Published online Mar 21, 2018. doi: 10.3748/wjg.v24.i11.1259
Peer-review started: January 11, 2018
First decision: February 26, 2018
Revised: March 1, 2018
Accepted: March 3, 2018
Article in press: March 3, 2018
Published online: March 21, 2018
To compare the effects of regular vs low-FODMAP rye bread on irritable bowel syndrome (IBS) symptoms and to study gastrointestinal conditions with SmartPill®.
Our aim was to evaluate if rye bread low in FODMAPs would cause reduced hydrogen excretion, lower intraluminal pressure, higher colonic pH, different transit times, and fewer IBS symptoms than regular rye bread. The study was a randomized, double-blind, controlled cross-over meal study. Female IBS patients (n = 7) ate study breads at three consecutive meals during one day. The diet was similar for both study periods except for the FODMAP content of the bread consumed during the study day. Intraluminal pH, transit time, and pressure were measured by SmartPill, an indigestible motility capsule.
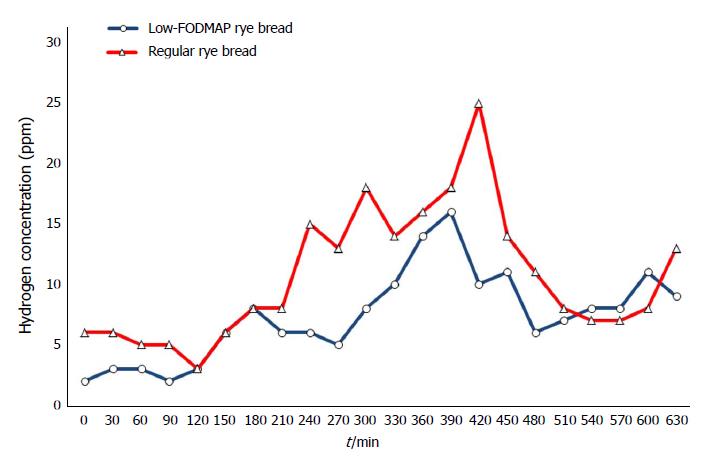
Hydrogen excretion (a marker of colonic fermentation) expressed as area under the curve (AUC)(0-630 min) was [median (range)] 6300 (1785-10800) ppm∙min for low-FODMAP rye bread and 10 635 (4215-13080) ppm∙min for regular bread (P = 0.028). Mean scores of gastrointestinal symptoms showed no statistically significant differences but suggested less flatulence after low-FODMAP bread consumption (P = 0.063). Intraluminal pressure correlated significantly with total symptom score after regular rye bread (ρ = 0.786, P = 0.036) and nearly significantly after low-FODMAP bread consumption (ρ = 0.75, P = 0.052). We found no differences in pH, pressure, or transit times between the breads. Gastric residence of SmartPill was slower than expected. SmartPill left the stomach in less than 5 h only during one measurement (out of 14 measurements in total) and therefore did not follow on par with the rye bread bolus.
Low-FODMAP rye bread reduced colonic fermentation vs regular rye bread. No difference was found in median values of intraluminal conditions of the gastrointestinal tract.
Core tip: Our study confirmed that low-FODMAP rye bread reduces colonic fermentation in irritable bowel syndrome (IBS) patients compared with regular rye bread. The observed correlation between increased intracolonic pressure and symptom severity underlines the central role of visceral sensitivity in IBS and suggests that some IBS symptoms might be exacerbated by any pathophysiological reason that leads to increased colonic pressure. The study also suggests that SmartPill might not be an optimal device to evaluate gastrointestinal circumstances during meal studies lasting less than 24 h, due to device’s inability to measure effects of a singular food bolus in a timely manner.
- Citation: Pirkola L, Laatikainen R, Loponen J, Hongisto SM, Hillilä M, Nuora A, Yang B, Linderborg KM, Freese R. Low-FODMAP vs regular rye bread in irritable bowel syndrome: Randomized SmartPill® study. World J Gastroenterol 2018; 24(11): 1259-1268
- URL: https://www.wjgnet.com/1007-9327/full/v24/i11/1259.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i11.1259
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder[1]. Symptoms include bloating, abdominal pain, flatulence, constipation, and diarrhea. Visceral sensitivity[2], low-grade inflammation[3], and impaired gas handling[4] contribute to the etiology of IBS.
Many patients consider food as a trigger of their symptoms[5,6] and half of IBS patients report postprandial exacerbation of symptoms[7]. FODMAPs (Fermentable Oligo-, Di-, Monosaccharides and Polyols) are poorly absorbable carbohydrates that are rapidly fermented in the proximal colon[8]. A low-FODMAP diet decreases colonic fermentation, which in turn parallels a reduction of IBS symptoms[9]. One of the major food groups excluded during a low-FODMAP diet is fiber-rich gluten-containing grain products[8]. Elimination of whole-grain products from the diet may, however, lead to decreased fiber intake and increase the risk of chronic diseases in the long term[10].
Rye, a widely-consumed grain in the Nordic countries, is very high in fiber that is mainly composed of arabinoxylan, lignin, cellulose, β-glucan, and fructans[11]. Fructans are classified as FODMAPs[8]. Typical rye bread contains more than 10% fiber and is the most important source of fiber (28%-35% of total intake) of Finnish adults[12]. Rye bread induces gastrointestinal symptoms in some individuals, possibly due to the high fructan content[13]. Therefore, IBS patients may avoid rye products.
Efforts are being made to develop grain products that are low in FODMAPs but high in other fibers[14]. In a recent study, an innovative low-FODMAP high-fiber (fiber > 10% of weight) rye bread caused less colonic fermentation and led to fewer IBS symptoms than regular rye bread[15]. The low-FODMAP rye bread was lower both in fructans and mannitol. Mannitol is a FODMAP compound that is formed during the rye sourdough breadmaking process.
A major challenge in the research of functional gastrointestinal disorders is the lack of objective markers of disease activity. Excretion of hydrogen and methane and colonic fermentation markers are among the rare easily available and objective markers of gastrointestinal circumstances during the consumption of FODMAPs or other poorly absorbable carbohydrates[16]. However, a recent study utilized a wireless motility capsule (SmartPill®) and demonstrated that pH in the colon of IBS patients is lower than in healthy subjects[17]. This may indicate more intensive fermentation in the colon of IBS patients. SmartPill measures intraluminal pH, temperature, motility, and pressure and might thus offer the means to gather objective data on gastrointestinal conditions in IBS patients. A combination of SmartPill data and measurements of perceived symptoms may improve our understanding on the etiology of IBS symptoms.
The aim of this pilot study was to compare a low-FODMAP rye bread and a regular rye bread with regards to postprandial abdominal symptoms, breath hydrogen concentration, and gastrointestinal transit times, pH, and pressure as measured by SmartPill. Our hypothesis was that a low-FODMAP rye bread would induce less hydrogen excretion, lower pressure, and increase pH in colon compared with regular rye bread, which would subsequently parallel with fewer IBS symptoms. The secondary objective was to evaluate the feasibility of the SmartPill capsule in associating IBS symptoms with physiologic responses in the gastrointestinal tract in a meal study.
Patients with IBS were recruited from the Helsinki metropolitan area via the Internet. The eligibility inclusion criteria were the following: (1) female; (2) aged 18 to 65 years; (3) BMI 18.5-30 kg/m2; and (4) IBS defined by the Rome III criteria[18]. The exclusion criteria were celiac disease, Crohn’s disease, diverticulitis, severe dyspepsia, stomach bezoar, bowel obstruction, severe constipation, medication used in the management of intestinal motility, major abdominal surgery, dysphagia, pregnancy or breastfeeding, regular smoking, implanted medical device, and hormonal, renal, hepatic, or hematologic disease or participation in another clinical trial during the past two months.
The study candidates were pre-screened with questionnaires on health and diet and IBS diagnostic criteria. Candidates meeting the preliminary inclusion criteria received laboratory tests (blood count, sedimentation rate, thyroid function tests, transglutaminase antibodies and immunoglobulins for celiac disease, calprotectin, and gene test for lactose intolerance) and a clinical evaluation by a gastroenterologist. Weight and height were also measured. All participants signed an informed consent form. The study protocol was approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District, Finland. The study was registered at ISRCTN registry (ISRCTN11005234).
This study was a randomized, double-blind, postprandial cross-over meal study. All participants attended on two occasions with a washout period of ≥ 2 wk between the study periods. Each study period consisted of a run-in period of 12 h (standardized dinner and overnight fast), a test day with study meals (breakfast, lunch, and dinner with low-FODMAP or regular rye bread) and a follow-up of 1 to 3 d depending on the transit time of the SmartPill capsule. The order of the interventions (low-FODMAP or regular rye bread) was randomized for each patient with a random number table. Both investigators and participants were blinded to the identity of the bread. The study events during the study period are detailed in Supplemental Table 1.
The diet was standardized from the evening (-12 h) before the test breakfast (0 h) until the following morning (+24 h). The standardized diet consisted of regular grocery products and had a low FODMAP content (Supplemental Table 1). The diet was similar for both study periods except for the FODMAP content of the bread eaten during the study day. Participants kept a food diary from the day before the test day until the end of the study period.
On the morning of the test day, the volunteers ingested the SmartPill capsule with water and ate four slices (approximately 120 g) of bread with spread, cheese, vegetables, and coffee or tea. Lunch with two slices of the test bread was consumed six hours later and dinner with an additional two slices of bread 10-12 h after the breakfast. Thus, participants consumed a total of eight slices (approximately 240 g) of bread during each study period. Breads were developed and supplied by Fazer Bakeries (Vantaa, Finland). The low-FODMAP rye bread was prepared using a specific sourdough that contains unique lactobacilli that efficiently consume fructans and also results in low mannitol content. The control rye bread was prepared using a traditional rye sourdough. The breads were similar in appearance and taste.
The nutrient composition of the breads (Table 1) was analyzed by Eurofins scientific Finland, Raisio (Food and Agro), Finland. The dietary fiber content of the breads was determined by using the AOAC method 2011.25 that discriminates soluble and insoluble, low, and high molecular weight dietary fibers. The mannitol content was analyzed by the HPLC method used by Eurofins Food and Agro, Lidköping, Sweden. Fructan content was measured by using the AOAC 999.03 method (Megazyme assay kit K-FRUC, Megazyme international Ireland Ltd, Bray, Ireland).
| Low-FODMAP rye bread | Regular rye bread | |||
| Per 100 g | Per slice (30 g) | Per 100 g | Per slice (30 g) | |
| Energy (kJ/kcal) | 1031/245 | 309/74 | 1037/246 | 311/74 |
| Fat (g) | 2.6 | 0.8 | 1.1 | 0.3 |
| Protein (g) | 7.5 | 2.3 | 7.5 | 2.3 |
| Carbohydrates (g) | 42.4 | 12.7 | 45.1 | 13.5 |
| Dietary fiber (g) | 10.8 | 3.2 | 12.8 | 3.8 |
| Soluble fiber (g) | 2.6 | 0.8 | 2.9 | 0.9 |
| Insoluble fiber (g) | 6.7 | 2.0 | 7.7 | 2.3 |
| Fructans (g) | 0.4 | 0.12 | 1.2 | 0.36 |
| Mannitol (g) | 0.09 | 0.03 | 0.26 | 0.08 |
SmartPill® (Given Imaging ltd, Yoqneam, Israel) is an indigestible wireless capsule that contains sensors for temperature, pH, and pressure. The capsule is 26.8 mm long and its diameter is 11.7 mm. The capsule sends the measured data to a receiver worn by the subject. Measurement data is uploaded to a computer and analyzed by MotiliGI® program. The program calculates mean pressure, median pH, contractions/min, and transit times based on changes in pH and temperature for the different parts of the gastrointestinal tract. In the present study, the capsule was swallowed with the test breakfast and the subject was prohibited from eating for six hours after the meal so the capsule would proceed to small intestine. Each study period ended when the capsule was defecated.
Breath hydrogen was analyzed with Gastrolyzer® (Bedfont Scientific Ltd, Kent, England) before the test breakfast (0 min) and every 30 min for 11 h (660 min) on the test day and every three hours on the following days. Breath hydrogen was analyzed as a marker for colonic fermentation[19].
IBS symptoms during the study periods were collected with a visual analogue scale (VAS) questionnaire. We did not formally validate the questionnaire, but it follows the concept as described by Francis et al[20]. A similar scoring system has been used previously in other diet studies in IBS[21,22]. Symptoms were recorded once before the test breakfast (0 min) and every 30 min for 11 h on the test day and every 3 h on the following days. The questionnaire consisted of nine individual 100 mm VAS lines of the following different symptom classes: abdominal pain, abdominal cramps, bloating, flatulence, belly rumbling, nausea, heartburn, unpleasant sensation in the upper abdomen, and continuous urge to defecate. Additionally, participants kept a diary of defecation times and form of stool.
Missing values in gastrointestinal symptoms and hydrogen measurements were imputed with the mean value of the previous and following measurements. Two subjects failed to report the 660 min measurements for all symptoms and hydrogen concentration, and thus values were included only until 630 min for all subjects when calculating the outcome variables. The baseline (0 min) of symptoms was not included in outcome variables as this was measured before eating the test breakfast.
The outcome variables calculated from the symptom questionnaire scores were the following: mean of the scores for each symptom during the follow-up period (30-630 min), sum of total symptom scores at each time point, and the area under the curve (AUC) of the total symptom score during the follow up (30-630 min). AUC of breath hydrogen was calculated using the absolute breath hydrogen values (0-630 min). AUC values were calculated following trapezoidal rule[23] without respect to increase because symptom severity and hydrogen concentration may have a baseline value of zero. Symptom and H2 measurements that were conducted after the test day were used to calculate the mean of total symptom score and mean of breath hydrogen content for different parts of the gastrointestinal tract.
Breath hydrogen was the primary outcome variable used in the study power calculations. Suitable previously published data was not available, and thus the study power was calculated based on a preliminary test performed in Fazer Bakeries in which healthy participants ate regular rye bread or low-FODMAP rye bread followed by analysis of breath hydrogen content during the six-hour postprandial period. The difference in breath hydrogen content (ppm) between fasting and at six hours was used to evaluate the number of subjects in the current study. Based on the power calculation, a sample size of eight would have 80% power to detect a 25-ppm difference in breath hydrogen using a paired t-test with a two-sided significance level of 0.05.
The patient characteristics and outcome variables are expressed as a median (range) and as number of cases for categorical variables. The difference in outcome variables between study periods was analyzed using the Wilcoxon signed-rank test for related samples. Correlations between mean symptom severity and mean breath hydrogen and between mean symptoms and SmartPill indices during the colonic phase were analyzed using the Spearman’s rho. Statistical analysis was performed with IBM SPSS Statistics (Version 23, IBM Co., New York, United States) and Microsoft Office Excel 2013 (Microsoft Co., Washington, United States).
A total of nine female subjects (median age 39 years, range 29-51 years) with IBS were recruited into the study, of whom two withdrew during the study for personal reasons. The BMI of the subjects was [median (range)], 26.4 (19.5-30.4) kg/m2. Three participants suffered from diarrhea-predominant IBS, two from constipation-predominant IBS and two from mixed type IBS. A flowchart of the recruitment process and the study is shown in Supplemental Figure 1.
Postprandial excretion of hydrogen expressed as AUC(0-630 min) was [median (range)] 6300 (1785-10800) ppm∙min for low-FODMAP rye bread and 10635 (4215-13080) ppm∙min for regular bread. The two bread tests differed significantly (P = 0.028), indicating more intensive colonic fermentation after consumption of the regular rye bread. Median expired hydrogen concentrations are shown in Figure 1.
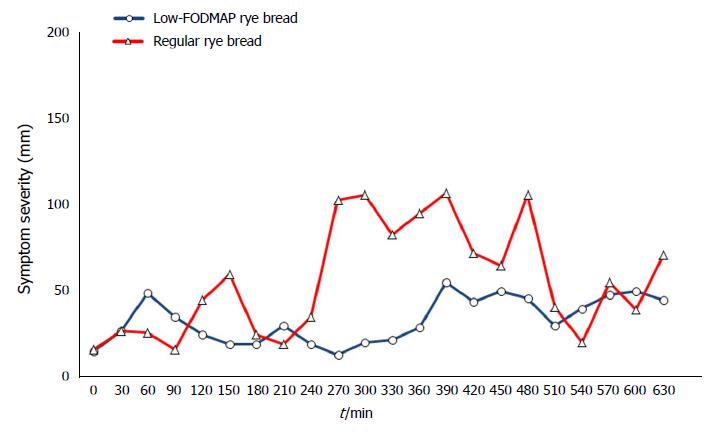
The means of the VAS measurements of individual gastrointestinal symptoms during the follow-up (30-630 min) did not reveal any statistically significant differences between the breads (Table 2). However, the flatulence severity was nearly significantly lower after low-FODMAP rye bread consumption (P = 0.06). Furthermore, there was a significant (P = 0.034) difference between the low-FODMAP bread (15 mm; range 5-34 mm) and the regular rye bread (34 mm; range 8-56 mm) in the maximum severity of flatulence (data for other maximum values not shown). Figure 2 shows the development of the total symptoms during the course of the test day. The difference in AUCs of total symptom score between low-FODMAP (23520 mm∙min; range 6885-113610 mm∙min) and regular rye breads (41130 mm∙min; range 10785-83220 mm∙min) was not statistically significant (P = 0.866).
| Low-FODMAP rye | Regular rye bread | P value1 | |
| Abdominal pain | 2.4 (0.0-28.2) | 4.8 (0.1-21.9) | 0.735 |
| Cramps | 1.2 (0.1-29.2) | 3.0 (0.1-10.0) | 0.917 |
| Bloating | 12.3 (1.0-48.4) | 23.1 (1.1-37.8) | 0.866 |
| Flatulence | 3.3 (0.5-7.6) | 4.2 (0.5-28.2) | 0.063 |
| Belly rumbling | 3.0 (0.1-6.6) | 3.8 (1.4-11.4) | 0.398 |
| Nausea | 1.0 (0.0-22.4) | 2.1 (0.0-15.5) | 1.000 |
| Heartburn | 1.4 (1.0-20.8) | 1.8 (0.1-20.3) | 1.000 |
| Unpleasant sensation in the uppemen | 7.4 (1.0-26.1) | 11.8 (1.2-23.2) | 0.310 |
| Urge to defecate | 1.5 (0.0-29.5) | 2.1 (0.3-22.4) | 0.735 |
All patients could swallow the SmartPill device without major challenges. In eight out of a total of 14 measurements the device stayed in the stomach for an unexpectedly long period (i.e., > 10 h; in 6 measurements out of a total of 14 the device resided in the stomach > 15 h, data not shown). SmartPill left the stomach in less than five hours only during one measurement. Therefore, the devices did not follow on par with the rye bread bolus. All devices were defecated within three days of swallowing.
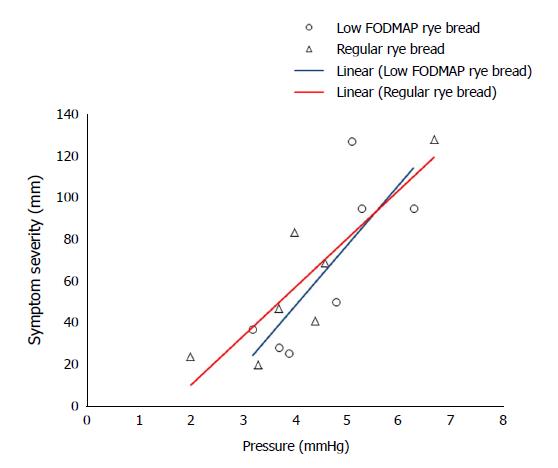
Transit times, median pH values, mean pressures, and contractions are shown in Table 3. SmartPill-derived transit times, pH values, mean pressure, or contractions/min in any part of the gastrointestinal tract did not differ between the bread tests. The association between colonic pressure and overall symptom severity during the time when the device was transiting the colon is shown in Figure 3. The correlation was significant after the participants had consumed regular rye bread (ρ = 0.786, P = 0.036) and was nearly significant after low-FODMAP bread consumption (ρ = 0.750, P = 0.052). The correlation coefficients between symptom severity and colonic contraction frequency were 0.775 (P = 0.041) and 0.786 (P = 0.036) after regular and low-FODMAP bread consumption, respectively. Colon pH and H2 excretion were associated with symptom severity after regular bread (ρ = 0.821, P = 0.023 and ρ = 0.857, P = 0.014, respectively) but not after low-FODMAP bread (ρ = 0.342, P = 0.452 and ρ = 0.536, P = 0.215, respectively) consumption.
| Low-FODMAP rye bread | Regular rye bread | P value1 | |
| Transit time (h) | |||
| Stomach | 18.1 (5.3-22.3) | 5.6 (4.5-18.0) | 0.091 |
| Small intestine | 4.0 (2.1-5.6) | 4.6 (3.2-6.6) | 0.866 |
| Colon | 25.2 (12.2-50.0) | 32.1 (14.7-47.6) | 0.176 |
| Whole GI tract | 46.5 (22.6-73.5) | 45.8 (24.3-70.4) | 0.612 |
| Median pH | |||
| Stomach | 1.5 (0.8-4.1) | 1.5 (1.0-2.4) | 0.671 |
| Small intestine | 7.5 (5.0-8.0) | 7.6 (7.0-7.8) | 0.915 |
| Colon | 7.2 (5.8-7.5) | 6.5 (5.9-8.5) | 0.612 |
| Mean pressure (mmHg) | |||
| Stomach | 2.2 (1.9-2.7) | 2.5 (2.0-3.0) | 0.610 |
| Small intestine | 3.1 (1.6-8.6) | 4.5 (2.4-7.0) | 0.398 |
| Colon | 4.8 (3.2-6.3) | 4.0 (2.0-6.7) | 0.310 |
| Contractions/min | |||
| Stomach | 1.2 (0.6-1.7) | 1.0 (0.8-1.8) | 0.553 |
| Small intestine | 3.2 (0.5-6.1) | 4.9 (1.9-6.5) | 0.176 |
| Colon | 1.7 (1.3-2.9) | 1.7 (0.6-3.3) | 0.495 |
In this meal study, we demonstrated that consumption of low-FODMAP rye bread leads to reduced hydrogen expiration (a marker of colonic fermentation) when compared with regular rye bread consumption. Furthermore, the maximum flatulence severity was lower for the low-FODMAP rye bread compared with regular rye bread. No difference was found in other symptoms, pH, contractions, or total gastrointestinal tract pressure. Interestingly, intracolonic pressure and contraction frequency, rather than total gastrointestinal pressure, were associated with symptom severity during the colonic transition period of SmartPill. This finding suggests that the colon is most affected and is the origin of IBS symptoms. The exceedingly long gastric transit time measured by SmartPill was an unexpected finding.
Our finding of lower hydrogen excretion during the low-FODMAP rye bread test is consistent with previous studies; grain products that are high in fructans increase hydrogen excretion (i.e., colonic fermentation) when compared with grain products low in fructans[15,24]. Additionally, the difference in the maximum flatulence value suggests a higher level of gas formation after regular rye bread consumption. There were no differences between the treatments in the perceived severity of other gastrointestinal symptoms, although the median values tended to favor the low-FODMAP rye bread. The perceived severity of symptoms was overall low. A larger sample size most likely would have been needed to reach statistical significance in symptom severity. The power calculation for the study was based on hydrogen excretion rather than change in symptoms.
A previous scintigraphy study has shown that after consumption of a medium-sized solid meal, 98%-99% of ingested food should leave the stomach within four hours and 84% within two hours[25]. The transition time of the SmartPill from stomach to small intestine varied from 4.5-22 h in the present study. These results are in contrast with previous studies[26,27], which have shown that gastric emptying of SmartPill takes place within five hours in most study subjects. On the other hand, sporadic prolonged gastric residence times of SmartPill in healthy volunteers have been previously reported[28,29]. SmartPill is a non-digestible capsule that presumably leaves the stomach during phase III of the migrating motor complex (MMC)[26]. The long gastric emptying times in the present study indicate that the six-hour lag time between the study breakfast and lunch was not long enough for the SmartPill to leave the stomach. The subsequent meal probably terminated the MMC cycle before the capsule was transported to the duodenum.
It is possible that gastric emptying times, at least those measured with SmartPill, are increased in IBS. Indeed, another study by Dupont et al[30] also reported gastric emptying times longer than 5 h in IBS patients. Furthermore, in study from Ringel-Kulka et al[17], patients with constipation-predominant IBS had prolonged gastric emptying times (mean 8 h). Although there were three bread meals during the study day, in many cases SmartPill followed the rye bolus with a delay of several hours. This is important information for future studies. SmartPill might fail to measure gastrointestinal circumstances in a timely manner, at least in patients with IBS. Based on our study, it is possible that SmartPill is more applicable for feeding studies lasting at least two days rather than one-day meal studies.
Due to the unexpectedly long residence of SmartPill in the stomach, the device was usually in the small intestine during the night. For this reason, we could not compare perceived symptoms and measured conditions in the small intestine. However, the residence time was longer in the large intestine, and thus we could link symptom ratings and conditions in the colon. Intracolonic pressure correlated with IBS symptom severity in our study. One of the key underlining reasons why IBS patients experience pain and discomfort is their lower threshold to sense pain and distension, a phenomenon called visceral hypersensitivity[2,31]. Therefore, any dietary or physio-anatomic factors that increase pressure in gut might worsen symptoms. Theoretically, intracolonic pressure might increase due to many factors, such as consumption of gas-forming FODMAPs, impaired handling of colonic gas, intestinal microbiota disturbances, fecal retention, difficulties in expelling gas or anatomical abnormalities that obstruct gas. However, with our methods we cannot explain the underlining cause of the increased intracolonic pressure. Nonetheless, hydrogen excretion did not correlate with intracolonic pressure in the present study, suggesting that other reasons such as intestinal abnormalities or microbiota disturbances might play a larger role. Intestinal abnormalities previously associated with IBS symptoms include small intestinal constriction, dilated transverse colon, and redundant colon[32,33]. Further research is warranted in the area of intracolonic pressure and its role in IBS symptoms. Interestingly, Rogers et al[34] have shown that intraluminal pressure was higher among subjects with IBS when compared with healthy subjects. These results, together with our findings, suggest that colonic gas amplitude may have a causal role in IBS symptom generation.
We also found a correlation between colonic contraction frequency and symptom severity during both bread periods. Previously, Hasler et al[35] demonstrated in their SmartPill study that colonic motor activity (i.e., colonic contraction frequency and duration of contractions) is increased in constipation-predominant IBS patients when compared with non-IBS subjects. Increased motoric activity might be one mechanism for how intracolonic pressure is increased in IBS. We also observed that pH and hydrogen excretion during the colonic phase were associated with symptom severity after consumption of regular rye bread; these findings might simply reflect the degree of colonic fermentation and act as surrogates of colonic pressure. Taken together, our findings with previous SmartPill-gathered data[17,35] provide further evidence for the role of intraluminal physiological conditions in triggering IBS symptoms. This observation is of relevance to clinicians as IBS is sometimes considered primarily as a psychosocial condition[36]. Interestingly, spasmolytics, such as peppermint oil capsules, relax smooth muscles of the intestinal wall and thus reduce colonic motility, which may explain the reductions in pain, discomfort, and feeling of bloating reported in several clinical trials[37]. Attempts to reduce intraluminal pressure and contractions might continue to be important therapeutic targets in IBS.
Our study has limitations. As stated previously, the number of subjects might have been too small to detect all true differences between the breads. The long residence of SmartPill in the stomach is a potential bias in our study. The observation period was rather short (630 min). On the basis of our experience in this study, we recommend longer studies when using SmartPill in diet-related research. The strengths of our study include the double-blind randomized setting and standardization of the evening snack before the trial and all food consumed during the first 24 h of the test period.
In conclusion, our meal study demonstrated that consumption of low-FODMAP rye bread led to reduced colonic fermentation and maximum flatulence values in IBS patients when compared with regular rye bread. No differences could be found in other symptoms, pH, colonic pressure, or gut contractions. Due to its inability to measure effects of a singular food bolus in a timely manner, this study also showed that SmartPill might not be an optimal device in evaluating gastrointestinal circumstances during meal studies lasting less than 24 h. The observed correlation between increased intracolonic pressure and symptom severity underlines the central role of visceral sensitivity in IBS. Further studies are needed to understand the role of intracolonic pressure formation in IBS.
FODMAPs are rapidly fermentable carbohydrates shown to aggravate gastrointestinal symptoms in irritable bowel syndrome (IBS). A major challenge in the research of IBS is the lack of objective markers of disease activity. Excretion of hydrogen and methane and colonic fermentation markers are among the rare easily available and objective markers of gastrointestinal circumstances during the consumption of FODMAPs or other poorly absorbable carbohydrates. Grains are often considered as triggers of irritable bowel syndrome symptoms but less is known about the effects of grain products with differing content of FODMAPs on gastrointestinal transit times, pH and intraluminal pressure in patients with IBS.
SmartPill, a motility monitoring capsule, which measures intraluminal pH, transit time and pressure, and might thus offer the means to gather objective data on gastrointestinal conditions in IBS patients. A combination of SmartPill data and measurements of perceived symptoms may improve our understanding on the etiology of IBS symptoms when consuming grains high in FODMAPs.
Our aim was to evaluate if rye bread low in FODMAPs would cause less hydrogen excretion, lower intraluminal pressure, higher colonic pH and less IBS symptoms than regular rye bread.
The study was conducted as a randomized double blind controlled cross-over meal study. Female IBS patients (n = 7) ate study breads on 3 consecutive meals. Intraluminal conditions were measured by SmartPill®, an indigestible motility capsule.
Postprandial hydrogen excretion, a marker of colonic fermentation, expressed as AUC(0-630 min) was [median (range)] 6300 (1785-10800) for low-FODMAP rye bread and 10635 (4215-13080) ppm∙min for regular bread (P = 0.028). The means of the visual analogue scale measurements of individual gastrointestinal symptoms did not show any statistically significant differences between the breads. Intraluminal pressure correlated significantly with total symptom score after regular rye bread (ρ = 0.786, P = 0.036) and nearly significantly after low-FODMAP bread consumption (ρ = 0.75, P = 0.052). We found no differences in pH, contractions or transit times between the breads. Gastric emptying of SmartPill was slower than expected on the basis of majority of research literature.
Our meal study demonstrated that low-FODMAP rye bread reduces colonic fermentation but no difference was found in median values of symptoms, pH, colonic pressure of gastrointestinal tract when compared to regular rye bread. Our observation on the correlation between increased intra-colonic pressure and symptom severity warrants further studies in IBS.
Our finding on the correlation of intracolonic pressure and symptom severity suggests that IBS symptoms might be worsened by any reason that leads to increased colonic pressure in IBS. Consequently, any therapeutic attempts to reduce intraluminal pressure and contractions might continue to be important therapeutic targets in IBS. The study also implied that SmartPill might not be an optimal device to evaluate the gastrointestinal circumstances during meal studies among IBS patients lasting less than 24 h, due to device’s inability to measure effects of a singular food bolus in a timely manner. Observation and feeding periods longer than 38 h are recommended for future research utilizing SmartPill, especially among people with IBS.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Finland
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiarioni G, Dumitrascu DL, Rostami K S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 360] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 2. | Simrén M, Törnblom H, Palsson OS, van Tilburg MAL, Van Oudenhove L, Tack J, Whitehead WE. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2017;pii:gutjnl-2016-312361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Agrawal A, Whorwell PJ. Review article: abdominal bloating and distension in functional gastrointestinal disorders--epidemiology and exploration of possible mechanisms. Aliment Pharmacol Ther. 2008;27:2-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Jones VA, McLaughlan P, Shorthouse M, Workman E, Hunter JO. Food intolerance: a major factor in the pathogenesis of irritable bowel syndrome. Lancet. 1982;2:1115-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 198] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Simrén M, Månsson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Björnsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 351] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Hayes PA, Fraher MH, Quigley EM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol. 2014;10:164-174. [PubMed] [Cited in This Article: ] |
| 8. | Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 9. | Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr. 2016;55:897-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Liu L, Wang S, Liu J. Fiber consumption and all-cause, cardiovascular, and cancer mortalities: a systematic review and meta-analysis of cohort studies. Mol Nutr Food Res. 2015;59:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Rakha A, Åman P and Andersson R. Characterisation of dietary fibre components in rye products. Food Chem. 2010;119:859-867. [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Helldán A, Raulio S, Kosola M, Tapanainen H, Ovaskainen ML, Virtanen S. Finravinto 2012 tutkimus-The National FINDIET 2012 Survey. ISBN 978-952-245-950-3 (printed); 978-952-245-951-0 (pdf) THL. Raportti 16/2013, 187p. Helsinki, Finland. 2013;. [Cited in This Article: ] |
| 13. | Paajanen L, Tuure T, Korpela R. Impaired tolerance of indigestible carbohydrates in adults with subjective milk intolerance. Scand J Nutr. 2004;48:131-135. [Cited in This Article: ] |
| 14. | Quigley EM. Editorial: balancing fibre and FODMAPs in IBS - a ‘rye’ look at an old problem. Aliment Pharmacol Ther. 2016;44:1134-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Laatikainen R, Koskenpato J, Hongisto SM, Loponen J, Poussa T, Hillilä M, Korpela R. Randomised clinical trial: low-FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:460-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Marteau P, Flourié B. Tolerance to low-digestible carbohydrates: symptomatology and methods. Br J Nutr. 2001;85 Suppl 1:S17-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, Galanko JA, Ringel Y. Altered Colonic Bacterial Fermentation as a Potential Pathophysiological Factor in Irritable Bowel Syndrome. Am J Gastroenterol. 2015;110:1339-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. Available from: http://www.romecriteria.org/assets/pdf/19_RomeIII_apA_885-898.pdf. [Cited in This Article: ] |
| 19. | Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281:122-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 331] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 973] [Cited by in F6Publishing: 1060] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 21. | Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 747] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 22. | Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320-328.e1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 23. | Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Nilsson AC, Ostman EM, Granfeldt Y, Björck IM. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr. 2008;87:645-654. [PubMed] [Cited in This Article: ] |
| 25. | Vasavid P, Chaiwatanarat T, Pusuwan P, Sritara C, Roysri K, Namwongprom S, Kuanrakcharoen P, Premprabha T, Chunlertrith K, Thongsawat S. Normal Solid Gastric Emptying Values Measured by Scintigraphy Using Asian-style Meal:A Multicenter Study in Healthy Volunteers. J Neurogastroenterol Motil. 2014;20:371-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Kuo B, McCallum RW, Koch KL, Sitrin MD, Wo JM, Chey WD, Hasler WL, Lackner JM, Katz LA, Semler JR. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Cassilly D, Kantor S, Knight LC, Maurer AH, Fisher RS, Semler J, Parkman HP. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | Timm D, Willis H, Thomas W, Sanders L, Boileau T, Slavin J. The use of a wireless motility device (SmartPill®) for the measurement of gastrointestinal transit time after a dietary fibre intervention. Br J Nutr. 2011;105:1337-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Diaz Tartera HO, Webb DL, Al-Saffar AK, Halim MA, Lindberg G, Sangfelt P, Hellström PM. Validation of SmartPill® wireless motility capsule for gastrointestinal transit time: Intra-subject variability, software accuracy and comparison with video capsule endoscopy. Neurogastroenterol Motil. 2017;29:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | DuPont AW, Jiang ZD, Harold SA, Snyder N, Galler GW, Garcia-Torres F, DuPont HL. Motility abnormalities in irritable bowel syndrome. Digestion. 2014;89:119-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, Gowland P, Spiller R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology. 2017;152:124-133.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 32. | Lam C, Chaddock G, Marciani Laurea L, Costigan C, Cox E, Hoad C, Pritchard S, Gowland P, Spiller R. Distinct Abnormalities of Small Bowel and Regional Colonic Volumes in Subtypes of Irritable Bowel Syndrome Revealed by MRI. Am J Gastroenterol. 2017;112:346-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Raahave D. Faecal retention: a common cause in functional bowel disorders, appendicitis and haemorrhoids--with medical and surgical therapy. Dan Med J. 2015;62:pii: B5031. [PubMed] [Cited in This Article: ] |
| 34. | Rogers J, Henry MM, Misiewicz JJ. Increased segmental activity and intraluminal pressures in the sigmoid colon of patients with the irritable bowel syndrome. Gut. 1989;30:634-641. [PubMed] [Cited in This Article: ] |
| 35. | Hasler WL, Saad RJ, Rao SS, Wilding GE, Parkman HP, Koch KL, McCallum RW, Kuo B, Sarosiek I, Sitrin MD. Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1107-G1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Lea R, Whorwell PJ. New insights into the psychosocial aspects of irritable bowel syndrome. Curr Gastroenterol Rep. 2003;5:343-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |