Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3522
Peer-review started: February 8, 2017
First decision: March 3, 2017
Revised: March 7, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: May 21, 2017
To determine the feasibility, safety, and oncological outcome of laparoscopic resection of gastric gastrointestinal stromal tumors (GISTs) based on favorable or unfavorable location.
Our hospital database included 207 patients who underwent laparoscopic removal of gastric GISTs from January 2004 to September 2015. Patient demographics, clinical presentation, surgery, histopathology, postoperative course, and oncological outcomes were reviewed and analyzed.
Gastric GIST in favorable locations was present in 81/207 (39.1%) cases, and in unfavorable locations in 126/207 (60.9%) cases. Overall mean tumor size was 3.28 ± 1.82 cm. No conversions occurred, and complete R0 resection was achieved in 207 (100%) cases. There were three incidences of iatrogenic tumor rupture. The feasibility and safety of laparoscopic surgery were comparable in both groups with no statistical difference between unfavorable and favorable location groups, respectively: for operative time: 83.86 ± 44.41 vs 80.77 ± 36.46 min, P = 0.627; conversion rate: 0% vs 0%; estimated blood loss: 27.74 ± 45.2 vs 29.59 ± 41.18 mL, P = 0.780; tumor rupture during surgery: 0.90% vs 2.82%, P = 0.322; or postoperative complications: 3.74% vs 7.04%, P = 0.325. The follow-up period recurrence rate was 1.89% with no significant differences between the two groups (3.03% vs 0%, P = 0.447). Overall 5-year survival rate was 98.76% and survival rates were similar between the two groups: 98.99% vs 98.39%, P = 0.623 (unfavorable vs favorable, respectively).
The laparoscopic approach for gastric GISTs is safe and feasible with well-accepted oncological surgical outcomes. Strategies for laparoscopic resection should be selected according to the location and size of the tumor. Laparoscopic treatment of gastric GISTs in unfavorable locations should not be restricted in gastrointestinal centers.
Core tip: In most guidelines laparoscopic surgery is suggested only for gastrointestinal stromal tumors (GISTs) in favorable locations, such as those in the greater curvature or anterior wall of the stomach. The feasibility, safety and oncological outcome of this technique for GISTs in unfavorable locations remain unclear. We aimed to determine the feasibility, safety, and oncological outcome of laparoscopic resection of gastric GISTs based on different location. To our knowledge, this retrospective study includes the largest series of patients with gastric GISTs treated with laparoscopic resection at a single center. We also used and describe three relatively new laparoscopic surgical techniques for GISTs.
- Citation: Liao GQ, Chen T, Qi XL, Hu YF, Liu H, Yu J, Li GX. Laparoscopic management of gastric gastrointestinal stromal tumors: A retrospective 10-year single-center experience. World J Gastroenterol 2017; 23(19): 3522-3529
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3522.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3522
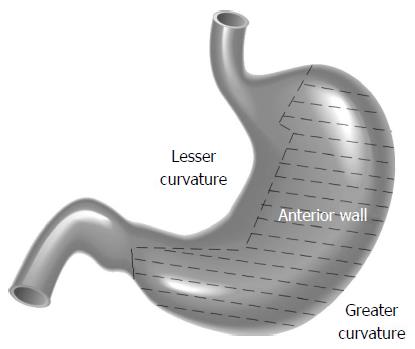
Gastrointestinal stromal tumors (GISTs) are the most common type of subepithelial tumor, with more than half (50%-60%) located in the stomach[1]. The standard treatment for localized GISTs is complete R0 surgical excision, avoiding tumor rupture, and without dissection of clinically-negative lymph nodes[2,3]. Although the feasibility and safety of the laparoscopic approach for GIST resection has been demonstrated in many retrospective studies[4], in the European Society for Medical Oncology, National Comprehensive Cancer Network (NCCN) and Asian GIST guidelines, laparoscopic surgery is suggested only for GISTs in favorable locations such as those in the greater curvature or anterior wall of the stomach[2,3,5]. In unfavorable locations, due to the difficulty in exposing tumor position, there is a risk of stenosis of the lumen postoperatively, and guaranteed R0 resection is still difficult with laparoscopic procedures. The feasibility, safety and oncological outcome of this technique for GISTs in unfavorable locations remain unclear[6,7].
We examined our most recent 10-year experience regarding the laparoscopic treatment of gastric GISTs based on different locations including favorable locations (tumors located in the greater curvature and anterior wall of the gastric body, fundus, and antrum) and unfavorable locations (tumors located in the lesser curvature or posterior wall of the gastric body, fundus, and antrum) (Figure 1) to determine the feasibility, safety, and oncological outcomes of laparoscopic resection of gastric GISTs. We also outline, herein, the technical details involved in the surgeries. To the best of our knowledge, this is the largest case series to date focusing on the laparoscopic management of GISTs.
All laparoscopically-resected gastric GISTs included in a maintained database at Nanfang Hospital, China, from January 2004 to September 2015, were retrospectively analyzed. All patients underwent preoperative endoscopy and abdominal computed tomographic imaging. The diagnosis of gastric GIST was established by positive immunohistochemical staining of CD117 and/or CD34 in the surgical specimens, and all operations were performed at our hospital by experienced laparoscopic surgeons. Data on patient demographics, clinical presentation, surgery, histopathology, postoperative course, and oncological outcomes were reviewed and analyzed. Hand-assisted cases not expected preoperatively were classified as conversions. Tumor size was defined as the maximal tumor dimension of the resected specimen, and R0 resection was defined as removal of all gross disease at surgery without microscopic disease. This study was approved by the ethics committee of Nanfang Hospital.
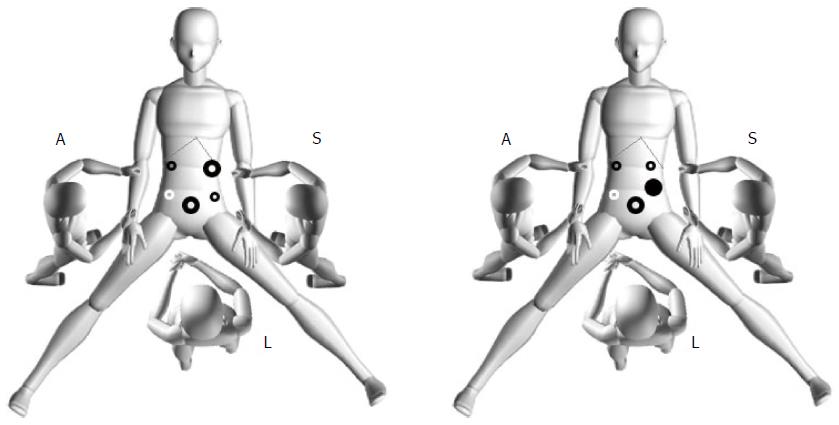
The surgical procedure following exploration was based on the location and size of the tumor. Laparoscopic procedures for managing GISTs were introduced in a previous study[7]. The options for laparoscopic surgery included gastrectomy (total, subtotal, distal, gastric stump, or proximal), wedge resection, transgastric resection, and seromuscular dissection and laparoscopic intragastric submucosal dissection. After conventional endotracheal intubation anesthesia, the patient was placed in the supine position with legs apart. The position of the surgeons and the location of the puncture hole are shown in Figure 2. The technical details depended on tumor location, size, and morphology. The tumor specimen was extracted through a minilaparotomy in an endoscopic retrieval bag. The details of laparoscopic wedge resection, transgastric resection, seromuscular dissection and laparoscopic intragastric submucosal dissection are described below.
Most lesions could be seen or palpated with laparoscopic instruments on the anterior side of the stomach, and wedge resection was performed preferentially to anatomical resection whenever possible. Exophytic GISTs were treated with wedge resection using a linear endoscopic stapler at the base. Endophytic lesions of the anterior wall of the stomach were resected with a margin of normal stomach using an ultrasonic scalpel or EndoGIA (United States Surgical Corporation, Norwalk, CT, United States).
An ultrasonic scalpel was used to incise the seromuscular layer at the lower edge of the tumor, and the incised seromuscular layer was grasped by noninvasive forceps to lift the tumor. The tumor was then detached from the mucosa from proximal to distal direction using the ultrasonic scalpel. Once the tumor was removed, the mucosal integrity was evaluated, and if the mucosa was penetrated, it was repaired with absorbable sutures. The seromuscular layer was then sutured laparoscopically using continuous 3-0 absorbable sutures, and the incision was observed during gastric endoscopy for the presence of bleeding or stenosis.
When the endogenous tumor was located in the mesangial side or near the posterior wall of the cardia, it was necessary to fully free the surrounding gastric wall, reposition the tumor, and incise the full-thickness stomach wall containing the tumor at the lower 1-cm edge of the tumor along the longitudinal axis. After tumor resection, the stomach wall was sutured with 3-0 absorbable suture vertically to the stomach longitudinal axis, and the seromuscular layer was then embedded intermittently. A laparoscopic linear cutter stapler can also be used to close the gastric wall opening. Recently, a “dumpling making” method was used in our center, which involves cutting the stomach in a half circle, lifting the tumor, using the cutter stapler to remove the stomach wall and tumor together and closing the incision at the same time.
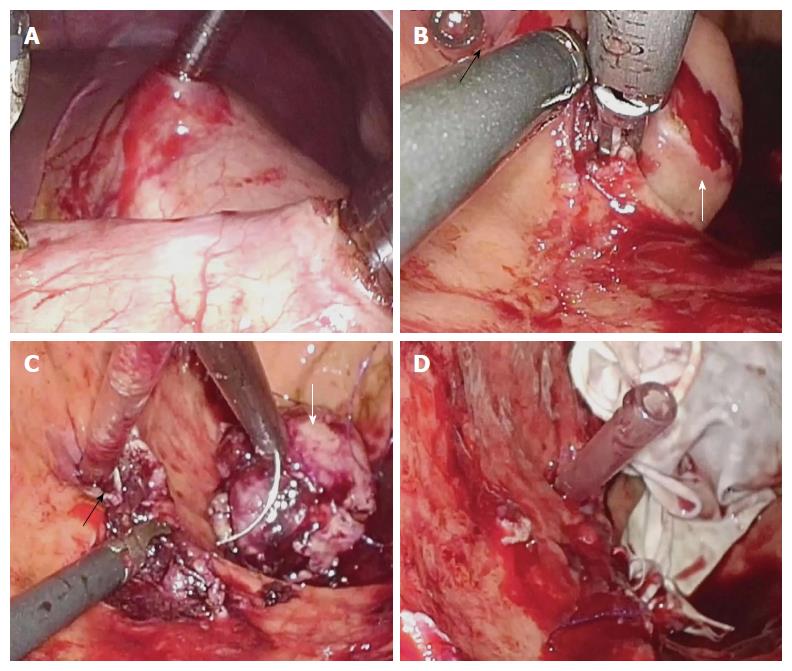
A small incision was made in the anterior wall of the gastric body near the greater curvature using the ultrasonic scalpel. A 12-mm cannula in the left lower abdomen was inserted into the stomach cavity and air was delivered into the cavity. A laparoscopic lens was inserted through the cannula to determine the position of the tumor and the puncture site for another accessory port and assistant cannula in the stomach wall. After placement, the laparoscopic instrument was moved into the stomach cavity. The mucosa at the edge of the tumor was then incised, and the tumor was stripped from the submucosal layer using the ultrasonic scalpel. Dissection of the tumor was performed while the intracardial port was monitored (Figure 3).
Following removal of the tumor, the mucosal incision was sutured with 3-0 absorbable suture. The gastric cannula provided guidance and support to prevent cardia stenosis caused by the sutures. The tumor was placed in the specimen bag, the stomach wall was lifted to the abdominal wall through the appropriately expanded cannula incision, and the specimen bag was then removed. The specimen bag could also be removed by intraoperative gastroscopy. The stomach wall incision was lifted through the cannula port outside the abdominal wall for suturing or was sutured directly under laparoscopy.
The majority of patients underwent close follow-up. However, 18 patients were lost to follow-up or had insufficient clinical records. Follow-up was conducted by telephone or outpatient visits, and follow-up data included adjunctive therapy, survival time, recurrence, and death.
Continuous variables are presented as mean ± SD and were compared using Student’s t-test. Categorical variables are expressed as valid percentages and were compared using the χ2 test or Fisher’s exact test, as appropriate. Recurrence and survival outcomes were calculated using the Kaplan-Meier method and were compared by the log-rank test. Univariate analysis of multiple clinicopathological variables was performed to determine the variables associated with poor outcomes. Factors deemed significant in univariate analyses were entered into multivariate analyses using logistic and Cox regression models. A P value < 0.05 was considered significant, and all statistical analyses were performed using SPSS 20.0 software (SPSS, Chicago, IL, United States).
During the study period, a total of 207 patients underwent laparoscopic resection of gastric GISTs. Patient and tumor characteristics are presented in Table 1. The tumors were in favorable locations in 81/207 (39.1%) cases, and in unfavorable locations in 126/207 (60.9%) cases. Overall mean tumor size was 3.28 ± 1.82 cm. According to the 2002 Fletcher’s criteria, 95/207 (55.7%) cases were in the low or very low risk group, 51/207 (30.2%) were in the intermediate risk group and 24/207 (14.1%) cases were in the high risk group. Twenty-seven (27/207, 13.0%) cases underwent endoscopy during surgery. No conversions occurred and complete R0 resection was achieved in all 207 (100%) cases. There were three incidences of iatrogenic tumor rupture and no major intraoperative complications. The overall postoperative complication rate was 0.51%. Nine cases had only mild complications, which resolved with observation and antibiotic treatment.
| Characteristics | |
| Age (yr) | 54.09 ± 12.53 |
| Male/female | 92/106 |
| Tumor location | |
| Favorable | 81 (39.1) |
| Unfavorable | 126 (60.9) |
| Tumor size (cm) | 3.28 ± 1.82 |
| Risk1 | |
| Very low | 38 (22.2) |
| Low | 57 (33.5) |
| Intermediate | 51 (30.2) |
| High | 24 (14.1) |
| Application of endoscopy during surgery | 27 (13.0) |
| Conversion | 0 |
| Tumor rupture during surgery | 3 (1.58) |
| Tumor resection margin R0 | 100% |
| Operative time (min) | 80.74 ± 38.96 |
| Estimated blood loss (mL) | 28.17 ± 44.99 |
| Postoperative exhaust time | 2.537 ± 0.88 |
| Time to liquid diet (d) | 2.91 ± 1.70 |
| Length of postoperative stay (d) | 6.10 ± 2.99 |
| Classification of postoperative complications2 | |
| Grade 1 | 3 (0.17) |
| Grade 2 | 6 (0.34) |
When comparing gastric GISTs in favorable locations with those in unfavorable locations according to the NCCN guidelines, we noted that the application of endoscopy in the unfavorable group was more frequent (20.72% vs 5.63%, respectively, P = 0.05). The feasibility and safety of laparoscopic surgery were comparable in both groups, with no statistical difference (favorable location group vs unfavorable location group, respectively) in operative time (83.86 ± 44.41 min vs 80.77 ± 36.46 min, P = 0.627), conversion rate, estimated blood loss (27.74 ± 45.2 mL vs 29.59 ± 41.18 mL, P = 0.780), tumor rupture during surgery (0.90% vs 2.82%, P = 0.322), or postoperative complications (3.74% vs 7.04%, P = 0.325) (Table 2).
| Unfavorable area | Favorable area | P value | |
| Age (yr) | 54.29 ± 12.29 | 55.59 ± 12.4 | 0.486 |
| BMI | 22.37 ± 3.28 | 23.17 ± 3.01 | 0.100 |
| Tumor size (cm) | 3.37 ± 1.85 | 4.07 ± 2.23 | 0.020 |
| Operative time (min) | 83.86 ± 44.41 | 80.77 ± 36.46 | 0.627 |
| Estimated blood loss (mL) | 27.74 ± 45.2 | 29.59 ± 41.18 | 0.780 |
| Conversion | 0 | 0 | |
| Application of endoscopy during surgery | 23 (20.72) | 4 (5.63) | 0.005 |
| Tumor rupture during surgery | 1 (0.90) | 2 (2.82) | 0.322 |
| Postoperative complications | 4 (3.74) | 5 (7.04) | 0.325 |
| Postoperative exhaust time | 2.64 ± 0.9 | 2.54 ± 0.86 | 0.452 |
| Time to liquid diet (d) | 3.05 ± 1.62 | 2.89 ± 1.79 | 0.516 |
| Time to semiliquid diet (d) | 4.17 ± 1.75 | 3.92 ± 1.83 | 0.345 |
| Postoperative hospital stay (d) | 7.17 ± 4.1 | 5.69 ± 2.46 | 0.007 |
| Recurrence | 3 (3.03) | 0 (0.00) | 0.447 |
| 5-yr overall survival rate | 98 (98.99) | 61 (98.39) | 0.623 |
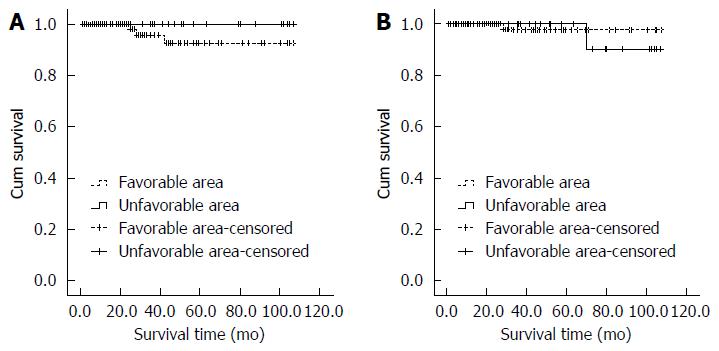
During the follow-up period (7-107 mo), GISTs recurred in three patients in unfavorable location group, but there were no recurrences in the favorable location group. The total recurrence rate was 1.89%. Two patients with recurrences had the history of tumor rupture during the laparoscopic surgery. No laparoscopic technique was performed in patients with recurrent disease. An exploratory laparotomy was performed in one patient with local recurrence after 22 mo of the first operation. Although the recurrence rate was higher in the unfavorable location group compared with the favorable location group, there were no significant differences between the two groups (3.03% vs 0%, P = 0.447) (Figure 4A). One patient in the favorable location group suffered from recurrence at 12 mo and died at 28 mo after the surgery. One patient in the favorable location group presented with tumor rupture during surgery and died 70 mo after the surgery. The overall 5-year survival rate was 98.76%, and overall survival rates were similar (98.99% vs 98.39%, P = 0.623) (Figure 4B) when comparing the unfavorable location group versus favorable location group, respectively.
Many guidelines recommend laparoscopic surgery for GISTs dependent on tumor location. Laparoscopic surgery has been performed for tumors in unfavorable locations, especially the cardia and antrum, and has been reported to be safe and effective for GISTs[8-13]; however, to our knowledge, no study has compared the safety and efficacy of laparoscopic surgery in GISTs in unfavorable locations vs favorable locations. With similar operative morbidity, conversion risk, blood loss, postoperative recovery, and oncological results, our series demonstrates that compared with GISTs of the greater curvature or anterior wall, the resection of gastric GISTs in other locations is also feasible and can be safely accomplished laparoscopically.
To our knowledge, this retrospective study includes the largest series of patients with gastric GISTs treated with laparoscopic resection at a single center. Laparoscopic resection for proven GISTs even at challenging locations had 100% negative resection margins and excellent long-term oncological outcomes. Compared with open surgery, laparoscopic surgery for GISTs has similar outcomes and advantages that include less pain, less invasiveness, early recovery, and better cosmetic results. Our results indicate that a laparoscopic approach should be considered in all patients with gastric GISTs who have no contraindications to this approach. Three systematic review and meta-analyses comparing laparoscopic versus open resections for gastric GISTs showed that laparoscopic resection for gastric GISTs is a safe and feasible procedure with superior postoperative outcomes[14]. Long-term survival was mainly associated with tumor stage and type, and laparoscopic surgery did not increase the risk of tumor relapse and metastasis[15]. The low morbidity, no conversion to open surgery, and the long-term disease-free interval observed in our study indicate that laparoscopic resection is safe and effective for gastric GISTs irrespective of tumor locations.
Endoscopic cooperative surgery is being described increasingly as a technique to resect gastric submucosal lesions in unfavorable locations, such as the cardia or pyloric region[16,17]. However, this approach requires facilities for endoscopic submucosal dissection and highly-advanced endoscopic skill, which limits the wide application of a combined laparoscopic-endoscopic approach[17]. In our experience, most cases underwent laparoscopic surgery alone, and diagnostic gastroscopy was used when it was difficult to delineate the tumor’s intraluminal extent and location. Different strategies for laparoscopic resection should be selected according to the tumor location.
Wedge resection is the most prevalent procedure for laparoscopic resection of GIST. Although wedge resection can be performed successfully in the majority of cases, the technique should be chosen carefully, especially in patients with GISTs near the cardia or pylorus[18]. The lesser curvature of the stomach and sites near the cardia or pylorus are considered unfavorable locations. When the tumor is located in these locations, wedge resection of the stomach wall can easily cause stenosis, and when a proximal or distal gastrectomy is performed, the scope of surgery for a stromal tumor increases.
In our center, we dissect the tumor from the gastric mucosa or incise and suture the gastric wall, which not only avoids gastric stenosis, but also maximizes retention of healthy stomach. Specifically, when tumors in unfavorable locations show exogenous growth, dissection of the tumor from the gastric mucosa should be chosen, and if the tumor shows endogenous growth, seromuscular dissection should be chosen when an intact mucosal layer surrounding the tumor is confirmed by preoperative gastroscopy.
For endogenous tumors treated with seromuscular dissection, mucosal layer identification is difficult, thus transgastric resection with suturing provides better visibility. When the tumor is located near the cardia or pylorus, if preoperative endoscopy or endoscopic ultrasonography suggests a clear tumor boundary, uniform texture, and endogenous growth, submucosal dissection of the tumor via the gastric cavity can be adopted. In these cases, surgery should be meticulous to prevent postoperative abdominal infection by placing gauze under the stomach wall incision and performing thorough aspiration of gastric contents. In patients with gastric retention, the stomach contents may flow into the abdominal cavity during gastric incision, thus preoperative gastrointestinal decompression and other relevant techniques should be performed.
Because this operation involves opening the gastric cavity, there may be potential risks of intraperitoneal infection; therefore, the safety of this treatment remains controversial. Conrad et al[6] operated on 11 cases using the following techniques: (1) a combined gastroscopic/laparoscopic approach when minimal manipulation of the lesion is needed; (2) multiport resection, which provides optimal triangulation and allows for resection of more complex lesions; (3) stapled removal of broad-based lesions; and (4) a single access technique with the device placed directly through the abdominal wall into the stomach. These techniques expand the surgeon’s armamentarium to address more complex intragastric processes safely.
Because the reported number of surgical resections for endogenous tumors via the gastric cavity is small, it can not be confirmed if this method is better than other methods for stromal tumors in special locations. However, theoretically, preventing exposure of the gastrointestinal tract within the abdominal cavity could reduce the occurrence of abdominal infection. Tumor resection via the stomach cavity has been performed in only a few cases, thus its safety remains to be verified.
The 2010 NCCN report suggested that gastric GISTs larger than 5 cm may be resected using a laparoscopic or laparoscopic-assisted technique[8]. Prior to 2015, a size limit in the NCCN guidelines for GISTs was not stated[3]. Size is also not emphasized in the Asian GIST guidelines[3]. On the other hand, the latest European Society for Medical Oncology practice guidelines clearly discourage a laparoscopic approach in patients with large tumors because of the risk of tumor rupture, which is associated with a very high risk of relapse[5].
In our study, which included tumors ranging in size from 0.5-11 cm, tumor rupture or bleeding did not occur in relatively large tumors intraoperatively. When it is necessary to retract the tumor during surgery, the tissue around the tumor should be clamped to avoid or reduce direct contact with the tumor. Tumor size should not be considered a limitation for experienced laparoscopic surgeons using the no-touch technique. Recent retrospective studies showed that laparoscopic surgery did not increase the risks of tumor relapse and metastasis[9,11]. Laparoscopic surgery should be considered as a standard approach in all cases irrespective of tumor size.
Despite recent advances in targeted oncological therapy for GISTs, for the majority of patients, complete surgical resection is sufficient to achieve long-term disease-free outcomes. Our experience echoes this fact. All curative-intent patients in this series underwent complete oncological resection with negative surgical margins on pathology. In this series, 146/170 (85.9%) of patients were classified as low or intermediate risk according to Fletcher’s criteria, and only a small number of these cases (6/146) received adjuvant therapy. To date, none has recurred during a median follow-up period of 4.1 years (7-107 mo).
These data reinforce the importance of proper oncological resection of GIST tumors, the primary merits of which are negative mucosal margins and avoidance of tumor rupture. Gastric GISTs recurred in three patients in the unfavorable location group, and there were no recurrences in the favorable location group. All of the patients who experienced recurrence belonged to the high risk group according to the Fletcher’s criteria, and one died at 28 mo after surgery without receiving imatinib therapy. Survival analysis showed no significant difference in the disease-free survival time between the two groups; therefore, the long-term efficacy was similar between the two groups.
Our study has certain limitations. First, although the study included the largest known case series, it was a single center study. Second, early data were not fully recorded. Although most demographic and clinicopathological characteristics were comparable between the two groups with tumors in different locations, we acknowledge the potential imbalance in unknown factors that may have compromised the validity of the results. Third, the lack of long-term follow-up restricts the evaluation of survival benefits. Fourth, wedge resection was still the most prevalent procedure for laparoscopic resection of GIST in our study. In this study, only two patients underwent laparoscopic intragastric submucosal dissection. The present clinical data failed to conduct an analysis for the relationship between different localization with different techniques. Fifth, our study was a non-randomized controlled pilot study with a small sample size. The major purpose of the study was to determine the perioperative safety and efficacy of laparoscopic management of GISTs.
In conclusion, this study showed that a laparoscopic approach for gastric GISTs is a safe and feasible procedure with well-accepted oncological surgical results. Different strategies for laparoscopic resection should be selected according to tumor location.
Laparoscopic surgery is recommended for gastrointestinal stromal tumors (GISTs) located in the greater curvature or anterior wall of the stomach. We retrospectively examined our most recent 10-year experience regarding the laparoscopic treatment of gastric GISTs with a focus on unfavorable locations such as the lesser curvature or posterior wall of the gastric body, fundus and antrum. We aimed to determine the feasibility, safety, and oncological outcome of laparoscopic resection of gastric GISTs based on a favorable or unfavorable location. We also used and describe three new laparoscopic surgical techniques for GISTs.
Although the feasibility and safety of the laparoscopic approach for GIST resection has been demonstrated in many retrospective studies, in the European Society for Medical Oncology, National Comprehensive Cancer Network (NCCN) and Asian GIST guidelines, laparoscopic surgery is suggested only for GISTs in favorable locations such as those in the greater curvature or anterior wall of the stomach. This study examined recent 10-year experience in a high value center regarding the laparoscopic treatment of gastric GISTs based on different locations including favorable locations and unfavorable locations to determine the feasibility, safety, and oncological outcomes of laparoscopic resection of gastric GISTs.
The laparoscopic approach for gastric GISTs is safe and feasible with well-accepted oncological surgical outcomes. Strategies for laparoscopic resection should be selected according to the location and size of the tumor.
The data in this study suggested that laparoscopic treatment of GISTs in unfavorable locations should not be restricted in gastrointestinal centers.
This is a very interesting study concerning new techniques of GIST resection in stomach localization. To date it is widely accepted (NCCN guidelines) that laparoscopy is safe, with good outcome in disease-free survival time.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Beltran MA, Guzel T S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH
| 1. | Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 2. | ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii21-iii26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 287] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 3. | von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Conrad EU, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:758-786. [PubMed] [Cited in This Article: ] |
| 4. | Koh YX, Chok AY, Zheng HL, Tan CS, Chow PK, Wong WK, Goh BK. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013;20:3549-3560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat. 2016;48:1155-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Conrad C, Nedelcu M, Ogiso S, Aloia TA, Vauthey JN, Gayet B. Techniques of intragastric laparoscopic surgery. Surg Endosc. 2015;29:202-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Xu C, Chen T, Hu Y, Balde AI, Liu H, Yu J, Zhen L, Li G. Retrospective study of laparoscopic versus open gastric resection for gastric gastrointestinal stromal tumors based on the propensity score matching method. Surg Endosc. 2017;31:374-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-S41; quiz S42-S44. [PubMed] [Cited in This Article: ] |
| 9. | Mueller CL, Braun J, Leimanis ML, Mouhanna J, Feldman LS, Ferri LE. Application of an individualized operative strategy for wedge resection of gastric gastrointestinal stromal tumors: Effectiveness for tumors in difficult locations. Surgery. 2016;160:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Chen K, Zhou YC, Mou YP, Xu XW, Jin WW, Ajoodhea H. Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg Endosc. 2015;29:355-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | de’Angelis N, Brunetti F, Felli E, Mehdaoui D, Memeo R, Carra MC, Zuddas V, Azoulay D. Laparoscopic versus open gastric wedge resection for primary gastrointestinal tumors: clinical outcomes and health care costs analysis. Surg Laparosc Endosc Percutan Tech. 2015;25:143-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Tabrizian P, Sweeney RE, Uhr JH, Nguyen SQ, Divino CM. Laparoscopic resection of gastric and small bowel gastrointestinal stromal tumors: 10-year experience at a single center. J Am Coll Surg. 2014;218:367-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Valle M, Federici O, Carboni F, Carpano S, Benedetti M, Garofalo A. Gastrointestinal stromal tumors of the stomach: the role of laparoscopic resection. Single-centre experience of 38 cases. Surg Endosc. 2014;28:1040-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Chen QL, Pan Y, Cai JQ, Wu D, Chen K, Mou YP. Laparoscopic versus open resection for gastric gastrointestinal stromal tumors: an updated systematic review and meta-analysis. World J Surg Oncol. 2014;12:206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Kim IH, Kim IH, Kwak SG, Kim SW, Chae HD. Gastrointestinal stromal tumors (GISTs) of the stomach: a multicenter, retrospective study of curatively resected gastric GISTs. Ann Surg Treat Res. 2014;87:298-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Chun SY, Kim KO, Park DS, Lee IJ, Park JW, Moon SH, Baek IH, Kim JH, Park CK, Kwon MJ. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc. 2013;27:3271-3279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: Advantages and hurdles. World J Gastrointest Endosc. 2015;7:192-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 54] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Khoo CY, Goh BK, Eng AK, Chan WH, Teo MC, Chung AY, Ong HS, Wong WK. Laparoscopic wedge resection for suspected large (≥5 cm) gastric gastrointestinal stromal tumors. Surg Endosc. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |