Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2785
Peer-review started: December 23, 2016
First decision: January 10, 2017
Revised: January 27, 2017
Accepted: March 20, 2017
Article in press: March 20, 2017
Published online: April 21, 2017
To investigate the functional effects of abnormal esophagogastric (EGJ) measurements in asymptomatic healthy volunteers over eighty years of age.
Data from 30 young controls (11 M, mean age 37 ± 11 years) and 15 aged subjects (9 M, 85 ± 4 years) were compared for novel metrics of EGJ-function: EGJ-contractile integral (EGJ-CI), “total” EGJ-CI and bolus flow time (BFT). Data were acquired using a 3.2 mm, 25 pressure (1 cm spacing) and 12 impedance segment (2 cm) solid-state catheter (Unisensor and MMS Solar GI system) across the EGJ. Five swallows each of 5 mL liquid (L) and viscous (V) bolus were analyzed. Mean values were compared using Student’s t test for normally distributed data or Mann Whitney U-test when non-normally distributed. A P value < 0.05 was considered significant.
EGJ-CI at rest was similar for older subjects compared to controls. “Total” EGJ-CI, measured during liquid swallowing, was increased in older individuals when compared to young controls (O 39 ± 7 mmHg.cm vs C 18 ± 3 mmHg.cm; P = 0.006). For both liquid and viscous bolus consistencies, IRP4 was increased (L: 11.9 ± 2.3 mmHg vs 5.9 ± 1.0 mmHg, P = 0.019 and V: 14.3 ± 2.4 mmHg vs 7.3 ± 0.8 mmHg; P = 0.02) and BFT was reduced (L: 1.7 ± 0.3 s vs 3.8 ± 0.2 s and V: 1.9 ± 0.3 s vs 3.8 ± 0.2 s; P < 0.001 for both) in older subjects, when compared to young. A matrix of bolus flow and presence above the EGJ indicated reductions in bolus flow at the EGJ occurred due to both impaired bolus transport through the esophageal body (i.e., the bolus never reached the EGJ) and increased flow resistance at the EGJ (i.e., the bolus retained just above the EGJ).
Bolus flow through the EGJ is reduced in asymptomatic older individuals. Both ineffective esophageal bolus transport and increased EGJ resistance contribute to impaired bolus flow.
Core tip: Disturbances in esophagogastric junction (EGJ) relaxation have previously been described in extreme older age (> 80 years). The functional consequences of such observations are not known. We investigated several novel metrics of EGJ function - EGJ-contractile integral (EGJ-CI), “total” EGJ-CI during swallowing and EGJ bolus flow time - in young controls and asymptomatic healthy older volunteers (> 80 years). Our findings indicate reduced swallow-induced EGJ relaxation and decreased EGJ bolus flow in older subjects. These findings confirm functional consequences for observations such as increased IRP measurements in older subjects and that caution applies when interpreting EGJ metrics in older patients.
- Citation: Cock C, Besanko LK, Burgstad CM, Thompson A, Kritas S, Heddle R, Fraser RJ, Omari TI. Age-related impairment of esophagogastric junction relaxation and bolus flow time. World J Gastroenterol 2017; 23(15): 2785-2794
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2785.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2785
The esophagogastric junction (EGJ) is an anatomically and physiologically complex region, with several functions such as preventing gastro esophageal reflux, while being able to allow bolus passage during swallowing, evacuation of air during belching or gastric contents during vomiting[1-3]. The EGJ consists of a combination of the lower esophageal sphincter (LES) and diaphragmatic crura. The LES may be anatomically aligned with the crural diaphragm (CD) or misaligned in the form of a hiatus hernia. The lower esophageal sphincter is a smooth muscle region in the lower esophagus, tonically contracted at rest, but with the capacity for swallow, reflex or distention-based relaxation[1,2]. The LES receives vagal input from the brainstem via myenteric non-adrenergic non-cholinergic neurons[4], which release primarily nitric oxide, but also vasoactive intestinal peptide in order to induce LES relaxation[5,6]. Passage of bolus through the EGJ region requires relaxation of the LES, aided by distraction of the LES region and CD by contraction of distal esophageal longitudinal muscle. The CD is thus an important constituent of the EGJ and therefore the term EGJ is thus preferred over LES to functionally describe this region[1].
The use of high-resolution manometry and the Chicago classification system for the description of esophageal pressure topography has necessitated the development of novel measures for the anatomical description of the EGJ, but also for the assessment of barrier function, swallow-induced relaxation and functional bolus clearance at the EGJ. Several novel metrics have been described in order to measure these different functional aspects at the EGJ. In terms of its barrier function at rest (preventing gastroesophageal reflux disease - GERD), the metric esophagogastric contractile integral (EGJ-CI) has been shown to be superior to other EGJ metrics in distinguishing GERD patients with and without proton-pump inhibitor response[7,8], as well as distal esophageal acid exposure[9] and could differentiate patients with achalasia or anti-reflux surgery from controls[10]. For the assessment of swallow-induced EGJ relaxation, Pandolfino et al[11] described the integrated relaxation pressure in four seconds (IRP4). This measure and specifically the time interval chosen, was shown to be superior to other iterations of IRP or metrics describing EGJ relaxation of clinical relevance[11,12]. More recently Lin et al[13,14] described the novel metric bolus flow time (BFT) at the EGJ. This metric determines bolus clearance at the EGJ and is reduced in achalasia[13] or other circumstances denoting reduced bolus clearance through the EGJ, such as ineffective esophageal motility[14].
We have recently described changes in the distal esophagus of individuals aged over eighty years, including reduced peristaltic vigor and clearance[15], as well as reduced EGJ relaxation in both healthy and dysphagic aged individuals[16-18]. Both reduced clearance and decreased EGJ relaxation mimic the circumstances under which a reduced bolus flow time had previously been described by Lin et al[13,14] and it would thus be of value to further assess EGJ function in the aged population, using the BFT. Furthermore, it is known that older individuals have reduced symptoms in relation to the severity of gastroesophageal reflux disease[19] and thus an assessment of EGJ barrier function would be additionally useful in this population, but the recent descriptions of EGJ barrier function metrics have not been assessed in this age cohort.
The aims of this study were to evaluate different aspects of esophagogastric junction function in asymptomatic individuals over eighty years of age compared to young controls: (1) EGJ barrier function at rest through the novel metric EGJ-CI; (2) Swallow induced EGJ-relaxation through the Chicago classification metric integrated relaxation pressure (IRP4); and (3) EGJ bolus flow through the pressure-flow metric bolus flow time (BFT) and bolus presence in the distal esophagus through bolus presence time (BPT).
Forty-five healthy volunteers (20 M, aged 20-93 years) were recruited through community advertisement. A screening history was performed in all subjects to exclude (1) past or present swallowing difficulties; (2) symptoms suggestive of a motility disorder; (3) upper gastrointestinal conditions including gastro-esophageal reflux disease; (4) diabetes mellitus; (5) previous history of gastrointestinal surgery; and (6) prescription medications known to affect GI motility. To further exclude underlying dysphagia, all potential subjects performed a previously validated Dakkak questionnaire[20] to assess the esophageal phase of swallowing for different food consistencies. Only subjects with a normal score were included in the study. Body weight and height, and current or past smoking history were also recorded. Enrolled subjects were stratified into the following two groups: younger controls and older subjects (> 80 years).
The study protocol was approved by the Southern Adelaide Clinical Human Research Ethics Committee (Approval No. 403.10). All participants gave written informed consent prior to enrolment, and studies were performed at the Repatriation General Hospital, Daw Park, South Australia. AIM pressure-flow analysis of the pharynx[21] and distal esophagus[15] had previously been reported in this cohort of subjects.
Subjects were studied in a sitting posture using an MMS Solar (Solar GI acquisition system, MMS, Enschede, The Netherlands) manometric assembly with a 10 French (3.2 mm diameter) unidirectional catheter (Unisensor Inc, Attikon, Switzerland) with 25 pressure (1cm spaced) and 12 impedance segments (2 cm length) straddling the esophagogastric segment with at least 2-3 sensors in the stomach. Pressure and impedance data were recorded at 20Hz.
Following nasal administration of co-phenylcaine forte spray and 2% lignocaine gel, subjects were intubated with the sensors in a posterior orientation. The HRIM assembly was positioned with the recording segment spanning the esophageal transition zone to proximal stomach. Following a 10-min accommodation period, subjects received five 5ml and 10 mL boluses of liquid (0.9% NaCl) and standardized viscous bolus (EFT Viscous Swallow Challenge Medium, viscosity 13000 cP; Sandhill Scientific, Denver, Co. United States) via a syringe and asked to swallow once on cue. Studies were performed in the upright posture with head in a neutral position.
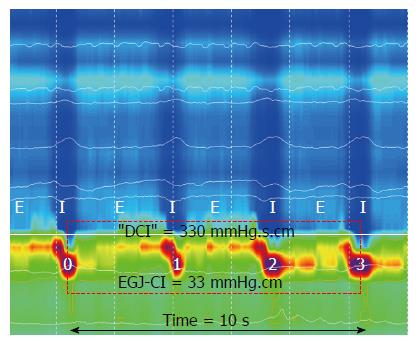
Method for the determination of EGJ-CIis described in Figure 1. EGJ-CI was measured by assessing EGJ function in the rest period, i.e., prior to the onset of swallowing boluses. During calculation of the EGJ-CI, EGJ pressure was measured relative to intra gastric pressure, as the distal esophageal/gastric pressure differential is an important determinant of distal esophageal acid reflux[22]. The isobaric contour was set at +2 mmHg above the intra gastric pressure. The distal contractile integral “box” was then placed around a three-respiratory cycle segment, starting with diaphragmatic contraction. The value obtained in the box (mmHg.s-1.cm-1) was divided by the total duration of the three respiratory cycles to calculate EGJ-CI (mmHg.cm-1). “Total” EGJ-CI[23] was determined by calculating the measurement within a “DCI”-box at the EGJ, using the 2 mmHg above intra gastric pressure isobar contour, during ten liquid swallows and dividing this value by the total duration in seconds.
Swallow induced EGJ relaxation was determined during liquid and viscous swallows by measuring the integrated relaxation pressure in four seconds at the EGJ. This value is determined as the lowest pressure for four contiguous or non-contiguous seconds within the ten seconds following swallow-induced LES relaxation, measured from UES onset, where visible, or impedance drop below 90% of the resting value at the most proximal impedance segment. In practice the IRP tool in the MMS software was used for this measurement. IRP4 is expressed in mmHg.
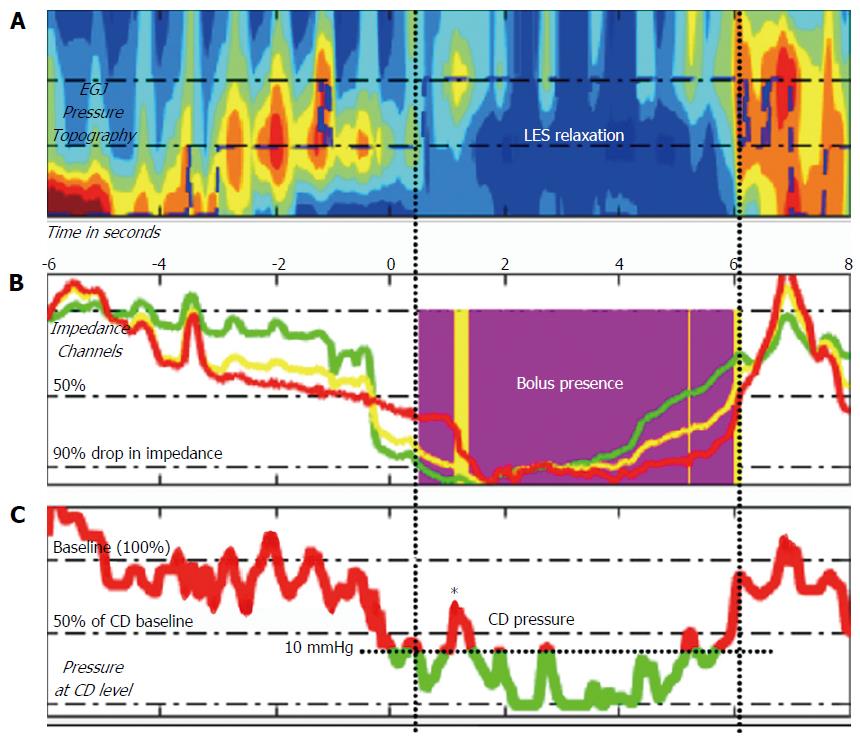
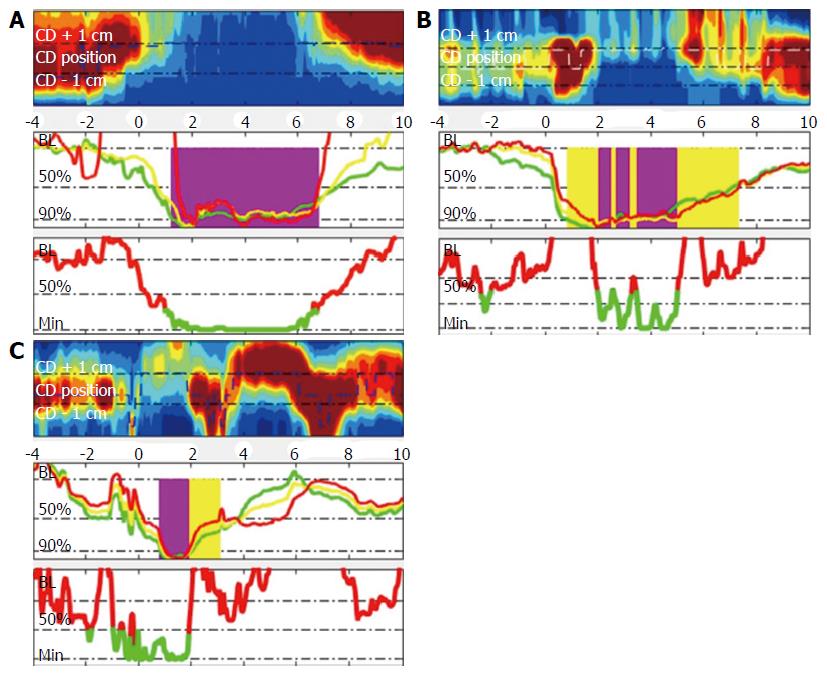
Method for the determination of bolus flow time (BFT) is described in Figure 2. Bolus flow time was determined based on the method originally described by Lin et al[14]. Text files were exported as thirty second segments including the swallow sequences. These files were then imported into Matlab and analysed using an adapted version of the script esophageal AIMplot version 5.0 (T.Omari, Flinders University; Adelaide, Australia). The methodology for esophageal AIMplot pressure flow analysis is described elsewhere[15,24,25]. Specifically, as relates to the measurement of BFT and BPT the method is as described below (Figure 2):
A virtual e-sleeve of pressure and impedance data at the EGJ was created. Pressure at the most proximal pressure channel in this region and intragastric pressure was used as reference values to determine bolus flow and directionality of such flow. Bolus flow from the esophagus to the stomach was determined to have occurred (with the commensurate time included in the BFT) when: (1) Impedance in the three impedance segments at and above the level of the EGJ dropped to below 90% of baseline (without having returned above 50% at which point flow ceases); (2) Pressure at the EGJ dropped to below 50% of baseline; and (3) Diaphragmatic crural contraction pressures were below 10 mmHg and remained at less than 50% of the baseline.
Bolus flow time and bolus presence time were reported in seconds. Impedance ratio is reported as implied as a ratio of the impedance at maximal luminal occlusion to that at maximal luminal distention.
Data were analysed using Sigmaplot 13 (Systat Software Inc., San Jose, CA, United States) and Prism Plus 6.0 (Graphpad, San Diego, CA, United States). Data was assessed for a normal distribution using the D’Agostino & Pearson omnibus test. Pairwise comparisons were done via independent sample t-test or Mann Whitney U-test when non-normally distributed. Fisher’s exact test was done to compare the proportions of subjects with different EGJ-subtypes. Data presented are mean ± SEM. A P value of < 0.05 considered statistically significant.
Characteristics of study participants are included in Table 1. All subjects tolerated the study procedure well and no adverse events were reported. The mean age of older subjects (n = 15; aged 85 ± 4 years, 9 M) was significantly higher than the younger group (n = 30; aged 37 ± 11 years, 11 M) (P < 0.001).
| Control | Older | P value | |
| Number (M/F) | 30 (11/19) | 15 (9/6) | 0.14 |
| Age ± SD (range) | 37 ± 11 (21-58) yr | 85 ± 4 (80-93) yr | < 0.001 |
| EGJ subtype | 0.69 | ||
| I | 18 (60) | 9 (60) | |
| II | 9 (30) | 3 (20) | |
| IIIa | 1 (3) | 1 (7) | |
| IIIb | 2 (7) | 2 (13) | |
| Proximal margin EGJ (cm) | 43 ± 0.6 | 45 ± 1 | 0.06 |
| Overall length EGJ1 (cm) | 3.2 ± 0.2 | 3.4 ± 0.3 | 0.34 |
There was no significant difference in the distribution of EGJ subtypes between controls and older subjects in a sitting posture (Table 1). Specifically, there was no increase in the Type III EGJ, associated with hiatus hernia, in the older subjects.
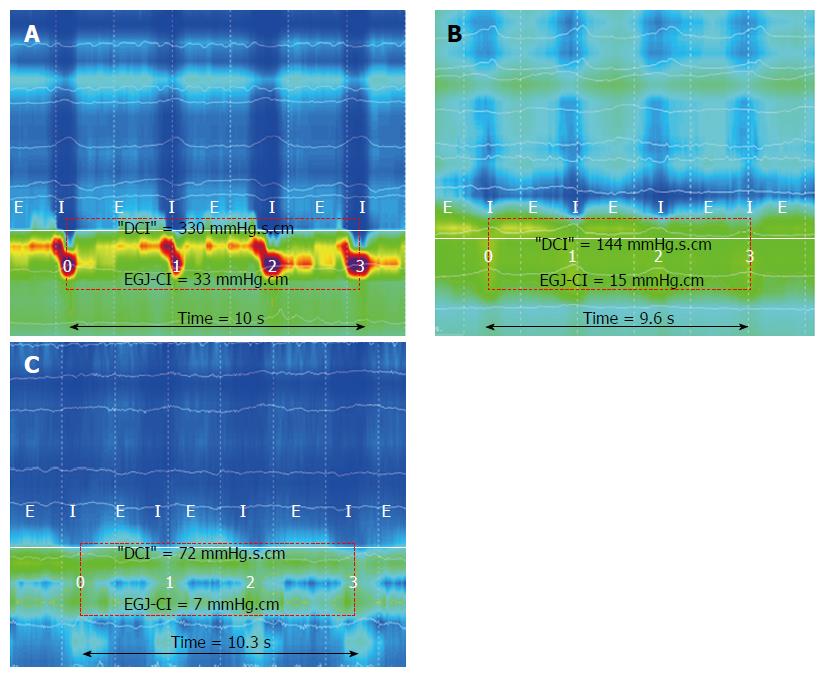
Examples of EGJ-CI calculation are shown in Figure 3. EGJ-CI was similar for older subjects (O) compared to younger controls (C) (O 34 ± 5 mmHg.cm vs C 25 ± 5 mmHg.cm, P = 0.18). Three older and six control subjects (20%) had EGJ-CI values below 20 mmHg.cm, within the range previously shown to be associated with gastro esophageal reflux disease[8]. Intragastric pressure was higher in older subjects compared to the younger group (Liquid: O 9 ± 2 mmHg vs C 2 ± 2 mmHg, P = 0.002; Viscous: O 11 ± 2 mmHg vs C 4 ± 2 mmHg, P = 0.005). Due to decreased swallow-induced relaxation, “total” EGJ-CI was increased in older individuals when compared to young controls (O 39 ± 7 mmHg.cm vs C 18 ± 3 mmHg.cm; P = 0.006).
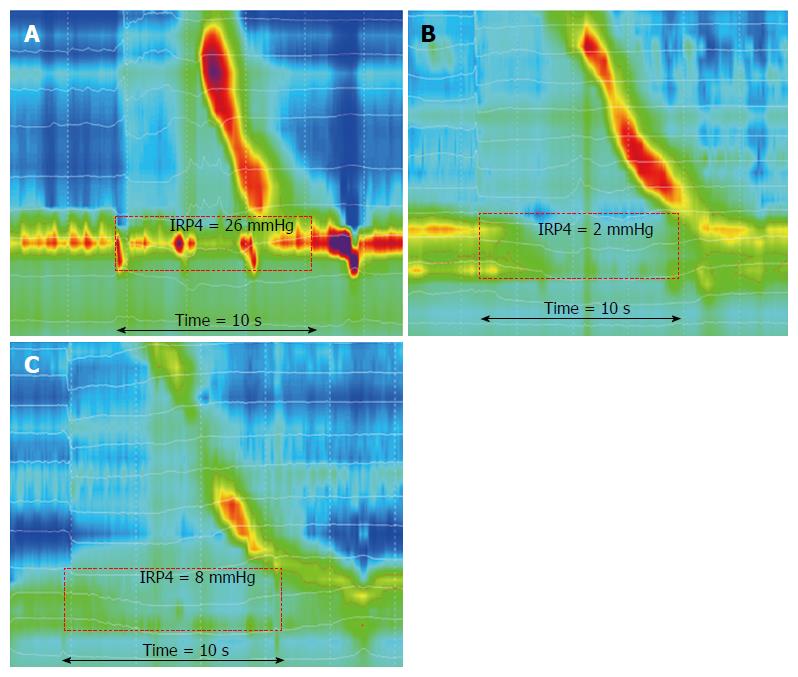
Examples of EGJ swallow-induced relaxation are shown in Figure 4. The EGJ relaxation pressure (IRP4) was significantly higher in older adults for both liquid (P = 0.02) and viscous (P = 0.02) swallows, when compared to the younger group (Table 2). Age had no effect on the nadir EGJ pressure for either bolus consistency. Despite increased IRP, older individuals did not display the esophagogastric outflow obstruction phenotype as defined by an increase in intrabolus pressure at the 30 mmHg isobar contour.
| Liquid swallows | Viscous swallows | |||||
| Control | Older | P value | Control | Older | P value | |
| IRP4 (mmHg) | 5.9 ± 1.0 | 11.9 ± 2.3 | 0.02 | 7.3 ± 0.8 | 14.3 ± 2.4 | 0.02 |
| GasP (mmHg) | 2.2 ± 1.5 | 9.4 ± 1.6 | 0.002 | 4.1 ± 1.6 | 11.2 ± 1.7 | 0.005 |
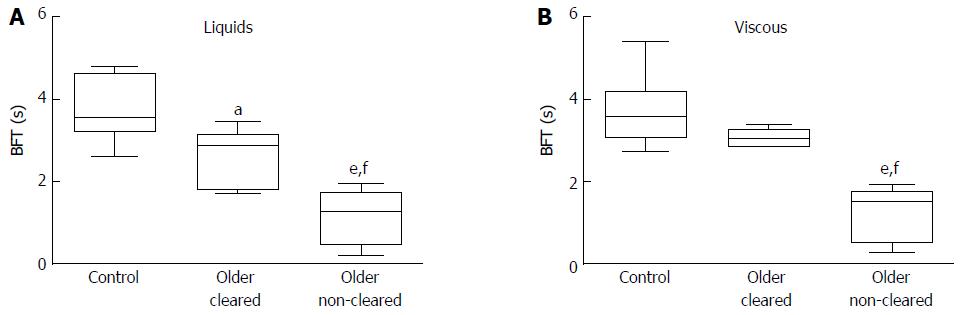
| BPT (s) | 5.5 ± 1.0 | 3.9 ± 0.5 | 0.01 | 4.5 ± 0.2 | 4.3 ± 0.5 | 0.19 |
| BFT (s) | 3.8 ± 0.2 | 1.7 ± 0.3 | < 0.001 | 3.8 ± 0.2 | 1.9 ± 0.3 | < 0.001 |
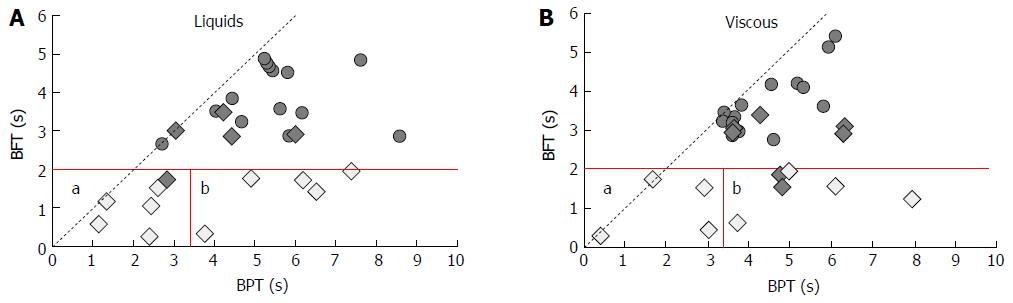
Data for EGJ bolus presence time (BPT) and bolus flow time (BFT) are shown in Table 2 and examples are shown in Figure 5. Bolus flow time is markedly reduced in older individuals for both consistencies (P < 0.001). There was a negative correlation between BFT and the IRP4 (r = -0.42, P = 0.02) for all subjects. Bolus flow time was lowest in older subjects with reduced impedance-based clearance (Figure 6).
This is the first study to report the influence of aging on several novel metrics assessing esophagogastric junction function. EGJ barrier function is assessed through the EGJ-CI, while swallow-induced EGJ relaxation is assessed through the IRP4 and associated bolus passage through bolus flow time (BFT). Our study shows evidence of (1) unchanged EGJ barrier function as measured via EGJ-CI; (2) reduced swallow-induced EGJ relaxation, which also increases the “total” EGJ-CI, which is measured during swallowing; and (3) reduced bolus flow time during both liquid and viscous swallowing, in aging. There is no evidence to support EGJ barrier dysfunction as a significant pathogenic factor in the increased incidence of gastro esophageal reflux disease reported in aging[26]. However, reduced EGJ relaxation in concert with greater intragastric pressure and reduced distal esophageal bolus clearance implies potential prolonged retention of gastric refluxate in the distal esophagus, potentially leading to greater mucosal damage by the refluxate. Due to an increased sensory threshold, reflux symptoms may not be perceived by the aging reflux patient[26,27]. A low threshold should be maintained for further clinical assessment (e.g., via endoscopy) of upper gastrointestinal symptoms in older subjects and a recognition that older subjects do not always present with typical symptoms.
Older patients with gastroesophageal reflux disease often present with atypical symptoms, including dysphagia[28], have an increased prevalence of erosive reflux disease[19,28] and also have associated motility disturbances[29]. Our findings that esophageal barrier function, as measured through EGJ-CI, is unchanged in older individuals have important implications for the assessment of aged patients with gastroesophageal reflux disease or undergoing high-resolution manometry for other indications. Whilst the resting EGJ-CI is congruent with “total” EGJ-CI in young subjects, this is not the case in subjects aged greater than eighty years. This is because decreased swallow-induced LES relaxation in these older subjects would increase the measured EGJ-CI during swallowing (“total” EGJ-CI). “Total” EGJ-CI would not be a reliable measurement of EGJ barrier function in older subjects and should not be clinically used to determine such function.
Our findings of a similar EGJ-CI in older subjects and younger controls are in keeping with those of Bardan et al[30] who showed similar LES resting pressure in healthy older volunteers as compared to younger in a supine posture. Other studies have shown either higher[16] or lower[17] resting pressures in aged individuals. In terms of the functional consequences of impaired EGJ barrier function, Lee et al[26] described increased distal esophageal acid exposure related to dysmotility and reduced acid clearance in older subjects with reflux disease. Their study also showed increased esophageal abdominal length[26]. Other studies have likewise shown increased prevalence of hiatus hernia in aging[28]. Our study in older subjects studied in a sitting posture did not find an increased prevalence of hiatus hernia as assessed by EGJ “subtypes”. Our study did, however, find decreased swallow-induced relaxation, discussed further below. In this context, care needs to be taken in exactly how the EGJ-CI is calculated, i.e., whether at rest or during swallowing, with only values at rest being clinically relevant in subjects over eighty, as discussed above.
Our findings demonstrating reduced swallow-induced EGJ relaxation in healthy aging, is consistent with a previous study by Besanko et al[18], which showed decreased swallow-induced relaxation, as measured through the IRP4, in healthy older adults over eighty years. Likewise, Jung et al[31] also showed a significant correlation of IRP4 with age and aging. The finding of decreased swallow-induced EGJ relaxation with aging is consistent with degeneration of myenteric lower motor neurons. Degeneration of such neurons have previously been demonstrated in aging animals[32] and humans[33]. Myenteric neurons and in particular cholinergic neurons[34], seem to represent a vulnerable subpopulation when compared to neuronal cells elsewhere in the body[35]. Furthermore, our findings indicating more proximal bolus retention[15] also implies decreased distal esophageal distention[36], decreasing the stimulus for nitrinergic distention-based EGJ relaxation[37]. Lastly, aged subjects have decreased esophageal sensory function and by implication a lesser perception of the stimulus for bolus/distention-based EGJ relaxation. The clinical implications of these findings are the potential for prolonged retention of refluxed contents, leading to the observed increase in erosive reflux disease in this population, but also longer esophageal retention of swallowed contents leading to a higher prevalence of “pill” esophagitis; and increased prevalence of esophageal dysphagia symptoms (or asymptomatic swallowing dysfunction) in the aging population. Esophageal bolus transit is reduced in this population[15] and thus an additional factor of decreased swallow-induced LES relaxation may change borderline bolus transport into clinically relevant dysphagia.
In our study, bolus flow time (BFT) at the EGJ was markedly reduced in older subjects when compared to controls. Further analyses revealed BFT was most markedly reduced in those individuals with impaired esophageal bolus clearance (Figure 6). Reduced EGJ bolus flow time has previously been shown in association with ineffective esophageal motility[14]. By adding bolus presence time (BPT) at the most distal impedance segments above the EGJ, we can draft a matrix (Figure 7) representing the main causes of reduced BFT, namely (1) reduced bolus clearance (reduction in both BPT and BFT) and (2) increased EGJ flow resistance, similarly to that in achalasia[13] (reduced BFT with increase in BPT). Our previous data suggested reduced overall esophageal bolus clearance[15] in older volunteers. Further assessment of the esophageal bolus clearance through a BFT/BPT matrix revealed an equivalent proportion of failed clearance due to ineffective esophageal motility and increased EGJ flow resistance, revealing that both these factors play a role in reduced esophageal bolus clearance in older individuals and as described above may lead to clinically relevant dysphagia.
Our study has some limitations. We did not record pH-metry, in order to assess the implications of potential changes in EGJ barrier function as measured in our study, as we could not justify acquiring pH-metry in asymptomatic volunteers. A specific assessment of EGJ metrics in aged individuals undergoing pH studies would be of value. Subjects were excluded if they reported reflux related symptoms or were on anti-reflux medications (other than occasional over the counter medications). We cannot exclude having inadvertently included asymptomatic individuals with reflux disease in our study. Furthermore, transient lower esophageal sphincter relaxations (TLESR’s) were not assessed during our study. Our study was not designed to assess TLESR’s, but a study of TLESR activity in aging would be of great value as to our knowledge, TLESR’s have never been specifically assessed in the aged population.
Our study showed evidence of similar EGJ barrier function at rest, but not during swallowing; reduced swallow-induced relaxation and markedly reduced bolus flow time (BFT) at the EGJ in older individuals. The use of a BPT/BFT matrix allowed us to determine different causes for reduced BFT in aging, indicating equivalent numbers being due to failed bolus clearance and increased EGJ flow resistance. Our study has important implications for better understanding mechanisms of failed bolus clearance in older individuals and in guiding investigation in older subjects with gastroesophageal reflux disease, where non-clearance of refluxate predisposes older subjects to increases in distal esophageal acid exposure, potentially explaining the increased prevalence of severe reflux esophagitis in older GERD patients[19]. Our study also implies the EGJ, in addition to the oropharynx and distal esophagus, should be a focus during investigation and may be a potential therapeutic target (e.g., for dilatation) in aged patients with dysphagia.
Decreased swallow-induced esophagogastric junction (EGJ) relaxation had previously been demonstrated in aged subjects over eighty years. We conducted a study to manometrically assess the functional consequences of such findings.
This study used novel analysis techniques of high-resolution impedance manometry recordings to determine the functional effects on bolus transport at the EGJ and postulate on the clinical implications in relation to reflux disease and dysphagia in older patients.
Novel analyses in determining EGJ barrier function, namely EGJ-contractile integrals are described for the first time in this population. Bolus flow time determined through pressure flow analysis at the EGJ is described for the first time in this population.
These data has clinical application when interpreting manometry recordings in older subjects over eighty years and may have further clinical utility in determining which patients may benefit from endoscopic dilatation of the EGJ (not measured in this study).
The study analyses functional effects of abnormal EGJ measurements in asymptomatic healthy volunteers over eighty years of age. EGJ functions were evaluated by using EGJ-contractile integral (EGJ-CI), “total” EGJ-CI and bolus flow time (BFT). A matrix of bolus flow and presence above the EGJ indicated reductions in bolus flow at the EGJ occurred due to both impaired bolus transport through the esophageal body and increased flow resistance at the EGJ. The authors concluded that this study has important implications for better understanding mechanisms of failed bolus clearance in older individuals and in guiding investigation in older subjects with gastroesophageal reflux disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ding XW, Reeh M S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Mittal RK, Goyal RK. Sphincter mechanisms at the lower end of the esophagus. GI Motility online. 2006. . [DOI] [Cited in This Article: ] |
| 2. | Mittal RK. Lower esophageal sphincter in Motor function of the Pharynx, Esophagus, and its sphincters. Morgan and Claypool Life Sciences. St Rafael, CA, 2011. . [Cited in This Article: ] |
| 3. | Hershcovici T, Mashimo H, Fass R. The lower esophageal sphincter. Neurogastroenterol Motil. 2011;23:819-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Goyal RK, Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest. 1975;55:1119-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 124] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Murray J, Du C, Ledlow A, Bates JN, Conklin JL. Nitric oxide: mediator of nonadrenergic noncholinergic responses of opossum esophageal muscle. Am J Physiol. 1991;261:G401-G406. [PubMed] [Cited in This Article: ] |
| 6. | Yamato S, Saha JK, Goyal RK. Role of nitric oxide in lower esophageal sphincter relaxation to swallowing. Life Sci. 1992;50:1263-1272. [PubMed] [Cited in This Article: ] |
| 7. | Tolone S, De Bortoli N, Marabotto E, de Cassan C, Bodini G, Roman S, Furnari M, Savarino V, Docimo L, Savarino E. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil. 2015;27:1423-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Nicodème F, Pipa-Muniz M, Khanna K, Kahrilas PJ, Pandolfino JE. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-Contractile Integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol Motil. 2014;26:353-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Xie C, Wang J, Li Y, Tan N, Cui Y, Chen M, Xiao Y. Esophagogastric Junction Contractility Integral Reflect the Anti-reflux Barrier Dysfunction in Patients with Gastroesophageal Reflux Disease. J Neurogastroenterol Motil. 2017;23:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Wang D, Patel A, Mello M, Shriver A, Gyawali CP. Esophagogastric junction contractile integral (EGJ-CI) quantifies changes in EGJ barrier function with surgical intervention. Neurogastroenterol Motil. 2016;28:639-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying EGJ morphology and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1033-G1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878-G885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Lin Z, Carlson DA, Dykstra K, Sternbach J, Hungness E, Kahrilas PJ, Ciolino JD, Pandolfino JE. High-resolution impedance manometry measurement of bolus flow time in achalasia and its correlation with dysphagia. Neurogastroenterol Motil. 2015;27:1232-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Lin Z, Imam H, Nicodème F, Carlson DA, Lin CY, Yim B, Kahrilas PJ, Pandolfino JE. Flow time through esophagogastric junction derived during high-resolution impedance-manometry studies: a novel parameter for assessing esophageal bolus transit. Am J Physiol Gastrointest Liver Physiol. 2014;307:G158-G163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Cock C, Besanko L, Kritas S, Burgstad CM, Thompson A, Heddle R, Fraser RJ, Omari TI. Impaired bolus clearance in asymptomatic older adults during high-resolution impedance manometry. Neurogastroenterol Motil. 2016;28:1890-1901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Andrews JM, Fraser RJ, Heddle R, Hebbard G, Checklin H. Is esophageal dysphagia in the extreme elderly (& gt; or=80 years) different to dysphagia younger adults? A clinical motility service audit. Dis Esophagus. 2008;21:656-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Besanko LK, Burgstad CM, Mountifield R, Andrews JM, Heddle R, Checklin H, Fraser RJ. Lower esophageal sphincter relaxation is impaired in older patients with dysphagia. World J Gastroenterol. 2011;17:1326-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Besanko LK, Burgstad CM, Cock C, Heddle R, Fraser A, Fraser RJ. Changes in esophageal and lower esophageal sphincter motility with healthy aging. J Gastrointestin Liver Dis. 2014;23:243-248. [PubMed] [Cited in This Article: ] |
| 19. | Johnson DA, Fennerty MB. Heartburn severity underestimates erosive esophagitis severity in elderly patients with gastroesophageal reflux disease. Gastroenterology. 2004;126:660-664. [PubMed] [Cited in This Article: ] |
| 20. | Dakkak M, Bennett JR. A new dysphagia score with objective validation. J Clin Gastroenterol. 1992;14:99-100. [PubMed] [Cited in This Article: ] |
| 21. | Omari TI, Kritas S, Cock C, Besanko L, Burgstad C, Thompson A, Rommel N, Heddle R, Fraser RJ. Swallowing dysfunction in healthy older people using pharyngeal pressure-flow analysis. Neurogastroenterol Motil. 2014;26:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Scheffer RC, Gooszen HG, Hebbard GS, Samsom M. The role of transsphincteric pressure and proximal gastric volume in acid reflux before and after fundoplication. Gastroenterology. 2005;129:1900-1909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Jasper D, Freitas-Queiroz N, Hollenstein M, Misselwitz B, Layer P, Navarro-Rodriguez T, Fox M, Keller J. Prolonged measurement improves the assessment of the barrier function of the esophago-gastric junction by high-resolution manometry. Neurogastroenterol Motil. 2017;29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Omari TI, Wauters L, Rommel N, Kritas S, Myers JC. Oesophageal pressure-flow metrics in relation to bolus volume, bolus consistency, and bolus perception. United European Gastroenterol J. 2013;1:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Rommel N, Van Oudenhove L, Tack J, Omari TI. Automated impedance manometry analysis as a method to assess esophageal function. Neurogastroenterol Motil. 2014;26:636-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Lee J, Anggiansah A, Anggiansah R, Young A, Wong T, Fox M. Effects of age on the gastroesophageal junction, esophageal motility, and reflux disease. Clin Gastroenterol Hepatol. 2007;5:1392-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Chen CL, Yi CH, Liu TT, Orr WC. Altered sensorimotor responses to esophageal acidification in older adults with GERD. Scand J Gastroenterol. 2010;45:1150-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Pilotto A, Franceschi M, Leandro G, Scarcelli C, D’Ambrosio LP, Seripa D, Perri F, Niro V, Paris F, Andriulli A. Clinical features of reflux esophagitis in older people: a study of 840 consecutive patients. J Am Geriatr Soc. 2006;54:1537-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Gutschow CA, Leers JM, Schröder W, Prenzel KL, Fuchs H, Bollschweiler E, Bludau M, Hölscher AH. Effect of aging on esophageal motility in patients with and without GERD. Ger Med Sci. 2011;9:Doc22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 30. | Bardan E, Xie P, Brasseur J, Dua K, Ulualp SO, Kern M, Shaker R. Effect of ageing on the upper and lower oesophageal sphincters. Eur J Gastroenterol Hepatol. 2000;12:1221-1225. [PubMed] [Cited in This Article: ] |
| 31. | Jung KW, Jung HY, Myung SJ, Kim SO, Lee J, Yoon IJ, Seo SY, Lee JH, Kim DH, Choi KD. The effect of age on the key parameters in the Chicago classification: a study using high-resolution esophageal manometry in asymptomatic normal individuals. Neurogastroenterol Motil. 2015;27:246-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Santer RM, Baker DM. Enteric neuron numbers and sizes in Auerbach’s plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988;25:59-67. [PubMed] [Cited in This Article: ] |
| 33. | de Souza RR, Moratelli HB, Borges N, Liberti EA. Age-induced nerve cell loss in the myenteric plexus of the small intestine in man. Gerontology. 1993;39:183-188. [PubMed] [Cited in This Article: ] |
| 34. | Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Saffrey MJ. Cellular changes in the enteric nervous system during ageing. Dev Biol. 2013;382:344-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Gregersen H, Pedersen J, Drewes AM. Deterioration of muscle function in the human esophagus with age. Dig Dis Sci. 2008;53:3065-3070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Brookes SJ, Chen BN, Hodgson WM, Costa M. Characterization of excitatory and inhibitory motor neurons to the guinea pig lower esophageal sphincter. Gastroenterology. 1996;111:108-117. [PubMed] [Cited in This Article: ] |