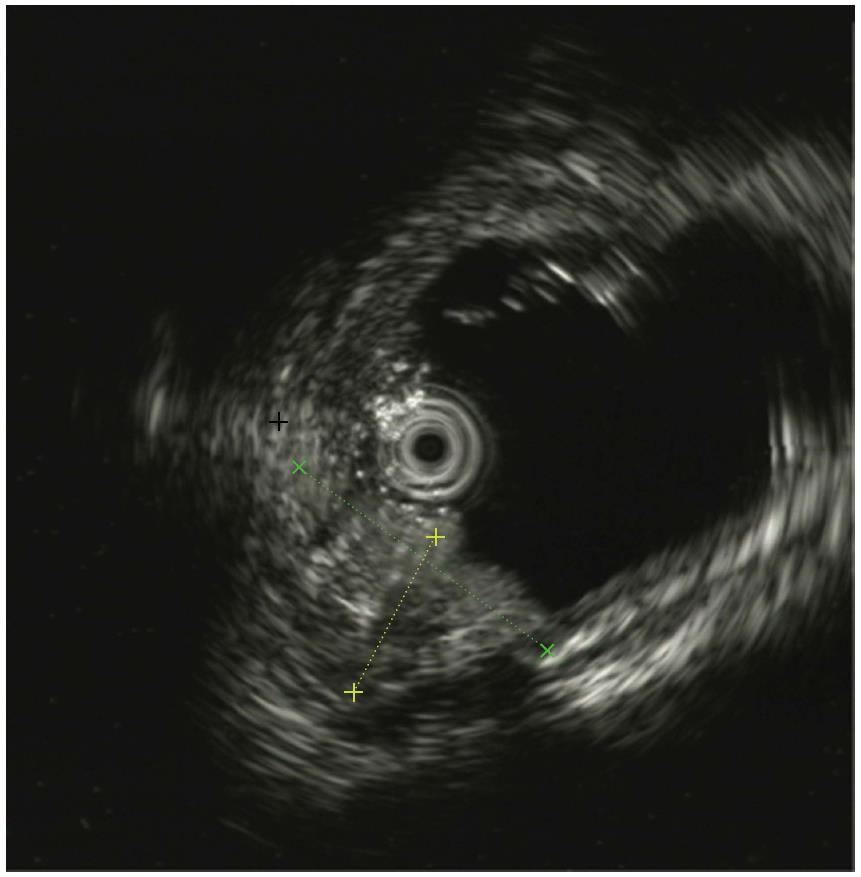
In this case, the NBI result showed the major gastric fundic-type mucosa (honey comb microvascular, small round microsurface) and focal pyloric-type mucosa (coiled microvascular, polygon microsurface) which lined the rectal mucosa. Endoscopic ultrasound (EUS) showed that the lesion originated from the mucosal layer, and partially involved the submucosal layer. Thickening of the mucosal layer with hypoechoic changes was observed in the intestinal wall of the lesion.
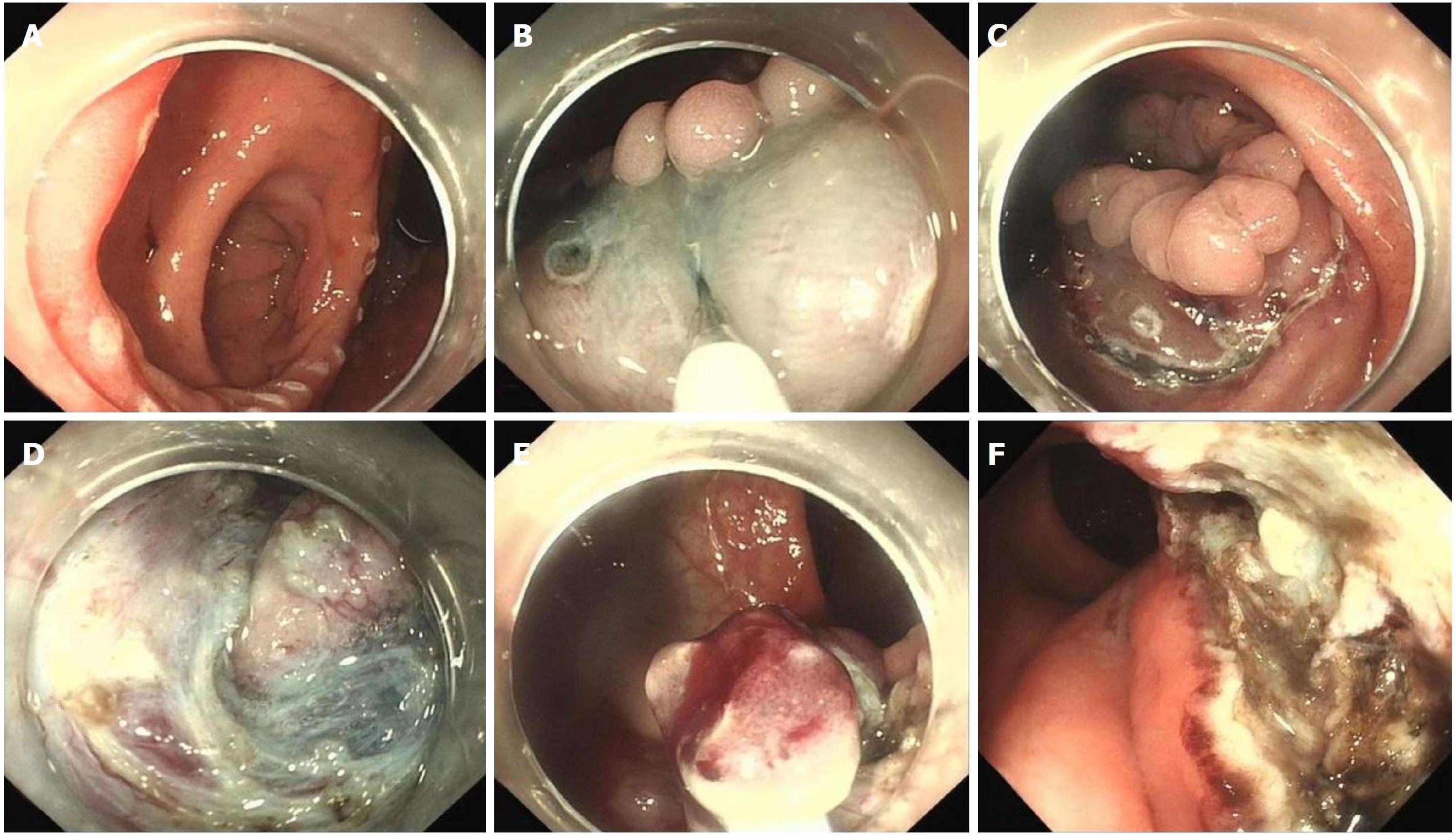
Although treatment with proton pump inhibitors or H2 receptor blockers may ameliorate the symptoms, we decided to remove the lesion by endoscopic resection due to repeated bleeding. As a result of its endoscopic features, ESD was performed in order to ensure complete resection. Unlike the routine ESD procedure used in a lateral spreading tumor (LST), the presence of the diverticulum greatly increased the technical difficulties. After the lesion was marked with a dual knife, a mixture of glycerol fructose solution, indigo carmine and epinephrine were injected into the submucosal layer. The lifting sign was positive in this lesion which indicated that the muscularis propria was not involved. A circumferential incision was then performed. The submucosal dissection was started from the peripheral margin to the base of the diverticulum. When the dissection reached the anal side of the lesion near the anal canal, lidocaine was injected into the submucosal layer to relieve anal pain. Due to the narrow space in the diverticular cavity, ESD could not be accomplished. At the base of the diverticulum, piecemeal resection with snare was performed. In order to reduce residual HGM in the diverticulum, argon plasma coagulation (APC) was carried out in the interior of the diverticulum. Due to the hypervascular characteristics of the rectum, the full extent of the wound was carefully inspected, and a hemostatic clamp was used to manage the remnants of vessels. Finally, the lesion was resected without bleeding, perforation or other adverse events. Histological evaluation of the resected specimen revealed ectopic gastric mucosa of the fundic-type.
This study was approved by the Ethics Committee and Institutional Review Board of the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China. Informed consent was obtained from the patient, and the record/information of the patient was anonymized and de-identified prior to analysis (Figures 1-5).
HGM has been reported to occur throughout the alimentary tract, but is mainly detected in the upper esophageal sphincter and is easily ignored by endoscopists if the gastroscope is withdrawn quickly without care. The incidence of HGM in the esophagus varies widely from 0.1% to 13.8% and HGM in the duodenum varies from 0.5% to 8.9% in published series[5]. HGM of the large bowel is infrequent, and is mostly reported in the rectum. Due to the limited number of patients, there is a lack of epidemiological studies and published data on HGM of the rectum. In a review of published reports, males were more commonly affected, and patients were predominantly children and adolescents[6,7]. Several hypotheses have been proposed to explain the origin of HGM, and false differentiation of pluripotent primitive endoderm stem cells is the most plausible and accepted hypothesis. Errors during embryonic development or metaplasia of rectal mucosa following mucosal injury are other mechanisms for the development of HGM[8].
The clinical presentation of HGM varies and depends on lesion size and location, and most are secondary to hydrochloric acid secretion. More than 90% of reported cases in the rectum were symptomatic[9]. The most common symptom was rectal bleeding, which also occurred in our case. Bleeding is usually painless, slight, intermittent and of varying duration. Other common symptoms include abdominal pain, diarrhea, tenesmus, perianal ulceration, anal pruritus, fistula, and bowel perforation. The report by Federico Iacopini showed that patients with specific anorectal symptoms were significantly younger than asymptomatic patients or patients with nonspecific abdominal symptoms and were frequently pediatrics (≤ 18 years)[10].
Endoscopically, HGM usually appears as an inlet patch in the proximal esophagus, a nodular mass in the duodenum, or a line in the interior of Meckel’s diverticulum[11]. In the rectum, HGM lesions may be identified as ulcers, polyps, diverticula, and flat or depression lesions with a reddish-appearance. HGM mimicking a rectal submucosal tumor has also been reported[12]. Our patient, who had a 5 cm superficial depressed lesion and a concomitant 1.5 cm deep diverticulum, is the first reported case with this condition. The lesion was well-circumscribed with a slightly raised border and hyperemia, erythematous, and nodule altered mucosa. In most reported cases, the HGM lesion in the large intestine was solitary. Murray described a girl with repeated episodes of rectal bleeding due to HGM in the rectum, sigmoid colon, ascending colon, and cecum[13]. HGM is generally localized in the right posterior wall of the rectum and more than 5 cm above the anal verge[14]. EUS can provide valuable information for the diagnosis of HGM and can indicate the appropriate management. In general, most lesions are found within the mucosal layer, and may involve the submucosal layer. EUS images have indicated a hypoechoic pattern change, and several small anechoic areas may be detected due to dilated gastric-type glands, which are characteristic of HGM[15]. Thickening of the mucosal layer can also be seen in EUS images. NBI and NBI-magnifying endoscopy are useful tools for the diagnosis of HGM and can differentiate gastric-type glands from the intestinal mucosa. To our knowledge, this case is also the first report of the use of NBI in HGM of the rectum. NBI clearly showed gastric fundic-type and pyloric-type mucosa lining the rectum and helped us to make a definitive diagnosis.
For the diagnosis of HGM in the rectum, histopathological examination is the gold standard and provides fragments of rectal mucosa coexisting with gastric mucosa. In a literature report, fundic-type mucosa was the most common histologic type of HGM, and was characterized by oxyntic features other than antral- or transitional-type[16]. Histopathology in our case also mainly showed fundic-type mucosa. Tc-99m pertechnetate abdominal scintigraphy is important in the diagnosis and localization of ectopic gastric mucosa, especially in Meckel’s diverticulum. In the study by Sciarra et al[17], the sensitivity of Tc-99m scintigraphy ranged from 50% to 91%, which may have depended on the location and size of the heterotopic tissue. Our patient had a negative result with no increased uptake of foci in the GI tract. In addition to NBI, confocal laser endomicroscopy (CLE) is a new endoscopic tool that allows magnification of 1000 times with the aid of a miniaturized confocal microscope inserted into the biopsy canal of a conventional endoscope. Through high-resolution and high-magnification assessment, it can provide images that can reliably predict histology without the need for biopsies[18]. Thus, HGM in the rectum may be correctly detected using CLE.
No current guidelines regarding the optimal treatment of HGM in the rectum are available due to reported sporadic cases. By the inhibition of gastric acid secretion, H2 receptor blockers or proton pump inhibitors may ameliorate or eliminate symptoms caused by HGM in the rectum. In most reported cases, rectal bleeding was successfully treated with proton pump inhibitor therapy and ulcer healing was achieved. However, recurrence can develop following the cessation of therapy and medication has failed to resolve heterotopic mucosa. In the surveillance report by Sauer et al[19], macroscopically the size of the lesion was unchanged after 4 mo of acid inhibition. Therefore, medical therapy relieves symptoms only in the short-term and could be used, to some extent, before definitive HGM excision. Endoscopic resection or surgical excision is generally considered the treatment of choice and has resulted in complete relief following removal of the HGM. Conventional endoscopic interventions such as endoscopic snare resection (polypectomy or endoscopic mucosal resection[20]) and ablation with APC are indicated in HGM patients mimicking polyps and small superficial lesions. ESD, enables en bloc resection of superficial lesions especially in early GI cancer and LST, and could also be used in the treatment of HGM. To date, HGM in the rectum treated with ESD has been reported in only 2 cases[10,12]. Surgery is another definitive treatment and can achieve complete resection of the lesion. However, given the concerns related to this benign entity, excessive surgery should be viewed with great caution, and would be the preferred approach in HGM with rectal duplication or serious complications such as major gastrointestinal bleeding, perforation, abscess or fistula, and for lesions following failed endoscopic resection.
In the present study, ESD was performed in our patient due to the large size and shape of the lesion. The presence of HGM in the diverticular cavity greatly increased the surgical difficulty. In addition, gastric acid secreted by HGM can induce chronic gastritis and ulcerative complications that may lead to submucosal fibrosis and further influence the submucosal dissection. Lesion marker and submucosal layer injection were performed used a dual knife as in conventional ESD. We attempted dissection from the oral side and anal side separately to the base of the diverticulum to the best of our ability. Based on our previous experience, lidocaine was injected into the submucosal layer in order to relieve anal pain during dissection of the rectum near the anal canal due to its rich nerve supply. En bloc resection could not be completed due to the narrow space in the diverticulum, and piecemeal resection with a snare was feasible. Finally, endoscopic ablation of the lesion with low wattage APC was performed to manage possible residual HGM in the interior of the diverticulum. The lesion should be resected completely if possible as residual HGM can produce gastric acid and induce repeated delayed hemorrhage of the wound. This is the first reported case of the resection of a large HGM lesion and a concomitant diverticulum in the rectum treated by ESD. Histological result revealed ectopic gastric mucosa of the fundic-type. No pyloric-type gastric mucosa was detected which was not in accordance with the results of NBI. This may have been due to less focal tissue of pyloric-type gastric mucosa in comparison with fundic-type gastric mucosa in the piecemeal resected lesion, the sampling or cutting site of the histological biopsy.
Recently, more and more new issues related to HGM in the rectum have been addressed. Many studies have reported the potential progression to malignancy in the esophagus and gallbladder. The risk and possibility of intestinal metaplasia, dysplasia and malignant progression to adenocarcinoma in the rectum have been incompletely evaluated. The study by Ko et al[21] examined a case of colonic adenocarcinoma arising from gastric heterotopia, which was the first case report in the literature. The colonization of H. pylori in rectal gastric heterotopic mucosa has been noted. In the review by Jarosław Swatek of 50 rectal gastric heterotopic mucosa patients, H. pylori was present in six patients[22]. It is of interest to note that H. pylori passed through the whole GI tract to the rectum. H. pylori infection may have resulted in chronic active gastritis, ulceration or bleeding in the rectum. Thus, in cases associated with H. pylori, eradication treatment should be prescribed as the first therapeutic option[23].
In summary, heterotopic gastric mucosa of the rectum is a very rare clinical entity which mainly presents as chronic painless rectal bleeding. The pathogenesis of this condition is unclear. The definitive diagnosis of HGM relies on histopathological examination. Endoscopy techniques such as NBI and CLE could help in the diagnosis of HGM without the need for biopsies. Endoscopic resection is thought to be the first-line treatment and surgical excision may be preferred in some patients. Further investigations on the pathogenesis, malignant transformation, H. pylori infection, and optimal treatment are necessary.
A 14-year-old girl visited our hospital due to a 5-year history of repeated hematochezia.
Heterotopic gastric mucosa (HGM) and a concomitant diverticulum in the rectum found through colonoscopy examination.
Ulcerative colitis.
CA 19-9 was 61.2 U/mL, hemoglobin was 116 g/L, and stool occult blood examination was weakly positive. Other laboratory results were close to normal.
At colonoscopy, a solitary superficial depressed lesion, approximately 5 cm in size, with a diverticular cavity was identified in the rectum. Abdominal computed tomography revealed no obvious gastrointestinal lesions.
The pathology test confirmed the diagnosis of HGM.
Endoscopic submucosal dissection.
To the best of our knowledge, this is the first report of large HGM lesion and a concomitant diverticulum in the rectum treated by endoscopic submucosal dissection (ESD).
HGM is the most commonly reported epithelial heterotopia and has been described as heteroplasia in organogenesis or metaplasia during the repair of damaged epithelium.
ESD is thought to be the safe and efficient treatment for HGM.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
P- Reviewer: Ikematsu H, Ishida T S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Dinarvand P, Vareedayah AA, Phillips NJ, Hachem C, Lai J. Gastric heterotopia in rectum: A literature review and its diagnostic pitfall. SAGE Open Med Case Rep. 2017;5:2050313X17693968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Salem GA, Fazili J, Ali T. Gastric heterotopia in the rectum. A rare cause of ectopic gastric tissue. Arab J Gastroenterol. 2017;18:42-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Cai J, Yu H. Giant polypoid gastric heterotopia in the small intestine in a boy: A case report and literature review. Medicine (Baltimore). 2017;96:e5854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Ewell GH, Jackson RH. Aberrant gastric mucosa in the rectum with ulceration and hemorrhage. Wis Med J. 1939;38:641-643. [Cited in This Article: ] |
| 5. | Yu L, Yang Y, Cui L, Peng L, Sun G. Heterotopic gastric mucosa of the gastrointestinal tract: prevalence, histological features, and clinical characteristics. Scand J Gastroenterol. 2014;49:138-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Huelsen A, Falvey J, Whitehead M, Ding S. Education and Imaging. Gastrointestinal: large heterotopic gastric mucosa in the rectum. J Gastroenterol Hepatol. 2012;27:1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Al-Hussaini A, Lone K, Al-Sofyani M, El Bagir A. Gastric heterotopia of rectum in a child: a mimicker of solitary rectal ulcer syndrome. Ann Saudi Med. 2014;34:245-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Wolff M. Heterotopic gastric epithelium in the rectum: a report of three new cases with a review of 87 cases of gastric heterotopia in the alimentary canal. Am J Clin Pathol. 1971;55:604-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 93] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Ikematsu H, Fu KI, Saito Y, Matsuda T, Shimoda T, Fujii T. Ectopic gastric mucosa in the rectum mimicking an early depressed cancer treated by endoscopic mucosal resection. Endoscopy. 2007;39 Suppl 1:E171-E172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Iacopini F, Gotoda T, Elisei W, Rigato P, Montagnese F, Saito Y, Costamagna G, Iacopini G. Heterotopic gastric mucosa in the anus and rectum: first case report of endoscopic submucosal dissection and systematic review. Gastroenterol Rep (Oxf). 2016;4:196-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Tai CM, Chang IW, Wang HP. Heterotopic gastric mucosa of the ileum. Endoscopy. 2015;47 Suppl 1:E423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Tan Y, Wang Y, Liu D. Heterotopic gastric mucosa mimicking a rectal submucosal tumor. Endoscopy. 2015;47 Suppl 1:E329-E330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Murray FE, Lombard M, Dervan P, Fitzgerald RJ, Crowe J. Bleeding from multifocal heterotopic gastric mucosa in the colon controlled by an H2 antagonist. Gut. 1988;29:848-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Limdi JK, Sapundzieski M, Chakravarthy R, George R. Gastric heterotopia in the rectum. Gastrointest Endosc. 2010;72:190-191; discussion 191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Eguchi K, Aoyagi K, Nimura S, Sakisaka S. Diagnostic value of endoscopic and endoscopic ultrasound characteristics of duodenal submucosal tumour-like heterotopic gastric mucosa. Can J Gastroenterol. 2011;25:365-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Fang Y, Chen L, Chen DF, Ren WY, Shen CF, Xu Y, Xia YJ, Li JW, Wang P, Zhang AR. Prevalence, histologic and clinical characteristics of heterotopic gastric mucosa in Chinese patients. World J Gastroenterol. 2014;20:17588-17594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Sciarra A, Hessler R, Godat S, Fraga M, Dromain C, Duran R, Halkic N, Sempoux C. Heterotopic Gastric Mucosa in a Duplication Cyst of the Common Hepatic Duct Mimicking Cholangiocarcinoma. Int J Surg Pathol. 2018;26:84-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | López-Cerón Pinilla M, Jimeno Ramiro M, Rodríguez de Miguel C, Pellisé Urquiza M. Heterotopic gastric mucosa diagnosed by confocal endomicroscopy. Rev Esp Enferm Dig. 2011;103:40-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Sauer CG, Bickston SJ, Borowitz SM. Gastric heterotopia of the rectum. J Pediatr Gastroenterol Nutr. 2010;50:329-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Soares J, Ferreira C, Marques M, Corujeira S, Tavares M, Lopes J, Carneiro F, Amil Dias J, Trindade E. Endoscopic Mucosectomy in a Child Presenting with Gastric Heterotopia of the Rectum. GE Port J Gastroenterol. 2017;24:288-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Ko H, Park SY, Cha EJ, Sohn JS. Colonic adenocarcinoma arising from gastric heterotopia: a case study. Korean J Pathol. 2013;47:289-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Swatek J, Wronecki L, Ciechanek R, Szumiło J. Asymptomatic gastric heterotopia in the rectum with Helicobacter pylori infection. Pol J Pathol. 2015;66:426-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Colsa-Gutiérrez P, Kharazmi-Taghavi M, Sosa-Medina RD, Berrío-Obregón JI, Ingelmo-Setién A. [Heterotopic gastric mucosa in the rectum: Report of a case]. Cir Cir. 2016;84:160-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |