Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7813
Peer-review started: October 7, 2017
First decision: October 25, 2017
Revised: November 4, 2017
Accepted: November 15, 2017
Article in press: November 15, 2017
Published online: November 28, 2017
The field of medical and surgical weight loss is undergoing an explosion of new techniques and devices. A lot of these are geared towards endoscopic approaches rather than the conventional and more invasive laparoscopic or open approach. One such recent advance is the introduction of intrgastric balloons. In this article, we discuss the recently Food and Drug Administration approved following balloons for weight loss: the Orbera™ Intragastric Balloon System (Apollo Endosurgery Inc, Austin, TX, United States), the ReShape® Integrated Dual Balloon System (ReShape Medical, Inc., San Clemente, CA, United States), and the Obalon (Obalon® Therapeutics, Inc.). The individual features of each of these balloons, the method of introduction and removal, and the expected weight loss and possible complications are discussed. This review of the various balloons highlights the innovation in the field of weight loss.
Core tip: This review has been elucidated through a comparison of the strengths and weaknesses of recent balloon approaches, highlighting the indications and possible complications.
- Citation: Vyas D, Deshpande K, Pandya Y. Advances in endoscopic balloon therapy for weight loss and its limitations. World J Gastroenterol 2017; 23(44): 7813-7817
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7813.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7813
Throughout the last decade, the treatment of obesity has slowly undergone a paradigm shift. The advent of endoscopic balloon therapy has had a profound impact on long-term weight management. Three intra gastric balloons have been recently approved by the Food and Drug Administration (FDA) for the treatment of Class 1 and 2 Obesity. (Body Mass Index, BMI 30-40 kg/m2).
Intragastric balloons have been used for the treatment of obesity since 1985. It was during this time that the FDA approved the Garren-Edwards Gastric Bubble, an orally inserted cylindrical device. The device was placed inside the stomach and filled with 220 cc of air. It was designed to be left in the stomach for 3 to 4 mo and then removed[1]. After the product’s approval, randomized clinical trials showed that its use did not result in significant weight loss when compared to diet and behavioral modification only. It was furthermore associated with a large number of clinical complications including migrations, erosions, and bowel obstructions[2,3]. The device was thus taken off the market in 1992.
Intragastric balloons have been used outside the US for overweight individuals (BMI 25-29.9 kg/m2), obese individuals (BMI 30-39.9 kg/m2) and morbidly obese individuals (BMI 40 kg/m2 and above) as a bridge therapy prior to definitive surgical procedures.
Currently, three intragastric balloon devices are FDA approved in the United States: Orbera™ Intragastric Balloon System (Apollo Endosurgery Inc, Austin, TX, United States), the ReShape® Integrated Dual Balloon System (ReShape Medical, Inc., San Clemente, CA, United States), and the Obalon ( ObalonⓇ Therapeutics, Inc.). These devices are indicated for patients with Class 1 and 2 obesity (BMI 30-40 kg/m2).
Intragastric balloon systems operate on the principle of inducing an anatomical sensation of fullness secondary to the space they occupy in the stomach cavity. Consequently, post-procedure patients remain full for longer periods of time between meals. The Orbera Intragastric Balloon and Reshape Integrated Dual Balloon is placed into the gastric cavity through the mouth via a gastroscope. The Obalon balloon is swallowed by the patient through guided fluoroscopy and endoscopy is required to remove the balloon[4-6].
The balloons in the ReShape Integrated Dual Balloon System have a fill volume of 750-900 cc and are designed to conform to the natural shape of the stomach. This dual balloon design reduces the potential for migration of the device from the stomach to the intestines if a balloon deflation occurs, thus reducing the risk of intestinal obstruction[7]. Methylene blue due is injected into the saline solution present inside the balloon, serving as an indicator of balloon deflation by turning the patient’s urine blue-green.
The Orbera system entails one balloon containing 400-700 cc of saline. Studies and trials have shown low rates of deflations in this system, leading to minimal migration and obstruction[8,9].
The Obalon balloon is a gas filled balloon system that functions using similar principles. It consists of up to 3 intragastric balloons placed over the first 3 mo. The patient swallows the catheter-balloon capsule, which also contains a radiopaque marker assisting in confirming its position under the gastroesophageal junction with fluoroscopy or X-ray. Once this is achieved, the catheter is used to inject gas (nitrogen-sulfur hexafluoride mixture) into the balloon. Each balloon has a volume of approximately 250 cc, totaling 750cc with 3 balloons[10].
The saline/air-filled End-Ball® and the Spatz Adjustable Balloon System (ABS) are two additional modalities that can be used and function in similar means to the approaches above. The SPATZ-ABS anchoring device is unique in preventing the migration of the balloon. This is especially advantageous when encountering acute angles where traditional metal anchoring modalities may not pass as easily.
Typically, balloons are kept in place for no more than 6 mo and then removed endoscopically. Monthly follow up is suggested for the 6 mo in which the balloon system is in place and for a 6 month period after the balloon is replaced. Thus, 12 mo of medical supervision from an experienced bariatric multidisciplinary team is required. During the appointments, an integrated approach is used to support the patient in adhering to the weight loss program. Specifically, goal setting, weight management, and progress follow up is tracked during these appointments.
As shown in the Table 1, the intragastric balloons provided up to 25%-29% excess body weight loss at 12 mo using the various balloons.
| OrberaTM | Reshape® | Obalon® | |
| Delivery/insertion | Needs endoscopy | Needs endoscopy | Patient swallows, X-ray |
| Removal | Needs endoscopy | Needs endoscopy | Needs endoscopy |
| Capacity | 400-700 cc ( 1 balloon) | 750-900 cc (2 balloons) | 750 cc ( 3 balloons) |
| Weight loss | 29% EWL at 12 mo | 25% EWL at 12 mo | 25.2% EWL at 12 mo |
Statistically significant and clinically pertinent comorbid improvements were observed in patients with diabetes, hypertension, and hyperlipidemia, and these improvements were sustained through 48 weeks of follow up in the REDUCE pivotal trial for the Reshape balloons[11].
Adverse events included post-implantation accommodative symptoms of nausea, (as high as 86.9%), vomiting, and abdominal pain. The most common adverse event was early removal of the device due to intolerance. The United States pivotal study showed a 4.25% rate of early removal of implanted devices[12].
Absolute contraindications for placement include previous gastric surgery, hiatal hernia > 5 cm, coagulation disorder, potential bleeding lesion of the foregut, pregnancy, alcoholism/drug addiction, and severe liver disease. Relative contraindications include esophagitis, Crohn’s disease, NSAID use, and uncontrolled psychiatric illnesses.
Balloon deflation has become a rare event since manufactures have improved the design of the devices. However, it is still imperative that patients and providers remain aware of this possibility and the need for immediate removal to avoid balloon migration.
The REDUCE trial showed deflation in up to 6% of patients and an absence of migrations. In order to detect the presence of deflation, the ReShape system monitors change in the color of urine from normal to blue-green[13].
Studies using the Orbera Intragastric Balloon System, showed an absence of any spontaneous deflations. Deflation in this system can be detected through patient-stated loss of satiety or weight changes, however, common practice dictates a relatively easy means of detection through monitoring the change in urine output.
The Obalon System did not report any deflations in the 336 patients that were studied as a part of the SMART clinical trial.
Overall, the newly FDA approved intragastric balloons provide a viable option for weight loss in patients with BMIs between 30-40 kg/m2. Studies have documented cases in which treatment with intragastric balloons have shown to incur better weight loss than diet and lifestyle modification alone. However, there is still much controversy on this topic, and the evidence is inconclusive for definitive guidelines, thus, further long-term monitoring and randomized control trials are needed to quantify benefits. The added benefit of patients being able to avoid surgical procedures such as gastric bypass or sleeve gastrectomy allows for this modality of treatment to appeal to certain patient groups. These may include patients that are not adequately fit or prepared to undergo a surgical procedure. It is imperative, however, to understand that the balloons work best when placed and cared for by an experienced multidisciplinary bariatric team, well equipped with not only handling complications of balloons but providing dietary and emotional support to these patients. Ultimately, these new devices have the potential to serve as a novel instrument in the tool box of the bariatric surgeon.
One of the biggest concerns noted is that the balloons are unable to provide long term, substantial weight loss when compared with traditional bariatric procedures. The bypass and the sleeve provide up to 60%-75% EWL at 1 year, when compared to the 25%-30% EWL with the balloon. Patients with substantially higher BMIs looking for a durable procedure for sustained weight loss may not benefit from the balloons. In addition, the co-morbidity resolution profiles of the gastric bypass and sleeve gastrectomy are superior to that of the balloons. It may be premature to compare rigorously tested established surgical procedures to the newly approved less invasive gastric balloons. Overall, the balloons have the potential to serve as a powerful tool in select niche patient populations.
Recently, a few cases have been documented by the FDA entailing five deaths with liquid-filled intragastric balloon systems used to treat obesity since 2016[14]. Of these deaths, four involved the Orbera Intragastric Balloon System and one involved the ReShape Integrated Dual Balloon System. The FDA, however, has also stated that the “root cause” of these case fatalities is not known, as the evidence only depicts a one month or less temporal relationship between balloon placement and death. It was thus uncertain if the cause of death was gastric or esophageal perforation, intestinal obstruction, or through an alternate means. As further study into the controversy unfolds, it is important to note the possibility of significant confounding variables such as pre-existing morbidities, operator placement errors, and spontaneous overinflation, in determining the root cause of the recent case fatalities.
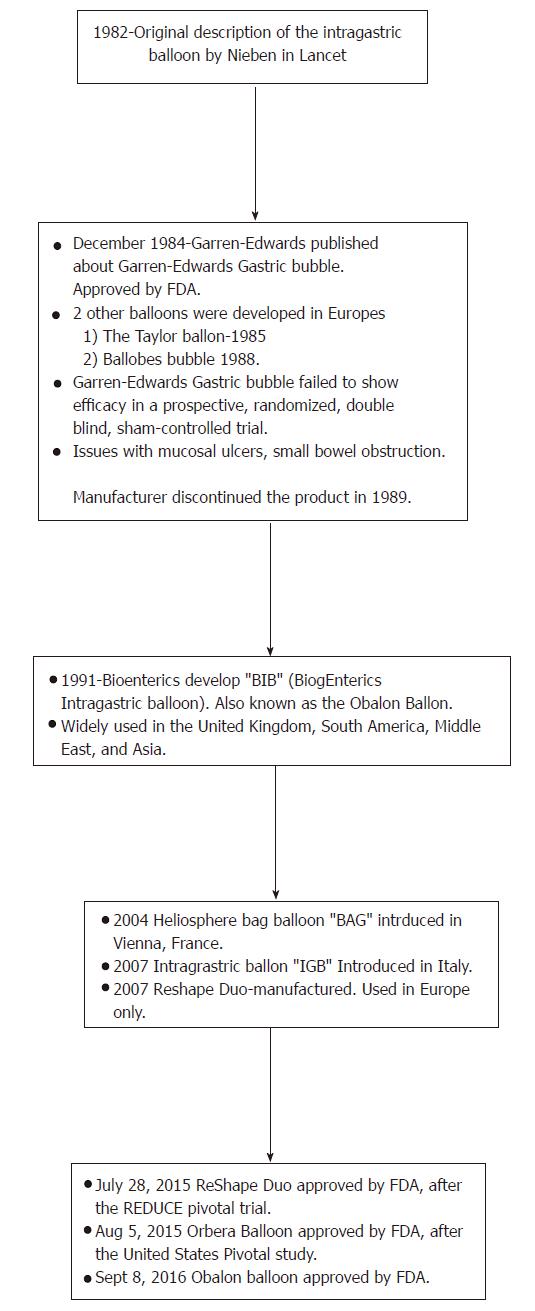
In order to better advance patient care and diagnostic as well as therapeutic approaches in gastroenterology, a meticulous analysis of endoscopic modalities is warranted. There is still much controversy regarding the post-intervention effects, however, modern advances have come a long way since the origin of the intragastric balloon, as highlighted in Figure 1. With new technologies and innovative devices such as the intragastric balloons, one has to be mindful about the legal aspects of introduction of the device and hospitals and clinics may need to institute a peer review process for credentialing and quality assurance purposes[15,16].
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Muguruma N, Rabago L, Wang HP S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ
| 1. | Ponce J. Impact of Different Surgical Techniques on Outcomes in Laparoscopic Sleeve Gastrectomies: First Report from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). Ann Surg. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Benjamin SB, Maher KA, Cattau EL Jr, Collen MJ, Fleischer DE, Lewis JH, Ciarleglio CA, Earll JM, Schaffer S, Mirkin K. Double-blind controlled trial of the Garren-Edwards gastric bubble: an adjunctive treatment for exogenous obesity. Gastroenterology. 1988;95:581-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis. 2013;9:290-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Dalton S. The dietitians' philosophy and practice in multidisciplinary weight management. J Am Diet Assoc. 1998;98:S49-S54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis. 2016;12:430-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18:841-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20:6357-6363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 8. | ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee, Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, Sullivan S, Thompson CC, Banerjee S. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425-438.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 281] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 9. | Ponce J. Response to the Letter to the Editor: “Comment on the REDUCE trial article: is it really as good as it sounds?”. Surg Obes Relat Dis. 2016;12:218-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Bennett MC, Badillo R, Sullivan S. Endoscopic Management. Gastroenterol Clin North Am. 2016;45:673-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ponce J, Woodman G, Swain J, Wilson E, English W, Ikramuddin S, Bour E, Edmundowicz S, Snyder B, Soto F. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11:874-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Kim J, Azagury D, Eisenberg D, DeMaria E, Campos GM; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11:739-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Ulicny KS Jr, Goldberg SJ, Harper WJ, Korelitz JL, Podore PC, Fegelman RH. Surgical complications of the Garren-Edwards Gastric Bubble. Surg Gynecol Obstet. 1988;166:535-540. [PubMed] [Cited in This Article: ] |
| 14. | Liquid-filled Intragastric Balloon Systems: Letter to Healthcare Providers - Potential Risks. US Food and Drug Administration 2017. . [Cited in This Article: ] |
| 15. | Vyas D, Cronin S. Peer Review and Surgical Innovation: Robotic Surgery and Its Hurdles. Am J Robot Surg. 2015;2:39-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Tate CM, Geliebter A. Intragastric Balloon Treatment for Obesity: Review of Recent Studies. Adv Ther. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |