Published online May 28, 2017. doi: 10.3748/wjg.v23.i20.3675
Peer-review started: December 6, 2016
First decision: February 9, 2017
Revised: February 23, 2017
Accepted: March 20, 2017
Article in press: March 20, 2017
Published online: May 28, 2017
To investigate the role of nuclear division cycle (NDC)80 in human hepatocellular carcinogenesis.
NDC80 gene expression was analyzed by real-time reverse transcription polymerase chain reaction in 47 paired hepatocellular carcinoma (HCC) and adjacent tissues. The HCC cell line SMMC-7721 was transfected with lentivirus to silence endogenous NDC80 gene expression, which was confirmed by real-time polymerase chain reaction and western blotting. The effects of NDC80 silencing on SMMC-7721 cell proliferation were evaluated by Cellomics ArrayScan VTI imaging. Cell cycle analysis and apoptosis were detected with flow cytometry. Colony formation was assessed by fluorescence microscopy.
NDC80 expression levels in HCC tissues were significantly higher than those in the adjacent tissues. Functional studies demonstrated that NDC80 silencing significantly reduced SMMC-7721 cell proliferation and colony formation. Knockdown of NDC80 resulted in increased apoptosis and cell cycle arrest at S-phase. NDC80 contributed to HCC progression by reducing apoptosis and overcoming cell cycle arrest.
Elevated expression of NDC80 may play a role in promoting the development of HCC.
Core tip: Nuclear division cycle (NDC)80 is a member of the NDC80 kinetochore complex and is highly expressed in cancer. NDC80 is a newly identified gene that is overexpressed in hepatocellular carcinoma (HCC). We analyzed the biological function of NDC80 in the proliferation and apoptosis of HCC cells, and provided new reference data and experimental support for HCC-targeting gene therapy.
- Citation: Ju LL, Chen L, Li JH, Wang YF, Lu RJ, Bian ZL, Shao JG. Effect of NDC80 in human hepatocellular carcinoma. World J Gastroenterol 2017; 23(20): 3675-3683
- URL: https://www.wjgnet.com/1007-9327/full/v23/i20/3675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i20.3675
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and the third most frequent cause of cancer mortality, following lung and stomach cancers[1,2]. Especially in China, which has a high incidence of hepatitis B, the magnitude of the problem should never be underestimated[3]. HCC has received considerable attention in recent years because of its rapidly increasing incidence[4,5]. Patients diagnosed with HCC have a poor prognosis because of the aggressive features of the disease. Only 10%-15% of HCC patients are suitable candidates for curative treatments, including surgical resection and liver transplantation. Surgical resection, ablation therapy, and liver transplantation are effective but only at an early stage of HCC development[5-8]. However, HCC is diagnosed at advanced stages in most patients when only limited therapeutic options are available. High metastasis and recurrence rates have become the major obstacles to improving long-term survival of HCC patients[8]. The molecular mechanisms of HCC development are not fully understood; thus, it is of importance to elucidate further the mechanisms of HCC and explore effective treatment. Gene therapy has emerged as a promising intervention against HCC. In this study, we investigated the role of a gene that has been previously associated with human HCC cells - nuclear division cycle (NDC)80 - in order to ascertain better its role in human hepatocellular carcinogenesis.
NDC80 (also called Hec1), a core component of the outer kinetochore and a mitotic regulator, is of particular interest because it clearly has an association with cancer progression. NDC80 forms a dumbbell-like heterotetramer with Nuf2, Spc24, and Spc25 to form the NDC80 complex[9-12]. NDC80 complexes are important to the spindle assembly checkpoint and participate in the regulation of mitosis[13-15]. NDC80 plays essential roles in chromosome segregation by mediating the spindle assembly checkpoint signaling and chromosome alignment. The spindle assembly checkpoint is a mechanism that ensures faithful chromosome segregation by monitoring the kinetochore-microtubule attachment[13,16]. Chromosome segregation dysfunction is one of the causes of chromosome instability. Chromosome instability is a common feature of tumor cells, and may be an important mechanism in tumor formation. In this study, we analyzed the biological function of NDC80 in the proliferation and apoptosis of HCC cells, and provided new reference data and experimental support for HCC-targeting gene therapy.
We used HepG2, Huh-7, SMMC-7721 and Hep3B cell lines, which are commonly used in HCC research, purchased from the Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences. All cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, United States) supplemented with 10% fetal bovine serum at 37 °C with 50 mL/L CO2. Forty-seven pairs of HCC and matched adjacent tissues were provided by Nantong Third People’s Hospital Affiliated to Nantong University. All patients had primary HCC and had not received any preoperative radiotherapy or chemotherapy. The diagnosis of all HCC patients was confirmed histopathologically. The pathological stage of HCC was determined according to the International Union Against Cancer Tumor-Node-Metastasis (TNM) Classification. The characteristics of the participants are summarized in Table 1. All tissues samples were frozen immediately after resection and stored in liquid nitrogen until use.
| Clinical variable | No. of patients |
| No. of patients | 47 |
| Age, yr, mean ± SD | 56 ± 11 |
| Sex | |
| Female | 18 (38.3) |
| Male | 29 (61.70) |
| Serum AFP, ng/mL | |
| ≤ 400 | 36 (76.60) |
| > 400 | 11 (23.40) |
| HBV infection | |
| Positive | 38 (80.9) |
| Negative | 3 (19.1) |
| Largest tumor diameter, cm | 5.7 ± 3.4 |
| TNM stage | |
| I | 2 (4.25) |
| II | 30 (63.83) |
| III | 15 (31.92) |
| Lymph node metastatic | |
| Positive | 20 (42.55) |
| Negative | 27 (47.45) |
Total RNA was extracted from tissue samples and HCC cell lines using TRIzol (Takara Bio, Dalian, China). RNA purity and concentration were determined by NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). RNA was reversely transcribed using a Prime ScriptTM RT Reagent Kit (Takara Bio). The reactions were performed in a Bio-Rad Real-Time polymerase chain reaction (PCR) Detection System using a SYBR® Green Master Mix (Vazyme Biotech, Nanjing, China). The primers used were as follows: NDC80: forward, 5’-CCTCTCCATGCAGGAGTTAAGA-3’, reverse, 5’-GGTCTCGGGTCCTTGAT TTTCT-3’; GAPDH: forward, 5’- TGACTTCAACAGCGACACCCA-3’, reverse, 5’- CACCCTGTTGCTGTAGCCAAA-3’. After a pre-denaturation step at 95 °C for 5 min, 40 cycles of PCR were performed as follows: 10 s denaturation at 95 °C and 30 s annealing at 60 °C. The fold amplification for each gene was calculated using the 2-ΔΔCt method.
The SMMC-7721 cell suspension was seeded onto six-well plates at 5 × 104 cells/well and incubated at 37 °C in 50 mL/L CO2 until 30% confluence was reached. Two experimental groups were constructed: NDC80-siRNA, which was transfected with NDC80-siRNA green fluorescent protein (GFP) lentivirus; and control group, which was transfected with empty GFP lentivirus. An appropriate amount of lentivirus was added according to the multiplicity of infection (MOI). The cells were repeatedly cultured in normal culture medium after 12 h. GFP-tagged gene expression was observed under a fluorescence microscope at 3 d after transfection, and cells with a transfection efficiency > 80% were selected for subsequent analyses.
The cultured cells were lysed in RIPA lysis buffer containing phosphatase inhibitor cocktail (Beyotime Institute of Biotechnology, Shanghai, China), and the protein concentrations were determined. Protein extracts were separated on 10% SDS-PAGE and then electrophoretically transferred to PVDF membranes at 100 V for 90 min. The membranes were incubated with antibody against NDC80 (1:1000; Abcam, Cambridge, MA, United States). The target proteins were examined using an electrochemiluminescence system (Thermo Fisher Scientific) and visualized with X-ray films. GAPDH was used as the control.
NDC80-siRNA and control cells were removed using 0.25% trypsin-EDTA and resuspended in standard medium after achieving logarithmic growth. Cells were seeded in five wells at 1000 cells/well, followed by further incubation at 37 °C and 50 mL/L CO2. A Cellomics ArrayScan VT1 (Thermo Fisher Scientific) was used to continuously measure GFP expression in each well over a 5-day period. In this study, statistical data were mapped and cell proliferation curves were drawn.
NDC80-siRNA and control cells were digested in 0.25% trypsin to reconstitute the single cell suspension at 5 × 104 cells/mL. A hemocytometer was used to assess the cell count, and cell suspensions were transferred into six-well plates at 800 cells/well. After approximately 2 wk incubation, colonies containing > 50 cells were scored as surviving colonies. Colonies were visualized under a fluorescence microscope (MicroPublisher 3.3RTV; Olympus, Tokyo, Japan). The supernatants were discarded, and cells were washed with phosphate-buffered saline (PBS) and fixed with paraformaldehyde (Sangon, Shanghai, China) for 30 min. All wells were washed with PBS and stained with 500 μL Giemsa solution (ECM550; Chemicon, Temecula, CA, United States) for 20 min. The cells were washed several times with deionized distilled water and allowed to air dry at room temperature. Colonies were counted and images were captured by a digital camera under light microscopy. The assay was repeated three times.
SMMC-7721 cells were plated in six-well plates under standard culture conditions. After treatment, cells were washed with PBS, fixed in 70% ice-cold ethanol and stored at -20 °C overnight. After being washed with PBS, the cells were incubated in 500 μL sample buffer containing 50 μg/mL propidium iodide (PI) and 0.25 mg/mL RNase A for 30 min at room temperature. Analysis of the apoptotic cells was performed by flow cytometry. The cells with sub-G1 DNA content were considered as apoptotic cells.
The cells were harvested with 0.25% trypsin and washed once with ice-cold PBS. After the cells were washed, the Annexin V-APC Apoptosis Detection Kit and PI (eBioscience, San Diego, CA, United States) were applied to assess apoptosis. Cells were centrifuged and resuspended in 500 μL binding buffer 106 cells/mL. And, 100 μL of the cell suspension was incubated with 10 μL PI and 5 μL Annexin V-FITC in a dark environment for 15 min at room temperature. Analysis of the apoptotic cells was performed by flow cytometry.
All tissues samples from the patients were taken after informed consent. The study was reviewed and approved by the Nantong Third People’s Hospital Affiliated to Nantong University Institutional Review Board.
GraphPad Prism version 6.0 was used for data analysis. Statistical significance was defined as P < 0.05. All data were presented as the mean ± SD. All the experiments were repeated at least three times.
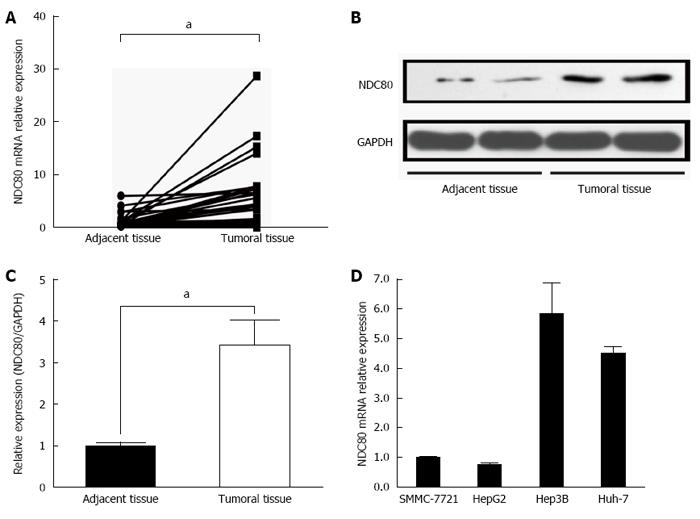
To investigate whether NDC80 expression was altered in HCC tissues, we detected its expression level by qRT-PCR in 47 paired tumor and adjacent tissues. NDC80 mRNA expression levels in the tumor tissues were drastically increased compared with those in the adjacent tissues (Figure 1A). A similar trend in NDC80 protein levels was observed by western blot analysis (Figure 1B and C). To reveal the potential role of NDC80 in HCC, we also examined NDC80 expression in four HCC cell lines: SMMC-7721, HePG2, Hep3B and Huh-7 (Figure 1D). The cell line must be lentivirus-friendly (MOI < 10) and enjoy a vigorous proliferation. Hence, the SMMC-7721 cell line was selected for future investigation. The NDC80 complex is comprised of NDC80, Nuf2, Spc24 and Spc25, which together form a dumbbell-like heterotetramer. We then detected the expression levels of Nuf2, Spc24 and Spc25 mRNA by qRT-PCR. The expressions of Nuf2 and Spc24 were significantly enhanced in HCC tissues compared with paired adjacent tissues (Supplementary Figure 1A and B). However, the expression of Spc25 mRNA was not changed between HCC tissues and adjacent tissues (Supplementary Figure 1C).
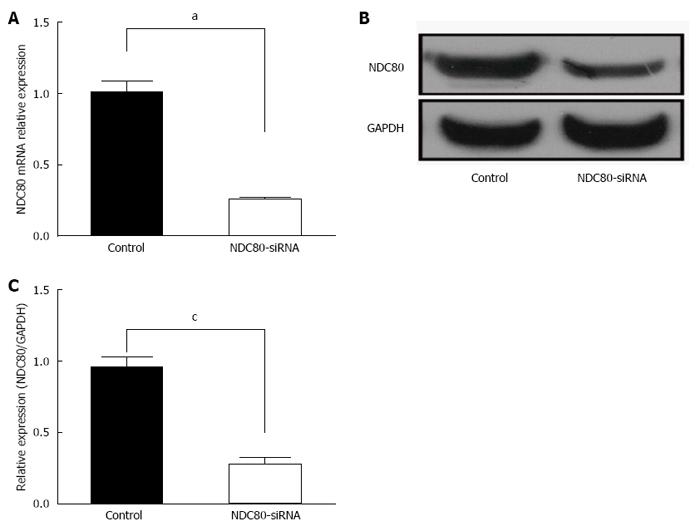
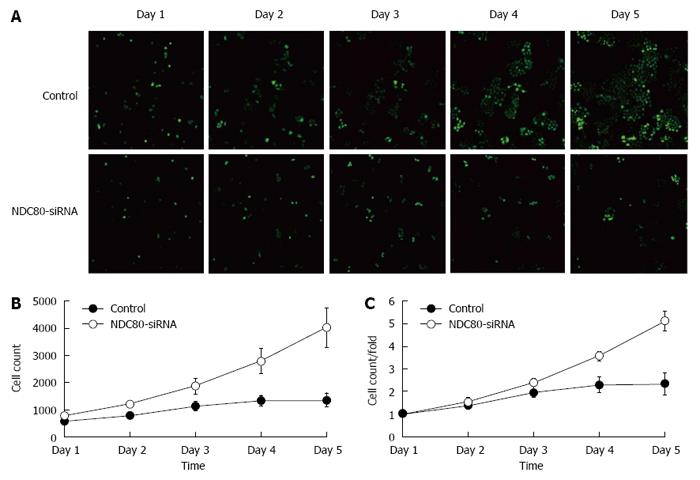
After NDC80-siRNA lentiviral transfection, RT-PCR analysis showed that NDC80-siRNA diminished the expression of the endogenous NDC80 mRNA by up to 80% (P = 0.0001) (Figure 2A). Correspondingly, the protein expression of NDC80 in NDC80-siRNA-treated cells was also suppressed (P = 0.0023) (Figure 2B and C). After transfection, cell proliferation was significantly inhibited in NDC80-siRNA-silenced cells relative to control cells, as shown by GFP-based Cellomics ArrayScan VTI imaging (Figure 3A). Cell numbers were monitored for 5 consecutive days. The number of cells and the fold-change in proliferation were markedly reduced in the NDC80-siRNA-silenced cells (Figure 3B). Accordingly, the results suggested that the silencing of NDC80 was associated with cell proliferation.
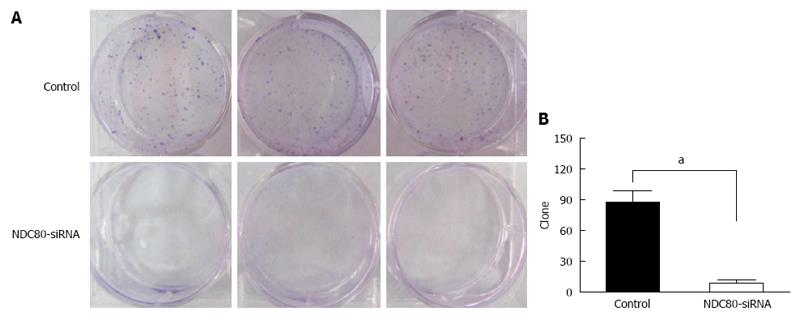
Silencing of NDC80 reduced the anchorage-independent growth of SMMC-7721 cells in soft agar (Figure 4A). The number of cell clones was significantly decreased in SMMC-7721 cells infected with NDC80-siRNA (P = 0.0005) (Figure 4B). The colony formation experiment confirmed that the silencing of NDC80 reduced the proliferative potential of SMMC-7721 cells.
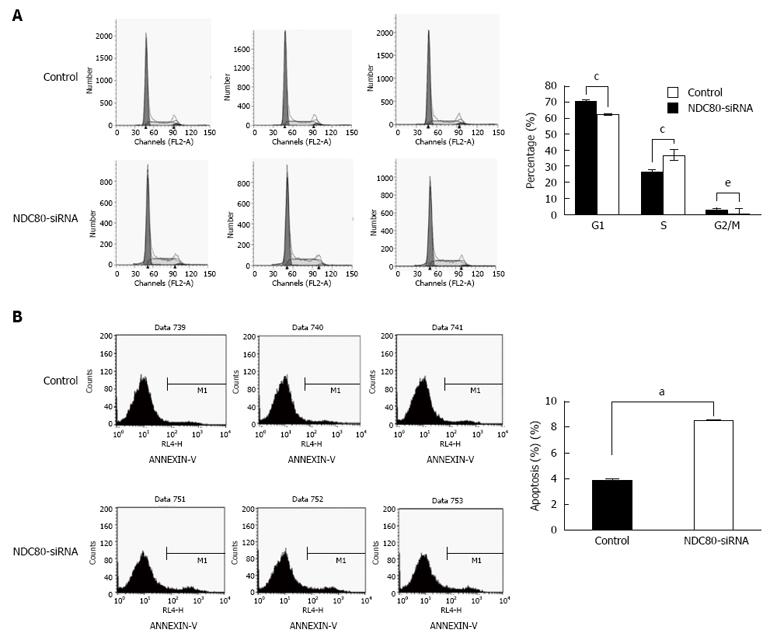
To elucidate further the growth-suppressing effect of NDC80-siRNA on SMMC-7721 cells, cell cycle distribution was analyzed by flow cytometry. In comparison with the control group, NDC80-siRNA significantly increased the fraction of S-phase cells, but decreased G1- and G2/M-phase cells in NDC80-siRNA group (Figure 5A). The result demonstrated that the silencing of NDC80 might induce cell cycle arrest at S-phase and that the effect of NDC80 on the cell cycle was time-dependent. Whether the silencing of NDC80 was related to the level of apoptosis in SMMC-7721 cells was further investigated. The apoptotic rate was assessed by flow cytometry using the Annexin V-APC Apoptosis Detection Kit. The proportion of apoptotic cells was significantly higher in NDC80-silenced cells than in the control cells (Figure 5B). These data suggested that the silencing of NDC80 interrupted cell cycle progression and affected cell survival.
Chromosomal instability has long been suggested to be a driving force for tumor development and progression. Accurate chromosome segregation requires that sister kinetochores of each mitotic chromosome interact with microtubules connected to opposite spindle poles, so that separated sister chromatids migrate toward opposite directions at anaphase onset[17-19]. NDC80 is a core component of the outer kinetochore, the function of which is intricately involved in the establishment of appropriate microtubule attachments. NDC80 protein maintains chromosome stability, and spindle checkpoint dysfunction, abnormal chromosome separation and cell cycle disorder may occur in cells with NDC80 overexpression, which perhaps leads to tumor initiation[18,20-22].
Expression of the mitotic regulator NDC80 is increased in various human malignancies, including HCC. Considering the significant overexpression of NDC80 in multiple human malignancies[23-27], studies on the mechanisms of NDC80 overexpression are significant. Liu et al[28] also reported that the expression level of NDC80 was remarkably up-regulated in HBV-related HCC tissues. In this study, we investigated the significance of the increased NDC80 expression in carcinogenesis in general and HCC in particular, by both loss and gain of function analysis. This study was conducted to ascertain the role of NDC80 in human hepatocellular carcinogenesis. Lentiviral packaging has become an ideal genetic engineering technology after years of optimization and improvement[29,30]. In this study, lentivirus-mediated siRNA provided an attractive approach to suppress NDC80 gene expression. We monitored the lentiviral infection efficiency by fluorescence microscopy, and confirmed the target gene knockdown by western blotting and real-time RT-PCR, which provided a basis for the continued observation of the role of NDC80 in SMMC-7721 cells. Real-time RT-PCR analysis showed that the NDC80 expression levels in the tumor tissues were significantly increased compared with those in adjacent tissues.
We found that cell proliferation and cell colony formation were significantly inhibited in NDC80-silenced HCC cells. Moreover, apoptosis was significantly increased in NDC80-silenced HCC cells. We performed a cell-cycle assay to illustrate the mechanism by which NDC80 promotes cell proliferation. We found that attenuation of NDC80 expression in carcinoma cells delayed cell-cycle progression through S-phase, signifying arrest at this phase. This suggests that abnormal NDC80 expression leads to severe disruption of cell-cycle progression. The present study also demonstrated that NDC80 knockdown induced HCC apoptosis. The apoptosis caused by NDC80 knockdown might be due to incomplete mitosis caused by severe mitotic spindle checkpoint dysfunction. Hence, NDC80 plays a significant role in maintaining the growth of HCC cells.
These findings strongly suggest that NDC80 contributes to the pathogenesis of HCC through its proliferative and anti-apoptotic effects. Our data suggest that NDC80 overexpression may be an early event in hepatocellular carcinogenesis. Therefore, NDC80 may represent a new target for HCC gene therapy. The results of this study provide a new theoretical basis for gene therapy in HCC.
In conclusion, the present study evaluates the association between NDC80 expression and HCC. NDC80 is highly expressed in HCC tissues relative to adjacent tissues. NDC80 significantly promotes HCC cell proliferation and colony formation and significantly inhibits apoptosis by arresting cells at the S phase. Therefore, we determined that NDC80 plays critical roles in tumor growth and HCC development. Although detailed mechanisms remain to be elucidated, the critical role of NDC80 in HCC development may provide evidence for development of novel therapeutics against NDC80 for the early detection and treatment of HCC.
We would like to thank Nantong Third People’s Hospital Affiliated to Nantong University for providing the HCC tissue samples.
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and the third leading cause of cancer mortality. High metastasis and recurrence rates have become the major obstacles to improving long-term survival of HCC. The molecular mechanisms of HCC development are not fully understood; thus, it is of importance to elucidate the mechanisms of HCC and explore effective treatment.
Nuclear division cycle (NDC)80 plays essential roles in chromosome segregation by mediating the spindle assembly checkpoint signaling and chromosome alignment. Chromosome instability is a common feature of tumor cells, and may be an important mechanism in tumor formation.
This is the first study which shows that NDC80 contributes to the pathogenesis of HCC through its proliferative and anti-apoptotic effects. The authors hypothesized that the critical role of NDC80 in HCC development could provide evidence for development of novel therapeutics against NDC80 for the early detection and treatment of HCC.
NDC80 may represent a new target for HCC gene therapy. The results of this study provide a new theoretical basis for gene therapy in HCC.
The authors demonstrated that NDC80 expression levels in the tumor tissues were significantly increased compared with those in the adjacent tissues. NDC80 significantly promotes HCC cell proliferation and colony formation and significantly inhibits apoptosis by affecting cell cycle S-phase arrest. However, the detailed mechanisms about the critical role of NDC80 in HCC development have not been elucidated.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Nogales E, Suzuki A, Yang SS S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
| 1. | Cui X, Li Z, Gao J, Gao PJ, Ni YB, Zhu JY. Elevated CXCL1 increases hepatocellular carcinoma aggressiveness and is inhibited by miRNA-200a. Oncotarget. 2016;7:65052-65066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Zhou B, Chen H, Wei D, Kuang Y, Zhao X, Li G, Xie J, Chen P. A novel miR-219-SMC4-JAK2/Stat3 regulatory pathway in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2014;33:55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Li J, Qiu X, Guo W, Yan B, Zhang S. Prospective analysis of tiopronin in prevention of sorafenib and antiviral therapy inducing liver toxicity in advanced hepatitis B virus-related hepatocellular carcinoma. Med Oncol. 2015;32:238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Qu C, He D, Lu X, Dong L, Zhu Y, Zhao Q, Jiang X, Chang P, Jiang X, Wang L. Salt-inducible Kinase (SIK1) regulates HCC progression and WNT/β-catenin activation. J Hepatol. 2016;64:1076-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Shirvani-Dastgerdi E, Schwartz RE, Ploss A. Hepatocarcinogenesis associated with hepatitis B, delta and C viruses. Curr Opin Virol. 2016;20:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Tsai CL, Hsu FM, Cheng JC. How to Improve Therapeutic Ratio in Radiotherapy of HCC. Liver Cancer. 2016;5:210-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kim KI, Chung HK, Park JH, Lee YJ, Kang JH. Alpha-fetoprotein-targeted reporter gene expression imaging in hepatocellular carcinoma. World J Gastroenterol. 2016;22:6127-6134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and Treatment of Hepatocellular Carcinoma in 2016: Standards and Developments. Visc Med. 2016;32:116-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Tang NH, Toda T. Ndc80 Loop as a protein-protein interaction motif. Cell Div. 2013;8:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Tang NH, Toda T. MAPping the Ndc80 loop in cancer: A possible link between Ndc80/Hec1 overproduction and cancer formation. Bioessays. 2015;37:248-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Matson DR, Stukenberg PT. Cdt1 throws kinetochore-microtubule attachments for a loop. Nat Cell Biol. 2012;14:561-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Tooley J, Stukenberg PT. The Ndc80 complex: integrating the kinetochore’s many movements. Chromosome Res. 2011;19:377-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Sun QY. Ndc80 complex: new evidence for the existence of spindle assembly checkpoint in mammalian oocyte meiosis. Cell Cycle. 2011;10:877-878. [PubMed] [Cited in This Article: ] |
| 14. | Wei RR, Sorger PK, Harrison SC. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc Natl Acad Sci USA. 2005;102:5363-5367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Mattiuzzo M, Vargiu G, Totta P, Fiore M, Ciferri C, Musacchio A, Degrassi F. Abnormal kinetochore-generated pulling forces from expressing a N-terminally modified Hec1. PLoS One. 2011;6:e16307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Tang NH, Toda T. Alp7/TACC recruits kinesin-8-PP1 to the Ndc80 kinetochore protein for timely mitotic progression and chromosome movement. J Cell Sci. 2015;128:354-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 550] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 18. | Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 19. | Diaz-Rodríguez E, Sotillo R, Schvartzman JM, Benezra R. Hec1 overexpression hyperactivates the mitotic checkpoint and induces tumor formation in vivo. Proc Natl Acad Sci USA. 2008;105:16719-16724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Sundin LJ, Guimaraes GJ, Deluca JG. The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore-microtubule attachment in mitosis. Mol Biol Cell. 2011;22:759-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Tien JF, Umbreit NT, Zelter A, Riffle M, Hoopmann MR, Johnson RS, Fonslow BR, Yates JR, MacCoss MJ, Moritz RL. Kinetochore biorientation in Saccharomyces cerevisiae requires a tightly folded conformation of the Ndc80 complex. Genetics. 2014;198:1483-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Wei R, Ngo B, Wu G, Lee WH. Phosphorylation of the Ndc80 complex protein, HEC1, by Nek2 kinase modulates chromosome alignment and signaling of the spindle assembly checkpoint. Mol Biol Cell. 2011;22:3584-3594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Mo QQ, Chen PB, Jin X, Chen Q, Tang L, Wang BB, Li KZ, Wu P, Fang Y, Wang SX. Inhibition of Hec1 expression enhances the sensitivity of human ovarian cancer cells to paclitaxel. Acta Pharmacol Sin. 2013;34:541-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Qu Y, Li J, Cai Q, Liu B. Hec1/Ndc80 is overexpressed in human gastric cancer and regulates cell growth. J Gastroenterol. 2014;49:408-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Gurzov EN, Izquierdo M. RNA interference against Hec1 inhibits tumor growth in vivo. Gene Ther. 2006;13:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R, Lau J, Chen PL, Lee WH. Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res. 2008;68:8393-8399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Huang LY, Lee YS, Huang JJ, Chang CC, Chang JM, Chuang SH, Kao KJ, Tsai YJ, Tsai PY, Liu CW. Characterization of the biological activity of a potent small molecule Hec1 inhibitor TAI-1. J Exp Clin Cancer Res. 2014;33:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Liu B, Yao Z, Hu K, Huang H, Xu S, Wang Q, Yang Y, Ren J. ShRNA-mediated silencing of the Ndc80 gene suppress cell proliferation and affected hepatitis B virus-related hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2016;40:297-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Yu HT, Lu PR. Effects of lentiviral short hairpin RNA silencing of Toll-like receptor 4 on the lens epithelial cell line HLEC. Genet Mol Res. 2016;15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Zhang L, Liu HJ, Li TJ, Yang Y, Guo XL, Wu MC, Rui YC, Wei LX. Lentiviral vector-mediated siRNA knockdown of SR-PSOX inhibits foam cell formation in vitro. Acta Pharmacol Sin. 2008;29:847-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |