Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2673
Peer-review started: December 30, 2014
First decision: January 19, 2017
Revised: February 13, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 21, 2017
To determine the effect of overexpression of fibrinogen-like protein 2 (FGL2) on regulatory T cell (Treg) and effector T (Teff) cell function on T cell-induced colitis in Rag1-/- mice.
Treg and Teff cells from fgl2-/-, fgl2+/+, and fgl2Tg mice were purified by FACS. They were studied in vitro for immunosuppressive activity and cell proliferation and in vivo for their effects on the development and prevention of T cell-induced colitis in Rag1-/- mice.
In vitro, fgl2Tg Treg had enhanced immunosuppressive activity, and fgl2Tg Teff had reduced proliferation to alloantigen stimulation. Transfer of Teff from C57Bl/6J mice (fgl2+/+) into Rag1-/- mice produced both clinical and histologic colitis with dense infiltrates of CD3+ T cells, crypt abscesses and loss of goblet cells. Fgl2Tg Treg prevented the development of T cell-induced colitis, whereas fgl2+/+ and fgl2-/- Treg were only partially protective. In mice that received fgl2Tg Treg, the ratio of Foxp3+ to CD3+ cells was increased both in the colon and in mesenteric lymph nodes, and Teff cell proliferation as determined by staining with Ki67 was reduced. Teff cells from fgl2Tg mice did not produce colitis.
Here we show that fgl2Tg Teff are hypoproliferative and do not induce colitis. We further demonstrate that fgl2Tg Treg prevent colitis in contrast to fgl2+/+ Treg, which were only partially protective. These studies collectively provide a rationale for exploring the use of FGL2 or Treg expressing high levels of FGL2 in the treatment of inflammatory bowel disease.
Core tip: This study investigates the effect of overexpression of fibrinogen-like protein 2 (FGL2) on T cell-induced colitis in mice. For these experiments, effector T cells (Teff) and regulatory T cells (Treg) were isolated from a newly generated line of transgenic mice that ubiquitously overexpress FGL2 (fgl2Tg). Following injection in Rag1-/- mice, fgl2Tg Treg were present in high numbers in mesenteric lymph nodes and were superior to fgl2+/+ Treg in preventing T cell-induced colitis. Fgl2Tg Teff were not capable of inducing colitis. This work is important in showing that the immunoregulatory molecule FGL2 may be useful in the treatment of colitis.
- Citation: Bartczak A, Zhang J, Adeyi O, Amir A, Grant D, Gorczynski R, Selzner N, Chruscinski A, Levy GA. Overexpression of fibrinogen-like protein 2 protects against T cell-induced colitis. World J Gastroenterol 2017; 23(15): 2673-2684
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2673
Inflammatory bowel disease (IBD) consists of a group of chronic relapsing inflammatory diseases of the gastrointestinal tract including Crohn’s disease (CD) and ulcerative colitis[1]. The onset of colitis is dependent on dysregulated innate and adaptive immune responses to bacterial flora[1-5]. A well-characterized model of IBD caused by infusion of CD4+CD25-CD45RBhigh effector T cells (Teff) into immunodeficient Rag1-/- mice has been used to study the pathogenesis of IBD[2,4,5]. Infusion of Teff cells into Rag1-/- mice leads to the development of colitis[5]. Infusion of CD4+CD25+CD45RBlow regulatory T cells (Treg) has been reported to be protective against the development of T-cell induced colitis[6,7]. It is now known that Treg are a subset of CD4+ T cells that are characterized by the expression of the transcription factor Foxp3[8]. Foxp3+ Treg are important in regulating host immune responses to pathogens and maintaining tolerance[9]. Foxp3+ Treg are comprised of functionally diverse subsets with distinct phenotypes and functions[10]. These cells are known to express a number of important suppressor molecules including IL-10, programmed cell death 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4)[11]. Recently, fibrinogen-like protein 2 (FGL2) has been identified to be an important effector molecule of Treg[10,12].
FGL2 is a member of the fibrinogen-like family of proteins, which was first isolated from a cytotoxic T cell library[13]. When expressed by macrophages and endothelial cells, FGL2 has prothrombinase activity, which contributes to the pathogenesis of experimental and human viral hepatitis[14-16]. When expressed by T cells, FGL2 has immunoregulatory activity[12,17]. The C-terminal of FGL2 contains a FRED domain, which accounts for the immunomodulatory activity and is important in regulating dendritic cell (DC) maturation, T cell proliferation and B cell apoptosis[12,17]. We and others have reported that Treg that express high levels of FGL2 lead to tolerance of fully mismatched heart allografts[18-20]. Studies showed that tolerant grafts contained large numbers of Treg that co-expressed Foxp3 and FGL2, whereas rejecting grafts contained Foxp3+ Treg that were FGL2 negative. Depletion of Treg by antibody to CD25 or by a non-depleting antibody to FGL2 led to loss of tolerance and severe allograft rejection[18,19].
We previously reported that mice lacking FGL2 (fgl2-/-) have a reduction in Treg immunosuppressive activity and develop autoimmune glomerulonephritis[12]. In those studies we showed that T cells from fgl2-/- mice are hyperproliferative and had a skewed Th1 profile, marked by increased IFN-γ and reduced IL-4 expression. Both the number of DC and maturation of DC were increased in fgl2-/- mice. Fgl2-/- mice had a higher percentage of CD4+Foxp3+ T cells in the thymus, spleen and lymph nodes compared with fgl2+/+ mice; however, fgl2-/- Treg had decreased immune-suppressive activity compared with fgl2+/+ Treg[12].
To further determine the role of FGL2 in immune function, we generated ubiquitous FGL2 over-expressing mice (fgl2Tg)[20]. Here we used these mice to determine the importance of FGL2 to the pathogenesis of IBD. We examined both the ability of fgl2Tg T effector cells to induce colitis and the ability of fgl2Tg Treg to prevent colitis. We hypothesized that the over-expression of FGL2 in Treg would lead to protection against T cell-induced colitis.
fgl2+/+, fgl2-/- and fgl2Tg mice were housed at the Ontario Cancer Institute Animal Resource Centre (Toronto, Canada). Rag1-/- and BALB/c mice were purchased from Jackson Laboratory (United States). Experiments were performed on mice 6-12 wk of age.
The generation of fgl2Tg mice has been previously reported[20]. Briefly, a prothrombinase inactive fgl2 gene was inserted into the iZ/EG targeting vector, which was electroporated into R1 ES cells[21]. Chimeric mice were generated using the tetraploid embryo aggregation technique[21]. Following germline transmission, fgl2LoxP mice were crossed with fgl2-/- mice using the Jackson Laboratory speed congenic service followed by two crosses with EIIa-cre; fgl2-/- mice to generate fgl2Tg mice that co-overexpress FGL2 and EGFP ubiquitously. Fgl2Tg mice used for these studies were further backcrossed to fgl2-/- mice on the C57BL/6 background to generate fully congenic mice (N10).
FGL2 concentrations in mouse plasma and culture supernatants were determined by a commercially available ELISA (BioLegend, United States).
Cells were stained using a standard method described by the manufacturer (eBioscience, United States). In brief, a single cell suspension was incubated with Fc Block (eBioscience) on ice for 20 min. Cells were stained with CD4-PC7, CD4-PE-Cy7, Foxp3-PE, CD45RB-APC (eBioscience) and CD25-PE (Miltenyi Biotec, United States) in the dark for 30 min at 4 °C. Viability staining was performed using eFluor450 (eBioscience). For intracellular staining, cells previously stained for membrane proteins were fixed and permeabilized using a Fix/Perm kit (eBioscience). Cells were visualized using the BD LSRII analyzer (BD Biosciences, United States) and data were analyzed using FlowJo software, version 9.6 (Tree Star, United States).
Single cell suspensions of BALB/c SMNC were generated using standard methods. BALB/c SMNC were irradiated with 2000cGy using a γ-irradiator. FACS sorted CD4+CD25- T cells were stained were labeled eFlour 670 dye as per manufacturer’s instructions (eBioscience). 4.0 × 105 BALB/c SMNC were incubated with 2.0 × 105fgl2+/+ or fgl2Tg CD4+ T cells in 96-well U-bottom plates at 37 °C for 3 d. Cells were then stained with anti-CD4-PE-Cy7 and eFluor 450 viability dye and analyzed using flow cytometry.
The Treg suppression assay was adapted from Shalev et al[12]. Fgl2+/+ or fgl2Tg lymphocytes were enriched for CD4+ T cells using the Negative T cell Isolation kit (Miltenyi Biotec). The CD4- lymphocyte fraction was used as antigen presenting cells in the suppression assay. The CD4+ enriched fractions were FACS sorted into CD4+CD25high Treg and CD4+CD25- T cells. All T cell populations were sorted to a purity > 98%. For the suppression assay, 2 × 105 CD4-fgl2+/+ antigen presenting cells were incubated with 0.4 × 105 CD4+CD25-fgl2+/+ T cells and serial dilutions of Treg (either fgl2+/+ or fgl2Tg) starting with a 1:1 ratio of CD4+CD25- T cells to CD4+CD25+ Treg. Cells were incubated in the presence of Concanavalin A at a final concentration of 1 μg/mL for 3 d at 37 °C in RPMI-10. To measure proliferation, 1 μCu of 3H-thymidine was added to culture supernatants and incubated for 18 h. Percent suppression was calculated as previously described[20].
The T cell-dependent model of colitis was adapted from Ostanin et al[5]. Briefly, single cell suspensions of SMNC were enriched for CD4+ T cells using the Negative T cell Isolation kit (Miltenyi Biotec). The CD4+ enriched fraction was stained with CD4-PC7, CD45RB-APC (eBioscience) and CD25-PE (Miltenyi Biotec) and sorted on a BD FACS Aria II cell sorter (BD Biosciences, United States) into CD4+CD25+CD45RBlow Treg and CD4+CD25-CD45RBhigh Teff. CD45RBlow Treg and CD45RBhigh Teff were adjusted to a concentration of 2 × 106 cells/mL and 1 × 107 cells/mL respectively in Hank’s balanced salt solution (HBSS). Sham treated mice were injected i.v. with 100 μL of HBSS; the “no Treg” (Teff only) group was infused with 0.5 × 106 CD45RBhighfgl2+/+ Teff cells; the fgl2+/+ and fgl2Tg Treg-treated groups were infused with 0.5 × 106 CD45RBhighfgl2+/+ Teff cells and 0.1 × 106 CD45RBlow Treg isolated from fgl2+/+ or fgl2Tg mice. Mice were weighed weekly and were sacrificed at 14 wk post cell transfer or when they had lost 20% of body weight.
Tissues were harvested and fixed in 10% buffered formalin solution. Following paraffin-embedding, tissues were sectioned at 4 μm. The hematoxylin and eosin (H&E) stains were employed using standard methods. Staining for CD3+ T cells and Foxp3+ Treg was performed with anti-mouse CD3 (17A2; eBioscience) and anti-mouse Foxp3 (FJK-16S; eBioscience) antibodies. T cell proliferation was assessed by staining formalin fixed tissues with a rat anti-mouse Ki67 (TEC-3; DAKO, Denmark) followed by anti-rat Ig (Vector Laboratories, Canada). Pathological scoring was performed by a blinded pathologist using the scoring system adapted from Aranda et al[3]. A maximum score of 12 points was awarded based on the inflammatory infiltrate in the lamina propria (0-3), the degree of mucin depletion in the large intestine (0-3), the degree of intra-epithelial lymphocytes in the crypts (0-3) and the % of surface area affected (0-3).
CD3 and Foxp3 stained slides were scanned using the Aperio ScanScrope Slide Scanner (Aperio Technologies, United States). Positively stained cells were counted with an algorithm developed with Spectrum software (Aperio Technologies).
All mice were housed in specific pathogen free conditions and fed a standard laboratory diet. Animals were treated in accordance with guidelines set by the Canadian Council for Animal Care and all appropriate measures were taken to minimize pain and discomfort. The animals were acclimatized to laboratory conditions (22 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access for food and water) for two weeks prior to experimentation. All animals were euthanized by barbiturate overdose for tissue collection.
Statistical significance was determined using Students t-test or a one-way or two-way ANOVA as indicated using PRISM v5a (GraphPad Software, United States). P values ≤ 0.05 were considered statistically significant.
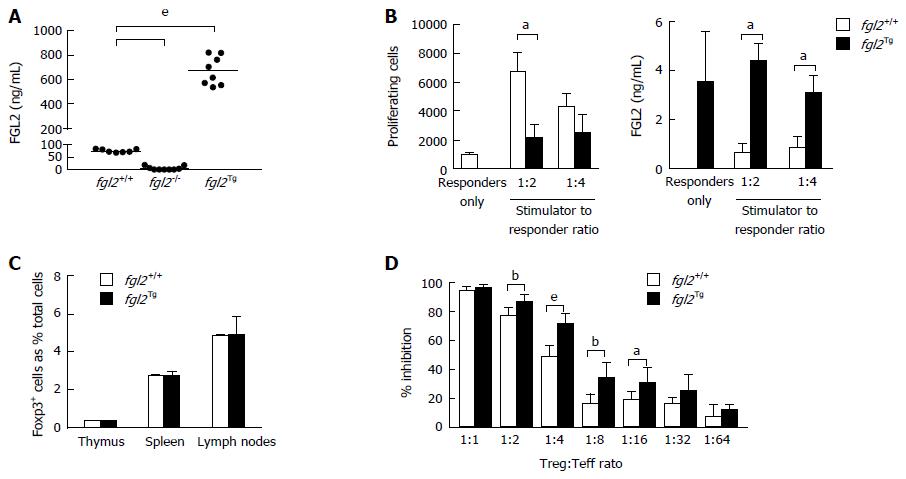
We previously reported on the generation of fgl2Tg mice that were backcrossed on a C57BL/6 background (N4)[20]. Here, we performed additional backcrosses to generate fully congenic fgl2Tg mice (N10). Congenic fgl2Tg mice maintained high expression of FGL2 with plasma levels of FGL2 that were approximately 9-fold higher than fgl2+/+ mice (672.40 ± 117.6 ng/mL vs 75.43 ± 6.24 ng/mL, respectively) (Figure 1A). To examine the effect of over-expression of FGL2 on T cell proliferation, CD4+ T cells were isolated from fgl2Tg mice and stimulated with irradiated BALB/c splenic mononuclear cells (SMNC). Consistent with previous data showing that addition of recombinant FGL2 inhibited T cell proliferation in-vitro, fgl2Tg CD4+ T cells were hypoproliferative compared with fgl2+/+ CD4+ T cells which is likely due to increased levels of FGL2 secreted by fgl2Tg CD4+ T cells (Figure 1B)[17]. Previously, we showed that fgl2-/- Treg have decreased immunosuppressive activity and that Treg suppressive activity could be inhibited with anti-FGL2 antibody[12]. Additionally, we showed that fgl2Tg Treg expressed significantly higher levels of FGL2[20]. Here we found that there were no changes in the frequency of Foxp3+ cells in fgl2Tg mice compared with fgl2+/+ mice (Figure 1C), but fgl2Tg Treg had increased immunosuppressive activity compared with fgl2+/+ Treg (Figure 1D).
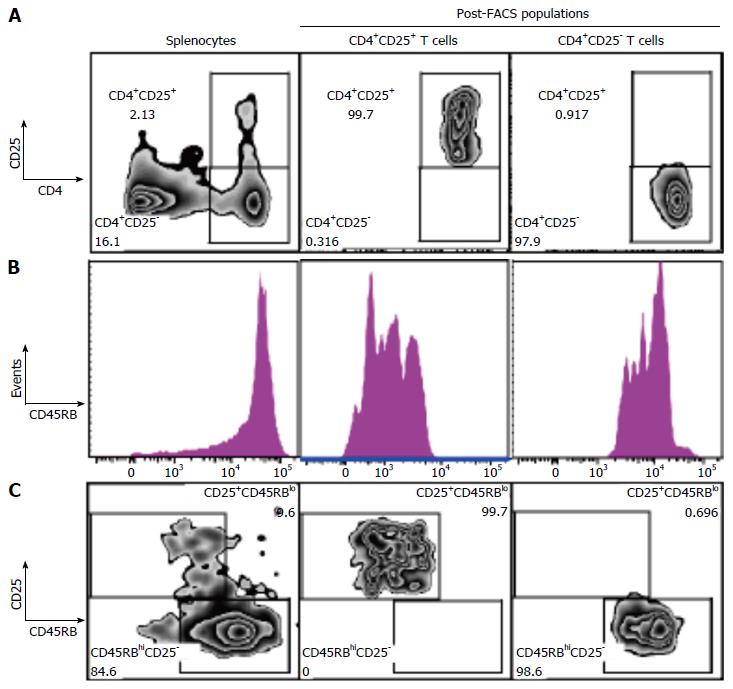
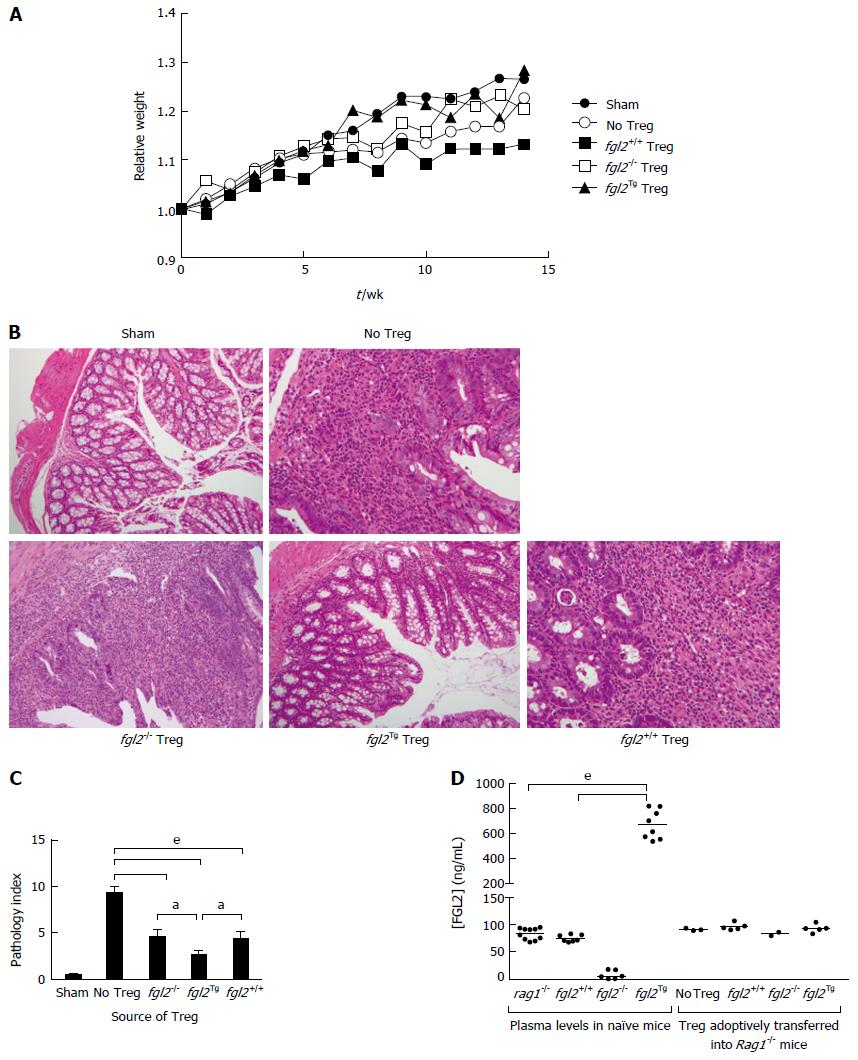
We next studied the effect of fgl2Tg Treg on the development of colitis[2-4]. For these studies, we isolated CD4+CD25+CD45RBlow Treg and CD4+CD25-CD45RBhigh Teff with a purity of > 98% using FACS (Figure 2). Rag1-/- mice received by tail vein injection 0.5 × 106 CD4+CD25-CD45RBhigh T cells isolated from fgl2+/+ mice and were sacrificed 14 wk post-injection. Mice were monitored for signs of disease including, lethargy, piloerection, hunching, dehydration and weight loss. Rag1-/- mice that received CD4+CD25-CD45RBhigh T cells developed clinical signs of disease, including decreased activity and piloerection. In contrast to sham mice, these mice did not gain weight post-transfer of Teff cells (Figure 3A). Mice that received fgl2-/- or fgl2+/+ Treg showed improved clinical activity within the first 4-6 wk, but these mice still demonstrated clinical signs of colitis including lethargy, piloerection and slight hunching. In contrast, all mice that received fgl2Tg Treg appeared clinically normal and had weight gain similar to sham control mice (Figure 3A).
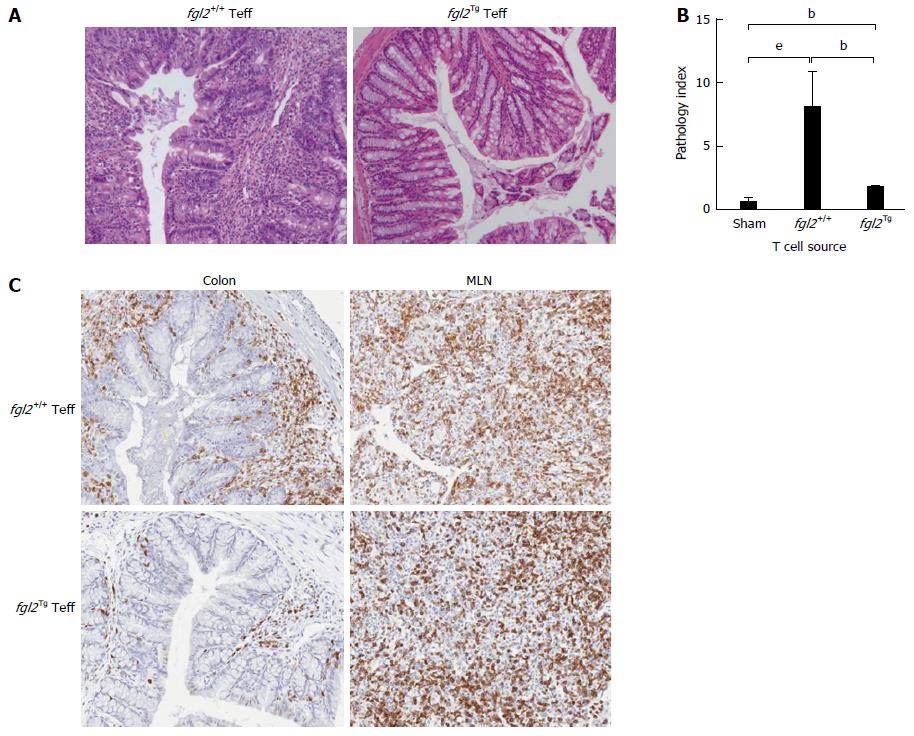
Tissues were harvested and examined histologically from the ileum, the proximal, medial and distal colon at 14 wk post cell transfer. In all groups of mice, the ileum was near normal similar to what has been reported previously by other investigators[5]. As expected, the sham group showed normal colonic architecture with large numbers of goblet cells and normal crypt architecture (Figure 3B). In contrast, colons from mice that received Teff but no Treg cells showed large, diffuse areas of parenchymal disease characterized by villous flattening, large cellular infiltrates, destruction of crypts, severe mucin depletion and loss of goblet cells (Figure 3B). Colons from mice that received fgl2-/- or fgl2+/+ Treg had improved histology but still had patchy areas of colitis marked by T cell infiltrates, destruction of epithelial crypts, mucin depletion and reduced numbers of goblet cells. Histologically, the colons from fgl2Tg Treg treated group were near normal with preserved goblet cells and few if any mononuclear cell infiltrates. Fgl2-/- mice had more severe disease compared to mice that received fgl2+/+ Treg, but by morphometry the difference in severity of colonic disease did not reach statistical significance (Figure 3B).
Changes in histology were quantified using a modified pathology index established by Aranda et al[3] with a maximum pathology score of 12. Mice in the no Treg group had the highest score indicative of a pan diffuse colitis with disease across the proximal, mid and distal colon associated with diffuse parenchymal destruction, crypt loss, mucin depletion and dense lymphocyte infiltrates (Figure 3C). Mice that received fgl2-/- or fgl2+/+ Treg had similar overall pathology scores, which were significantly better than mice in the no Treg group. Colons from mice that received fgl2Tg Treg were near normal with normal numbers of crypt goblet cells; however, there were occasional patchy foci of inflammation and small numbers of crypt abscesses seen especially in the mid colon. These mice had a pathology score that was significantly better than mice that received either fgl2-/- or fgl2+/+ Treg (Figure 3C).
To determine if adoptive transfer of fgl2Tg Treg could alter systemic levels of FGL2 we measured plasma levels of FGL2 in the Rag1-/- mice. Interestingly, we found that there was no change in plasma levels of FGL2 following transfer of fgl2Tg Treg (Figure 3D). These data suggest that the adoptively transferred fgl2Tg Treg are working locally as opposed to systemically to limit colonic inflammation.
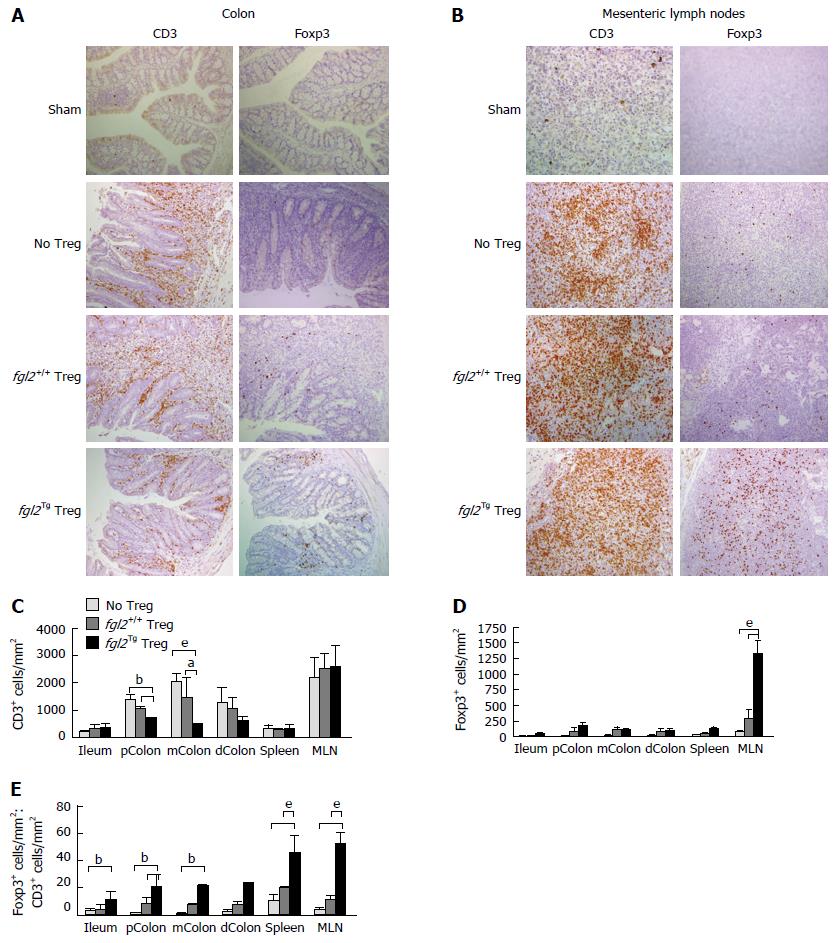
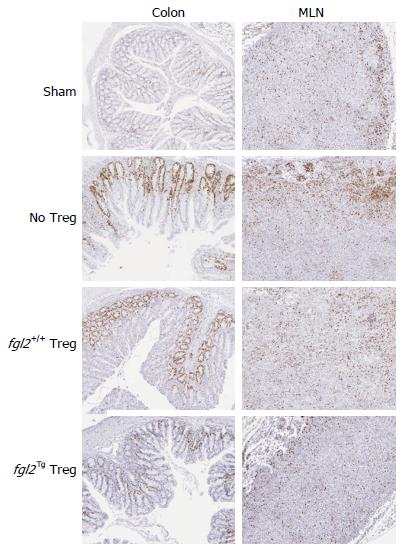
The colon and mesenteric lymph nodes (MLN) from all groups of mice were stained for the presence of CD3+ T cells and Foxp3+ Treg cells. As expected, colons from the sham group of mice had no CD3+ T cells (Figure 4A). Mice that received only Teff contained large numbers of CD3+ T cell infiltrates in association with areas of colitis (Figure 4A). Mice that received both Teff cells and fgl2+/+ Treg had reduced numbers of CD3+ T cells compared to mice that were treated with Teff cells only, but CD3+ T cells were still present in the lamina propria in proximity to areas of colitis. In contrast, mice that received Teff cells and fgl2Tg Treg had very few CD3+ T cells within the colon (Figure 4A). Colon sections were also stained for Foxp3 (Figure 4A). As expected, Foxp3+ Treg were not found in sham control mice or mice that only received Teff. In the fgl2+/+ and fgl2Tg Treg treated groups Foxp3+ cells were found throughout the colon (Figure 4A). These findings were confirmed by morphometry (Figure 4C-E). The ratio of Foxp3+ to CD3+ cells was highest in mice that received fgl2Tg Treg (P < 0.001) (Figure 4E).
Mesenteric lymph nodes (MLN) from sham mice had very few CD3+ but did not stain for Foxp3. Large numbers of CD3+ T cells were seen in the MLN of all the groups of mice that were infused with Teff cells consistent with reconstitution of the immune system. Very few Foxp3+ T cells were seen in mice that received Teff alone or Teff and fgl2+/+ Treg. In contrast, large numbers of Foxp3+ cells were seen in the MLN of mice that received fgl2Tg Treg, leading to a high Foxp3+ to CD3+ cell ratio (Figure 4B-E).
To examine the influence of Treg on Teff cell proliferation, Ki67 staining was performed as described in the methods and by others[22]. In sham mice, Ki67+ cells were seen primarily in the cortex of the MLN, and in mice that received Teff alone, there were increased clusters of Ki67+ cells, primarily localized to the cortex of the MLN. MLN from mice that received fgl2+/+ Treg also contained significant numbers of Ki67+ cells, although they were more diffusely spread within the MLN. In contrast, mice that received fgl2Tg Treg had only small numbers of Ki67+ cells similar to sham mice. As opposed to sham mice, mice that were reconstituted with Teff alone had large numbers of Ki67+ cells both within the lamina propria and epithelium, coincident with areas of histologic colitis. Mice that received fgl2+/+ Treg also had foci of Ki67+ cells in the lamina propria and epithelium. In contrast, no Ki67+ staining was seen in these areas in mice that received fgl2Tg Treg. Ki67+ staining was seen in the colonic crypts of all groups of mice as expected (Figure 5).
To examine the effect of FGL2 over expression of Teff function, Teff cells were isolated from fgl2Tg or fgl2+/+ mice and infused into Rag1-/- recipients. As discussed above, all mice that received fgl2+/+ Teff, developed severe colitis, whereas none of the mice that received fgl2Tg Teff developed clinical or histologic signs of colitis (Figure 6A and B). MLN from mice receiving either fgl2Tgor fgl2+/+ Teff were repopulated with CD3 cells; however, CD3+ cell infiltrates were only seen in the colons of mice that received fgl2+/+ Teff in association with areas of severe colitis (Figure 6C).
IBD consists of a group of chronic relapsing inflammatory diseases of the gastrointestinal tract that include CD and ulcerative colitis[1]. The onset of colitis has been shown to be dependent on dysregulated innate and adaptive immune responses to bacterial flora[1-5]. Here we investigated the effect of overexpression of the Treg immunosuppressive effector molecule FGL2 in the T cell-induced mouse model of colitis. For these studies, we isolated Treg and Teff cells from transgenic mice that ubiquitously overexpress FGL2, which were recently generated in our laboratory[20]. In vitro, fgl2Tg Treg had enhanced suppressive activity compared with fgl2+/+ Treg, and fgl2Tg CD4+ T cells had reduced proliferative potential compared with fgl2+/+ CD4+ T cells. In vivo, fgl2Tg Treg were superior to fgl2+/+ Treg in preventing colitis. This was accompanied by increased ratios of Foxp3+ Treg to CD3+ T cells in the colon and MLN. In mice treated with fgl2Tg Treg, there was also reduced proliferation of Teff cells as assessed by Ki67 staining. Furthermore, fgl2Tg Teff cells failed to induce colitis.
Treg are known to regulate the differentiation and proliferation of Teff by several mechanisms, including bystander suppression and/or by altering the cytokine milieu[11]. IL-10 has been shown to be important in the prevention of experimental IBD through binding with its receptor leading to expansion of Treg. Interestingly, Treg from IL-10 knock mice (IL-10-/-) are as effective as Treg from normal mice in protecting against IBD suggesting that although IL-10 is important in protection against colitis, the source of IL-10 need not be from Treg[23]. FGL2 has previously been described as an important Treg immunosuppressive effector molecule[12]. Recently, FGL2 was shown to be critical for the immune suppressive activity of a major subset of Treg that express T cell immunoglobulin and ITIM domain (TIGIT)[10]. In those studies, TIGIT+ Treg were shown to highly suppress TH1 and TH17 cell responses while preserving TH2 responses, which was dependent on FGL2 expression[10]. Both TIGIT-/- Treg and TIGIT+ Treg inhibited T cell-induced colitis, but the role of FGL2 in colitis was not examined in their report[10].
Others have reported that Treg can ameliorate Teff induced colitis in the Rag1-/- mouse model[2]. Although mice infused with Treg had markedly reduced colitis, Treg treated mice still had evidence of disease as reflected by histology and pathologic scoring[2]. The results presented here demonstrate that fgl2Tg Treg are superior to fgl2+/+ Treg both in suppressive activity in vitro and in prevention of colitis in vivo. Interestingly, adoptive transfer of fgl2Tg Treg compared with fgl2+/+ Treg led to greater number of Treg in the MLN and less Teff proliferation in the MLN. These data are supportive of the hypothesis that fgl2Tg Treg in comparison to fgl2+/+ Treg prevent colitis by preventing activation of Teff in the MLN, which then inhibits homing of Teff to the colon[10]. The increased numbers of fgl2Tg Treg compared with fgl2+/+ Treg in the MLN may be the result of either enhanced homing of fgl2Tg Treg cells to the MLN, changes in fgl2Tg Treg survival/proliferation or the induction of Treg from naïve T cells. Recently, we reported that Treg from fgl2Tg mice promote tolerance in a fully MHC mismatched mouse model of heart transplantation[20]. We also reported that tolerant heart allografts contain increased numbers of FGL2+ Treg whereas they were near absent in rejecting allografts[18]. Taken together, these data suggest Treg secreting high levels of FGL2 are critical to the establishment and maintenance of tolerance in both allo-transplantation and autoimmune disease. Clinical trials are currently underway to test the safety and efficacy of Treg populations in the treatment of IBD[24,25]. We propose that expansion of FGL2high Treg may be a highly effective approach to treating patients with autoimmune disease, including IBD.
Through the generation of fgl2Tg mice, we have confirmed that FGL2 is an important immune modulator that regulates Teff cell function and proliferation. We demonstrate here that fgl2Tg Teff in contrast to fgl2+/+ Teff are not capable of inducing colitis in Rag1-/- mice. Previously, we demonstrated that the inhibitory Fcγ receptor (FcγRIIB), which is expressed on antigen presenting cells (APC) such as DC and B cells, is the receptor for FGL2[26,27] . We also showed that recombinant FGL2 inhibits the maturation of bone marrow-derived DC and promotes B cell apoptosis[26]. It is unlikely that FGL2 acts directly on Teff as T cells express little if any FcγRIIB[27]. Consistent with this, we have observed that recombinant FGL2 did not inhibit T cell proliferation when purified T cells were stimulated with anti-CD3 and anti-CD28 (data not shown). However, recombinant FGL2 inhibits T cell proliferation in mixed lymphocyte reactions when APC are present[28]. The inhibition on DC maturation by FGL2 may explain why mice infused with fgl2Tg Teff had reduced numbers in the colon. We hypothesize that the increased expression of FGL2 by fgl2Tg Teff inhibits the maturation of DCs encountered in the inflamed tissue which, in turn, inhibits the activation and expansion of the same Teff in a negative feedback loop. We cannot rule out at this time, however, that there is an intrinsic defect in fgl2Tg CD4+ T cells due to overexpression of FGL2.
A recent paper has demonstrated increased mucosal biopsy staining for FGL2 and increased plasma levels of FGL2 in patients with active IBD (CD and ulcerative colitis)[29]. In these patients, endothelial cells and infiltrating inflammatory cells in mucosal biopsy specimens stained strongly for FGL2. Together with our studies showing the immunosuppressive effects of FGL2, these data suggest that expression of FGL2 is an important regulator of mucosal immunity and may represent a feedback mechanism to limit inflammation in patients with active IBD[29].
Collectively, the studies presented here confirm that FGL2 is an important immunosuppressive effector. Teff from fgl2Tg mice are hypoproliferative and fail to induce colitis when injected into Rag1-/- mice. Treg from fgl2Tg have increased immunosuppressive activity in vitro and protect mice from T cell-mediated colitis. These studies support the concept that FGL2 expressing Treg are critical for the maintenance of tolerance and provide a rationale for exploring the use of recombinant FGL2 or Treg expressing high levels of FGL2 in the treatment of autoimmune disease.
Inflammatory bowel disease (IBD) consists of a group of chronic relapsing inflammatory diseases of the gastrointestinal tract that include Crohn’s disease and ulcerative colitis. Regulatory T cells (Treg) have been shown to be important regulators of disease activity in IBD and can ameliorate disease in the T cell-induced model of colitis in immunodeficient Rag1-/- mice.
Fibrinogen-like protein 2 (FGL2) is a newly described immunoregulatory molecule that is an effector molecule of Treg. The effect of overexpression of FGL2 in the T cell-induced model of colitis has not been studied previously. We recently generated a transgenic line of mice that ubiquitously overexpress FGL2 (fgl2Tg). Here we isolated Treg and effector T cells (Teff) from fgl2Tg mice and compared these cells to wildtype (fgl2+/+) cells in the T cell-induced colitis model.
The authors found that fgl2Tg Treg have enhanced immune suppressive activity compared with fgl2+/+ Treg in vitro. Following injection in Rag1-/- mice, fgl2Tg Treg were present in high numbers in mesenteric lymph nodes and were superior to fgl2+/+ Treg in preventing T cell-induced colitis. Fgl2Tg Teff were hypoproliferative in vitro and were not capable of inducing colitis.
Overexpression of FGL2 by either Treg or Teff prevents T cell-mediated colitis. These studies collectively provide a rationale for exploring the use of either recombinant FGL2 or Treg expressing high levels of FGL2 in the treatment of inflammatory bowel disease.
FGL2 is an immunoregulatory molecule that has been shown to be an effector molecule of Treg.
This is an excellent, but descriptive, paper. The experiment proposed in the comments to authors may provide a link between the two disparate observations described.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cordero OJ, Cordero PU, Yankee T S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1158] [Cited by in F6Publishing: 1204] [Article Influence: 66.9] [Reference Citation Analysis (1)] |
| 2. | Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461-1471. [PubMed] [Cited in This Article: ] |
| 3. | Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464-3473. [PubMed] [Cited in This Article: ] |
| 4. | Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237-244. [PubMed] [Cited in This Article: ] |
| 5. | Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135-G146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 6. | Singh B, Read S, Asseman C, Malmström V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190-200. [PubMed] [Cited in This Article: ] |
| 7. | Zhang N, Schröppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 8. | Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 660] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 9. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6077] [Cited by in F6Publishing: 6156] [Article Influence: 293.1] [Reference Citation Analysis (0)] |
| 10. | Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 607] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 11. | Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1257] [Cited by in F6Publishing: 1294] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 12. | Shalev I, Liu H, Koscik C, Bartczak A, Javadi M, Wong KM, Maknojia A, He W, Liu MF, Diao J. Targeted deletion of fgl2 leads to impaired regulatory T cell activity and development of autoimmune glomerulonephritis. J Immunol. 2008;180:249-260. [PubMed] [Cited in This Article: ] |
| 13. | Koyama T, Hall LR, Haser WG, Tonegawa S, Saito H. Structure of a cytotoxic T-lymphocyte-specific gene shows a strong homology to fibrinogen beta and gamma chains. Proc Natl Acad Sci USA. 1987;84:1609-1613. [PubMed] [Cited in This Article: ] |
| 14. | Ding JW, Ning Q, Liu MF, Lai A, Leibowitz J, Peltekian KM, Cole EH, Fung LS, Holloway C, Marsden PA. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. J Virol. 1997;71:9223-9230. [PubMed] [Cited in This Article: ] |
| 15. | Parr RL, Fung L, Reneker J, Myers-Mason N, Leibowitz JL, Levy G. Association of mouse fibrinogen-like protein with murine hepatitis virus-induced prothrombinase activity. J Virol. 1995;69:5033-5038. [PubMed] [Cited in This Article: ] |
| 16. | Chan CW, Chan MW, Liu M, Fung L, Cole EH, Leibowitz JL, Marsden PA, Clark DA, Levy GA. Kinetic analysis of a unique direct prothrombinase, fgl2, and identification of a serine residue critical for the prothrombinase activity. J Immunol. 2002;168:5170-5177. [PubMed] [Cited in This Article: ] |
| 17. | Chan CW, Kay LS, Khadaroo RG, Chan MW, Lakatoo S, Young KJ, Zhang L, Gorczynski RM, Cattral M, Rotstein O. Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow-derived dendritic cells. J Immunol. 2003;170:4036-4044. [PubMed] [Cited in This Article: ] |
| 18. | Urbanellis P, Shyu W, Khattar R, Wang J, Zakharova A, He W, Sadozai H, Amir AZ, Shalev I, Phillips MJ. The regulatory T cell effector molecule fibrinogen-like protein 2 is necessary for the development of rapamycin-induced tolerance to fully MHC-mismatched murine cardiac allografts. Immunology. 2015;144:91-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Li XL, Ménoret S, Bezie S, Caron L, Chabannes D, Hill M, Halary F, Angin M, Heslan M, Usal C. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol. 2010;185:823-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Bartczak A, Chruscinski A, Mendicino M, Liu H, Zhang J, He W, Amir AZ, Nguyen A, Khattar R, Sadozai H. Overexpression of Fibrinogen-Like Protein 2 Promotes Tolerance in a Fully Mismatched Murine Model of Heart Transplantation. Am J Transplant. 2016;16:1739-1750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 453] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 23. | Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 613] [Cited by in F6Publishing: 646] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 24. | Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143:1207-1217.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | Lord JD. Promises and paradoxes of regulatory T cells in inflammatory bowel disease. World J Gastroenterol. 2015;21:11236-11245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Liu H, Shalev I, Manuel J, He W, Leung E, Crookshank J, Liu MF, Diao J, Cattral M, Clark DA. The FGL2-FcgammaRIIB pathway: a novel mechanism leading to immunosuppression. Eur J Immunol. 2008;38:3114-3126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 818] [Cited by in F6Publishing: 801] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 28. | Liu H, Yang PS, Zhu T, Manuel J, Zhang J, He W, Shalev I, Zhang L, Cybulsky MI, Grant DR. Characterization of fibrinogen-like protein 2 (FGL2): monomeric FGL2 has enhanced immunosuppressive activity in comparison to oligomeric FGL2. Int J Biochem Cell Biol. 2013;45:408-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Dong X, Ye X, Chen X, Chen T, Xie S, Li Q, Lin X, Huang Z. Intestinal and peripheral fibrinogen-like protein 2 expression in inflammatory bowel disease. Dig Dis Sci. 2014;59:769-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |