Published online Dec 14, 2016. doi: 10.3748/wjg.v22.i46.10084
Peer-review started: August 17, 2016
First decision: September 20, 2016
Revised: October 12, 2016
Accepted: November 15, 2016
Article in press: November 16, 2016
Published online: December 14, 2016
Mammalian sirtuins are seven members belonging to the silent information regulator 2 family, a group of Class III histone/protein deacetylases. Sirtuins (SIRT 1-7) have different subcellular localization and function and they regulate cellular protein function through various posttranslational modifications. SIRT1 and 3, the most studied sirtuins, use the product of cellular metabolism nicotinamide adenine dinucleotide as a cofactor to post-translationally deacetylate cellular proteins and consequently link the metabolic status of the cell to protein function. Sirtuins have been shown to play a key role in the development and rescue of various metabolic diseases including non-alcoholic fatty liver disease (NAFLD). NAFLD is currently the most chronic liver disease due mainly to high-calorie consumption and lower physical activity. No pharmacological approach is available to treat NAFLD, the current recommended treatment are lifestyle modification such as weight loss through calorie restriction and exercise. Recent studies have shown downregulation of sirtuins in human as well as animal models of NAFLD indicating an important role of sirtuins in the dynamic pathophysiology of NAFLD. In this review, we highlight the recent knowledge on sirtuins, their role in NAFLD and their unique potential role as novel therapeutic target for NAFLD treatment.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease with no effective pharmacological therapy. The discovery of treatment is hindered by the insufficient understanding of the pathophysiology of the disease. Sirtuins are key players in hepatic carbohydrate and lipid metabolism, insulin signaling, and inflammation and hence may represent a novel therapeutic target for NAFLD. However, the particular role for each sirtuin, the cross talk between sirtuins in different cell compartments or within a given organelle, and the development of selective sirtuins activators/inhibitors still need further investigation.
- Citation: Nassir F, Ibdah JA. Sirtuins and nonalcoholic fatty liver disease. World J Gastroenterol 2016; 22(46): 10084-10092
- URL: https://www.wjgnet.com/1007-9327/full/v22/i46/10084.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i46.10084
Non-alcoholic fatty liver diseases (NAFLD) is emerging as the leading cause of chronic liver diseases affecting one billion of people in the world. The current model for NAFLD pathophysiology, “the multiple-hit hypothesis”, characterizes NAFLD as the manifestation of both genetic and environmental factors, dysfunction of various organs and organelles, as well as the complex interaction between hepatocytes and other cells (e.g., Kupffer and stellate cells) in the liver[1]. Moreover, the liver is a hub for many metabolic pathways making NAFLD a multistage, progressive disease with systemic consequences. NAFLD is commonly associated with obesity, insulin resistance and enhanced risk of cardiovascular disease and mortality[2-6]. Importantly, cardiovascular diseases are the main cause of morbidity in NAFLD patients. High-calorie consumption and lower physical activity have contributed to the rise in the prevalence of NAFLD. To date, no approved pharmacological approaches are available to treat NAFLD, the current confirmed recommendations for NAFLD are lifestyle modifications such as weight loss through caloric restriction (CR) and increased physical activity[7-9]. Therefore, a pressing need for developing new novel pharmacological treatments, is still remaining. An inclusive pharmacological approach would be one that addresses the pathogenic complexity of NAFLD. Currently, sirtuins have been under intense investigation as a novel therapeutic target for the treatment of NAFLD. In this review, we summarize the current knowledge on the pathophysiology of NAFLD and on the sirtuins as a potential target for the treatment of NAFLD.
NAFLD is a spectrum of liver diseases that occurs in the absence of excessive alcohol intake or viral infection. It includes hepatic steatosis (> 5% of fat in the liver), nonalcoholic steatohepatitis (NASH, fat deposit with inflammation), cirrhosis and hepatocellular carcinoma[9-12]. NAFLD is currently the most widespread form of liver disease affecting 10%-30% of all ages from childhood to adult population, and is predicted to be the leading cause of liver pathology and liver failure in the coming years[13,14]. NAFLD is more prominent in obese and insulin resistant individuals affecting 70%-90% in these populations[15,16]. NAFLD is also present in 10%-20% of the general pediatric population; this proportion increases to 50% in obese children in western society[13,17-22]. A more recent study suggests that metabolic derangements may start early in life, even in utero. Exposure to excess fuel in fetal life may result in NAFLD in the offspring[23,24].
Our understanding of the mechanisms involved in the pathophysiology of NAFLD are insufficient to pinpoint the major determinants involved in the development and progression of the disease and to develop therapeutic strategies for NAFLD. Studies on genetic and molecular factors involved in NAFLD clearly implicate lipid and glucose metabolism in the development of the disease. Moreover, functional studies implicate the different cell population in the liver as well as interaction between the liver, adipose tissue, gut and the muscle in the pathogenesis of NAFLD. In contrast to the “two-hit hypothesis” proposed by Day[25] in which hepatic accumulation of triglyceride (“1st hit”) sensitizes the liver to additional insults such as oxidative stress and pro-inflammatory cytokines (“2nd hit”) resulting in NASH. The current understanding, “the multiple parallel hypothesis”, refers to NAFLD as a systemic, multifactorial disease involving multiple organs, such as adipose tissue, muscle and the intestine, and organelles such the endoplasmic reticulum and the mitochondria.
Hepatic steatosis, which is previously considered as the benign form of NAFLD, results from an imbalance between influx of fatty acids to the liver from the diet, adipose tissue lipolysis or de novo lipogenesis; and their oxidation or export in the circulation as very low density lipoproteins (VLDL)[9]. Failure of insulin to suppress lipolysis in insulin resistant adipose tissue is commonly associated with NAFLD[26,27]. Moreover, it is estimated that in NAFLD patients, roughly 60% of fatty acids in the liver originate from adipose tissue, 25% from de novo lipogenesis, and 15% from the diet[28]. Interestingly, both β-oxidation of fatty acids in the liver and VLDL secretion, are initially upregulated in non-alcoholic fatty liver in an attempt to compensate for the rise in fatty acids in the liver[29-32]. However, this short term compensatory mechanism is insufficient to sustain the ongoing influx of fatty acid to the liver leading to liver injury[30-32]. NASH patients have lower VLDL secretion and lower fatty acid oxidation (FAO) than patients with fatty liver[30,31].
Non-alcoholic steatohepatitis (NASH) is a more severe form of NAFLD that is generally defined by the presence of steatosis with inflammation and cellular damage. Fibrosis is commonly described as an irreversible scarring of liver tissue with excessive presence of extracellular matrix. The presence of fibrosis is one of the most important predictors of NAFLD related mortality[10,33]. The current understanding of NASH pathogenesis follows a multiple hits model[34,35] that implicate multiple stressors. Lipotoxicity, endoplasmic reticulum stress, adipose tissue derived adipokines (TNFα and IL6), gut endotoxins and LPS produced by gut microbiota that drift into to the liver through the portal vein due to changes in the intestinal permeability in NAFLD, and oxidative stress trigger inflammatory response and progressive liver damage. Inflammation can sometimes precede steatosis, and patients with NASH can present without much steatosis suggesting that inflammation can sometimes occur first. Recent studies have also shown that individuals with hepatic steatosis may progress to fibrosis in a relatively short period of time (3-7 years)[36,37]. NAFLD patients may be classified into two categories, slow and fast progressors. The slow progressors may develop NASH but no fibrosis while the fast progressors may develop fibrosis and sometimes skip NASH stage of the disease[38]. Changes in mitochondrial function is an important mechanism that may drive the switch from hepatic steatosis to NASH. Several reports indicate that mitochondrial respiration is elevated in NAFLD patients[29,30]. However, in humans with NASH, respiration may be uncoupled from ATP production, causing significant increases in reactive oxygen species (ROS)[30]. Importantly, elevated ROS production was associated with an increase in detoxification and antioxidant capacity in hepatic steatosis, but not in NASH, indicating that mechanisms to cope with excess ROS generation may be insufficient in NASH[30].
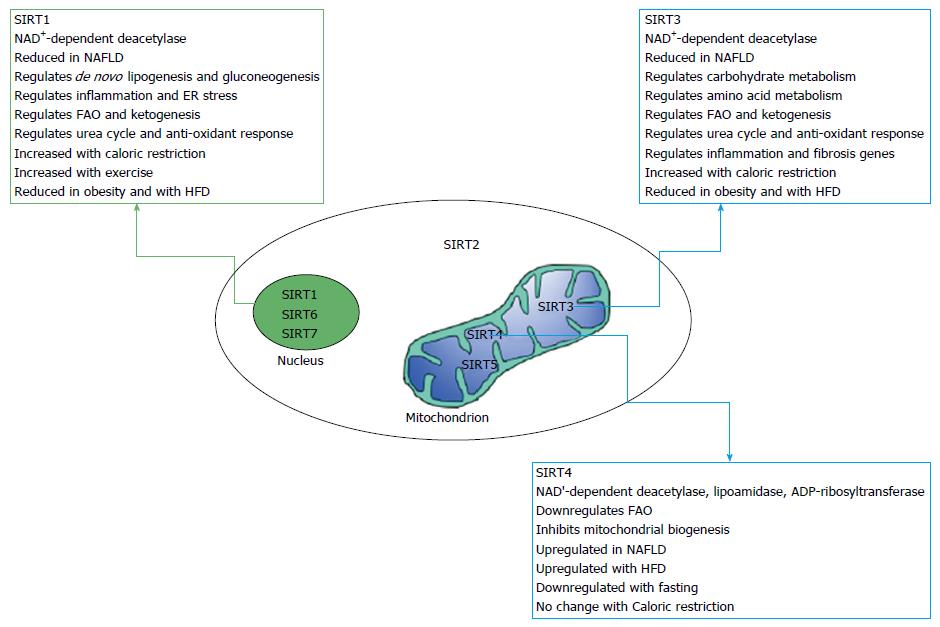
Sirtuins are a group of proteins that belong to the family of silent information regulator 2. Sirtuins have been shown, in recent years, to play an important role in the pathophysiology of various metabolic diseases including NAFLD[39]. Sirtuins are implicated in many cellular and physiological functions including hepatic glucose and fatty acid metabolism, mitochondrial function, hepatic gluconeogenesis, insulin secretion and the maturation of fat cells[40,41] as illustrated in Figure 1. Sirtuins regulate protein function through a growing list of posttranslational modification including deacetylation, succinylation and malonylation[42,43]. Seven mammalians sirtuins (SIRT1-SIRT7) have been identified and shown to share the same conserved NAD binding site and catalytic core domain but with different N and C termini[44]. The different sirtuins have various subcellular localization and expression[44]. SIRT 1, 6, and 7 are localized mainly in nucleus while SIRT 3, 4 and 5 are localized to the mitochondrial matrix and SIRT2 predominantly cytoplasmic[44]. Recent studies have shown reduced levels of most sirtuins in NAFLD. Direct evidence came from Wu et al[45] who demonstrated decreased expression of SIRT1, SIRT3, SIRT5, and SIRT6 in NAFLD patients compared to the control group. This was associated with increased expression of lipogenic genes including sterol regulatory element binding protein-1, fatty acid synthase, and acetyl-CoA carboxylase. In contrast to the other sirtuins, the expression of SIRT4 was upregulated in NAFLD patients[45]. Interestingly, in a recent study, Bruce et al[46] indicated that exposure to excess dietary fat during early and post-natal life increases the susceptibility to develop NASH in adulthood and this was associated with reduced sirtuin abundance. Offspring fed a high fat diet (HFD) developed NAFLD while HFD-fed offspring of mothers fed a HFD diet developed NASH in combination of reduced NAD+/NADH, SIRT1, SIRT3 and increased expression of genes involved in lipid metabolism[46]. SIRT1 and SIRT3 are the most studied sirtuins; we will focus mainly on these two sirtuins, their mode of action and their role in NAFLD.
Both SIRT1 and SIRT3 are NAD+-deacetylase that use NAD as a cofactor to deacetylate cellular proteins. Lysine acetylation is a reversible, dynamic reaction of adding acetyl groups to lysine residues. Acetylation affects all proteins in the cell and has recently been shown to be abundant in the mitochondria where it plays a key role in the dynamic regulation of proteins and thereby cell metabolism[43,47-54]. Dysregulation of lysine acetylation plays a pathogenic role in diverse conditions such as metabolic syndrome, aging, cancer and NAFLD[55-58].
Studies from our group and others document strong involvement of the mitochondria in the pathogenesis of NAFLD[59-62]. SIRT3 is the most investigated mitochondrial sirtuin, while SIRT1 has been shown to be expressed in various metabolic tissues including liver, adipose tissue, skeletal muscle, pancreas and brain. SIRT1 plays a key role in the development of NAFLD through its involvement in the regulation of both lipid and carbohydrate metabolism[45,46,63-66]. Studies in mice and in cultured cells have characterized SIRT1 as a metabolic sensor that has the potential to improve NAFLD.
Inhibition of SIRT1 signaling in human fetal hepatocytes resulted in an increase in intracellular glucose and lipid levels with upregulation of de novo lipogenesis and gluconeogenesis related genes[66]. In mice, liver specific deletion of SIRT1 as well as SIRT1 downregulation using small hairpin RNA resulted in hepatic steatosis, inflammation and endoplasmic reticulum stress[67,68]. Hepatocyte-specific deletion of SIRT1 impaired PPARα signaling and decreased FAO. However, SIRT1 overexpression increased levels of PPARα and increased FAO[67].
SIRT1 is reduced by HFD while CR resulted in an increase in hepatic SIRT1 expression and improvement in NAFLD histology[69]. Overexpression of SIRT1 in mice provided protection against HFD induced hepatic steatosis through upregulation of FAO and downregulation of lipogenesis[64]. Moreover, treatment of mice fed a HFD with resveratrol (RSV), a polyphenol found in red wine and other plants, improved lipid metabolism, and decreased NAFLD and inflammation in the liver[70]. Interestingly, it has been documented that inhibition of SIRT1 signaling in human fetal hepatocytes resulted in an increase in intracellular glucose and lipid levels[66]. SIRT1 is also modulated in obesity. Recent studies have shown a correlation between plasma SIRT1 and NAFLD in obese patients. SIRT1 was significantly lower in an obese group with severe liver steatosis compared to a group with mild steatosis, and both groups had lower SIRT1 in the plasma compared to control lean patients[71]. Phenotypic similarities exist between CR and SIRT1 overexpression. Mice overexpressing SIRT1 are leaner and resistant to hepatic steatosis and insulin resistance[72]. Together, these studies indicate a potential therapeutic use of SIRT1 in hepatic steatosis[66].
SIRT3 is a soluble protein located in the mitochondrial matrix and has been shown as a major regulator of mitochondrial protein acetylation and function[44,73]. SIRT3 regulates carbohydrate metabolism, ketogenesis, β-oxidation, and amino-acid metabolism and stress-related pathways[73-77]. The protein is encoded by the nuclear genome and is translated as a 45-kDa protein with an N-terminal mitochondrial targeting sequence that is cleaved to give the 28-kDa enzymatically active protein[78]. SIRT3 is expressed in many tissues including the liver, adipose tissue, heart, brain and kidney[44]. Although SIRT3-KO mice are metabolically undistinguishable from WT controls under basal conditions, they show increased hyperacetylation of mitochondrial proteins in the liver and the heart[54,74,75,79]. About 65% of all mitochondrial proteins have at least one acetylated lysine[48,54,73]. SIRT4 and SIRT5 are also localized to the mitochondria and unlike SIRT3-KO mice, SIRT4 and SIRT5-KO mice did not display the global increase in hepatic mitochondrial acetylation observed in SIRT3-deficient animals.
Mitochondria play a key role in the adaptation to CR and SIRT3 has been identified as an important regulator in CR-associated metabolic changes[54]. The expression of SIRT3 is considerably increased in response to CR or prolonged fasting[75,80,81]. SIRT3 regulates the function of several mitochondrial proteins involved in oxidative phosphorylation, FAO, the urea cycle, and the antioxidant response system[73,75,82-85]. Unlike wild-type mice where FAO is upregulated with fasting, fasted SIRT3 deficient mice display reduced FAO and ATP production with increased hepatic TG content[75].
SIRT3 also regulates the acetylation levels of mitochondrial electron transport complex I and regulates ATP synthesis[77]. ATP levels were reduced by more than 50% in the heart, liver and kidney of mice lacking SIRT3[77]. Succinate dehydrogenase (SDH) (one of complex II subunits of the electron transport chain) has been identified as a direct target of SIRT3, suggesting a role of SIRT3 in the regulation of complex II[86,87]. Increased succinate concentrations is involved in hepatic stellate cells (HSCs) activation. The expression of SIRT3 and SDH activity are decreased in isolated liver and HSCs from methionine- and choline-deficient (MCD) diet-induced NAFLD. Suppression of SIRT3 using siRNA exacerbated HSC activation while SIRT3 overexpression attenuated HSC activation in vitro[88]. Interestingly, liver- and muscle-specific SIRT3-KO mice show no detectable changes in their metabolic phenotype in response to HFD[89] suggesting more studies are needed to ascertain the role of tissue specific function of SIRT3[76,89].
Published studies document that both obesity and chronic HFD reduce SIRT3 activity, induce hyperacetylation of various mitochondrial proteins and impair mitochondrial function[58,75,90]. HFD has been shown to induce SIRT3 expression and FAO early after initiation of high-fat feeding[58]. However, chronic HFD suppress SIRT3 expression, increase mitochondrial protein acetylation, and ultimately reduce FAO. Wild type mice fed a HFD develop obesity, hyperlipidemia, type 2 diabetes mellitus, and NASH[91-93]. These effects of HFD feeding are significantly accelerated in SIRT3 deficient mice[58]. Our unpublished data also show that overexpression of SIRT3 rescues NAFLD in mice heterozygous for the mitochondrial trifunctional protein, an animal model of mitochondrial dysfunction generated by our group[94].
SIRT3-KO mice subjected to MCD diet exhibit increased serum ALT levels, increased hepatic content, higher expression of inflammatory and fibrogenic genes, and reduced (SOD2) activity. However, overexpression of SIRT3 resulted in opposite effects suggesting that SIRT3 ablation aggravates MCD induced NASH while SIRT3 overexpression alleviates the MCD induced phenotype[95].
Palmitate modulated oxygen consumption and enhanced ROS levels and apoptosis in SIRT3 deficient mouse primary hepatocytes and SIRT3 siRNA-depleted hepatocytes[96]. Recent studies using HFD induced NAFLD in mice identified a differentially expressed microRNA (miRNA) in livers of NAFLD mice compared with controls. The expression of miRNA-421 was significantly upregulated in mice with NAFLD and SIRT3 was identified as target for this micro-RNA. Overexpression of miRNA-421 in hepatocytes decreased SIRT3 and FOXO3 protein levels, and reduced oxidative damage while suppression of this miRNA had opposite effects[97]. Interestingly, exposure of fetuses to maternal obesity contributes to early perturbations in whole body and liver energy metabolism, and this was associated with reduced SIRT3 and reduced hepatic FAO. These findings suggest that changes in SIRT3 activity precedes the development of obesity associated insulin resistance and NAFLD in the offspring[98].
Weight loss through CR and exercise have been shown to improve insulin resistance and inflammation. Based on the beneficial effect of CR on NAFLD and other diseases and the associated increase in sirtuins levels or activity, the development of molecules that activate or inhibit sirtuins is of great interest[99].
The discovery of selective and potent sirtuins activators and inhibitors is still in its early stages. A list of Sirt1 activators that were tested in human and animal NAFLD is shown in Table 1[100-114]. RSV, a natural polyphenol found in grapes and other plants, mimicks CR and enhances sirtuins activity[102,109]. However, due to its poor bioavailability, reformulated forms of RSV-related compounds have been developed such as resVida, Longevinex®, SRT50 along with other RSV unrelated molecules such as SRT1720, SRT2104, and SRT2379. The formulated form of RSV resVida (150 mg/d RSV) showed beneficial effects, similar to CR effect, in healthy obese men including reduced intrahepatic lipid, plasma glucose, TG, alanine-aminotransferase and inflammation markers[104]. SRT1720 was the most potent SIRT1 activator; it enhanced SIRT1 activity by 750% at 10 μmol/L although other studies by Pacholec et al[106] concluded that neither SRT1720 nor RSV are direct activators of SIRT1 and one study reported that RSV does not have beneficial effects in NAFLD patients[112]. Administration of SRT1720 to diet-induced obesity rodent models protected from obesity and insulin resistance by enhancing oxidative metabolism in the liver, muscle, and adipose tissues[105,107,111]. As in CR, SIRT1720 induced mitochondrial biogenesis, increase mitochondrial respiration and ATP levels[110]. Moreover, SRT1720 reduced levels of hepatic liver content and aminotransferase and the expressions of lipogenic genes[101]. Recent studies, however, indicate that the activation of SIRT1 by RSV is indirect and is mediated by activation of AMPK[40,115]. Sirtuins are themselves regulated by the cofactor NAD+ as well as their reaction product nicotinamide (NAM) from NAD+. NAM (the amide form of vitamin B3, nicotinic acid) is a water-soluble sirtuin inhibitor. NAM binds to a conserved region in the sirtuin catalytic site and favors a reverse reaction instead of the deacetylation reaction[116]. Computational studies indicate that NAM inhibition of SIRT3 involves apparent competition between the inhibitor and the enzyme cofactor NAD+ while the inhibition of other sirtuins activity was non-competitive[117]. More detailed review on sirtuins inhibitors and activators is found in[99,118]. More studies are needed to develop more potent and specific activators and inhibitors of sirtuins activity.
| SIRT1 activators | Ref. |
| Resveratrol | Howitz et al[109], 2003 |
| Wood et al[102], 2004 | |
| Timmers et al[104], 2011 | |
| Smith et al[105], 2009 | |
| Milne et al[107], 2007 | |
| Amiot et al[113], 2013 | |
| Yoshino et al[100], 2012 | |
| Chachay et al[112], 2014 | |
| SRT1720 | Feige et al[111], 2008 |
| Funk et al[110], 2010 | |
| Yamazaki et al[101], 2009 | |
| Pacholec et al[106], 2010 | |
| SRT2104 | Libri et al[108], 2012 |
| Venkatasubramanian et al[103], 2013 | |
| Hoffmann et al[114], 2013 |
Sirtuins represent potential targets for treatment of NAFLD due to the role they play in cellular pathways involved in hepatic lipid and carbohydrate metabolism, insulin signaling, and inflammation. Additional studies are urgently needed to further our understanding of the interaction among various sirtuins in NAFLD and to develop selective activators/inhibitors of sirtuins.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Geng TY, Joven J, Trovato GM S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [PubMed] [Cited in This Article: ] |
| 2. | Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389-1397. [PubMed] [Cited in This Article: ] |
| 3. | Misra VL, Khashab M, Chalasani N. Nonalcoholic fatty liver disease and cardiovascular risk. Curr Gastroenterol Rep. 2009;11:50-55. [PubMed] [Cited in This Article: ] |
| 4. | Sanyal AJ. NASH: A global health problem. Hepatol Res. 2011;41:670-674. [PubMed] [Cited in This Article: ] |
| 5. | Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384-387. [PubMed] [Cited in This Article: ] |
| 6. | Perry RJ, Zhang D, Zhang XM, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science. 2015;347:1253-1256. [PubMed] [Cited in This Article: ] |
| 7. | Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L, Younossi ZM. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: Systematic review. World J Gastroenterol. 2016;22:6318-6327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 111] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 8. | Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190-1204. [PubMed] [Cited in This Article: ] |
| 9. | Nassir F, Rector RS, Hammoud GM, Ibdah JA. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol Hepatol (N Y). 2015;11:167-175. [PubMed] [Cited in This Article: ] |
| 10. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [PubMed] [Cited in This Article: ] |
| 11. | Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848-854. [PubMed] [Cited in This Article: ] |
| 12. | Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837-858. [PubMed] [Cited in This Article: ] |
| 13. | Temple JL, Cordero P, Li J, Nguyen V, Oben JA. A Guide to Non-Alcoholic Fatty Liver Disease in Childhood and Adolescence. Int J Mol Sci. 2016;17. [PubMed] [Cited in This Article: ] |
| 14. | López-Velázquez JA, Silva-Vidal KV, Ponciano-Rodríguez G, Chávez-Tapia NC, Arrese M, Uribe M, Méndez-Sánchez N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann Hepatol. 2014;13:166-178. [PubMed] [Cited in This Article: ] |
| 15. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [PubMed] [Cited in This Article: ] |
| 16. | Younossi ZM, Diehl AM, Ong JP. Nonalcoholic fatty liver disease: an agenda for clinical research. Hepatology. 2002;35:746-752. [PubMed] [Cited in This Article: ] |
| 17. | Alisi A, Manco M, Panera N, Nobili V. Association between type two diabetes and non-alcoholic fatty liver disease in youth. Ann Hepatol. 2009;8 Suppl 1:S44-S50. [PubMed] [Cited in This Article: ] |
| 18. | Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58:1218-1229. [PubMed] [Cited in This Article: ] |
| 19. | Valenti L, Romeo S. Destined to develop NAFLD? The predictors of fatty liver from birth to adulthood. J Hepatol. 2016;65:668-670. [PubMed] [Cited in This Article: ] |
| 20. | Lawlor DA, Callaway M, Macdonald-Wallis C, Anderson E, Fraser A, Howe LD, Day C, Sattar N. Nonalcoholic fatty liver disease, liver fibrosis, and cardiometabolic risk factors in adolescence: a cross-sectional study of 1874 general population adolescents. J Clin Endocrinol Metab. 2014;99:E410-E417. [PubMed] [Cited in This Article: ] |
| 21. | Ayonrinde OT, Olynyk JK, Marsh JA, Beilin LJ, Mori TA, Oddy WH, Adams LA. Childhood adiposity trajectories and risk of nonalcoholic fatty liver disease in adolescents. J Gastroenterol Hepatol. 2015;30:163-171. [PubMed] [Cited in This Article: ] |
| 22. | Moran JR, Ghishan FK, Halter SA, Greene HL. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. 1983;78:374-377. [PubMed] [Cited in This Article: ] |
| 23. | Brumbaugh DE, Friedman JE. Developmental origins of nonalcoholic fatty liver disease. Pediatr Res. 2014;75:140-147. [PubMed] [Cited in This Article: ] |
| 24. | McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323-335. [PubMed] [Cited in This Article: ] |
| 25. | Day CP. NASH-related liver failure: one hit too many? Am J Gastroenterol. 2002;97:1872-1874. [PubMed] [Cited in This Article: ] |
| 26. | Granér M, Seppälä-Lindroos A, Rissanen A, Hakkarainen A, Lundbom N, Kaprio J, Nieminen MS, Pietiläinen KH. Epicardial fat, cardiac dimensions, and low-grade inflammation in young adult monozygotic twins discordant for obesity. Am J Cardiol. 2012;109:1295-1302. [PubMed] [Cited in This Article: ] |
| 27. | Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. [PubMed] [Cited in This Article: ] |
| 28. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [PubMed] [Cited in This Article: ] |
| 29. | Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804-810. [PubMed] [Cited in This Article: ] |
| 30. | Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739-746. [PubMed] [Cited in This Article: ] |
| 31. | Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, Endo H, Takahashi H, Inamori M, Kobayashi N. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772-780. [PubMed] [Cited in This Article: ] |
| 32. | Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424-431. [PubMed] [Cited in This Article: ] |
| 33. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [PubMed] [Cited in This Article: ] |
| 34. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [PubMed] [Cited in This Article: ] |
| 35. | Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704-20728. [PubMed] [Cited in This Article: ] |
| 36. | Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550-556. [PubMed] [Cited in This Article: ] |
| 37. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [PubMed] [Cited in This Article: ] |
| 38. | Haas JT, Francque S, Staels B. Pathophysiology and Mechanisms of Nonalcoholic Fatty Liver Disease. Annu Rev Physiol. 2016;78:181-205. [PubMed] [Cited in This Article: ] |
| 39. | Bedalov A, Chowdhury S, Simon JA. Biology, Chemistry, and Pharmacology of Sirtuins. Methods Enzymol. 2016;574:183-211. [PubMed] [Cited in This Article: ] |
| 40. | Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056-1060. [PubMed] [Cited in This Article: ] |
| 41. | Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913-2921. [PubMed] [Cited in This Article: ] |
| 42. | Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol. 2014;15:536-550. [PubMed] [Cited in This Article: ] |
| 43. | Hirschey MD, Zhao Y. Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation. Mol Cell Proteomics. 2015;14:2308-2315. [PubMed] [Cited in This Article: ] |
| 44. | Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479-1514. [PubMed] [Cited in This Article: ] |
| 45. | Wu T, Liu YH, Fu YC, Liu XM, Zhou XH. Direct evidence of sirtuin downregulation in the liver of non-alcoholic fatty liver disease patients. Ann Clin Lab Sci. 2014;44:410-418. [PubMed] [Cited in This Article: ] |
| 46. | Bruce KD, Szczepankiewicz D, Sihota KK, Ravindraanandan M, Thomas H, Lillycrop KA, Burdge GC, Hanson MA, Byrne CD, Cagampang FR. Altered cellular redox status, sirtuin abundance and clock gene expression in a mouse model of developmentally primed NASH. Biochim Biophys Acta. 2016;1861:584-593. [PubMed] [Cited in This Article: ] |
| 47. | Norvell A, McMahon SB. Cell biology. Rise of the rival. Science. 2010;327:964-965. [PubMed] [Cited in This Article: ] |
| 48. | Still AJ, Floyd BJ, Hebert AS, Bingman CA, Carson JJ, Gunderson DR, Dolan BK, Grimsrud PA, Dittenhafer-Reed KE, Stapleton DS. Quantification of mitochondrial acetylation dynamics highlights prominent sites of metabolic regulation. J Biol Chem. 2013;288:26209-26219. [PubMed] [Cited in This Article: ] |
| 49. | Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci USA. 2002;99:13653-13658. [PubMed] [Cited in This Article: ] |
| 50. | Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607-618. [PubMed] [Cited in This Article: ] |
| 51. | Chen Y, Zhao W, Yang JS, Cheng Z, Luo H, Lu Z, Tan M, Gu W, Zhao Y. Quantitative acetylome analysis reveals the roles of SIRT1 in regulating diverse substrates and cellular pathways. Mol Cell Proteomics. 2012;11:1048-1062. [PubMed] [Cited in This Article: ] |
| 52. | Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834-840. [PubMed] [Cited in This Article: ] |
| 53. | Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000-1004. [PubMed] [Cited in This Article: ] |
| 54. | Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49:186-199. [PubMed] [Cited in This Article: ] |
| 55. | Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stančáková A, Goetzman E, Lam MM, Schwer B. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177-190. [PubMed] [Cited in This Article: ] |
| 56. | Finley LW, Haigis MC. Metabolic regulation by SIRT3: implications for tumorigenesis. Trends Mol Med. 2012;18:516-523. [PubMed] [Cited in This Article: ] |
| 57. | Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32-42. [PubMed] [Cited in This Article: ] |
| 58. | Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, Watson PA, Birdsey N, Bao J, Gius D. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433:505-514. [PubMed] [Cited in This Article: ] |
| 59. | Nassir F, Ibdah JA. Role of mitochondria in nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:8713-8742. [PubMed] [Cited in This Article: ] |
| 60. | Yue P, Chen Z, Nassir F, Bernal-Mizrachi C, Finck B, Azhar S, Abumrad NA. Enhanced hepatic apoA-I secretion and peripheral efflux of cholesterol and phospholipid in CD36 null mice. PLoS One. 2010;5:e9906. [PubMed] [Cited in This Article: ] |
| 61. | Rector RS, Morris EM, Ridenhour S, Meers GM, Hsu F-F, Turk J. Selective hepatic insulin resistance in a murine model heterozygous for a mitochondrial trifunctional protein defect. Hepatology. 2013;57:2213-2223. [PubMed] [Cited in This Article: ] |
| 62. | Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, Wagner JD, Matern D, Rinaldo P, Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128:1381-1390. [PubMed] [Cited in This Article: ] |
| 63. | Geng C, Zhang Y, Gao Y, Tao W, Zhang H, Liu X, Fang F, Chang Y. Mst1 regulates hepatic lipid metabolism by inhibiting Sirt1 ubiquitination in mice. Biochem Biophys Res Commun. 2016;471:444-449. [PubMed] [Cited in This Article: ] |
| 64. | Colak Y, Yesil A, Mutlu HH, Caklili OT, Ulasoglu C, Senates E, Takir M, Kostek O, Yilmaz Y, Yilmaz Enc F. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. J Gastrointestin Liver Dis. 2014;23:311-319. [PubMed] [Cited in This Article: ] |
| 65. | Colak Y, Ozturk O, Senates E, Tuncer I, Yorulmaz E, Adali G, Doganay L, Enc FY. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit. 2011;17:HY5-HY9. [PubMed] [Cited in This Article: ] |
| 66. | Tobita T, Guzman-Lepe J, Takeishi K, Nakao T, Wang Y, Meng F, Deng CX, Collin de l’Hortet A, Soto-Gutierrez A. SIRT1 Disruption in Human Fetal Hepatocytes Leads to Increased Accumulation of Glucose and Lipids. PLoS One. 2016;11:e0149344. [PubMed] [Cited in This Article: ] |
| 67. | Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327-338. [PubMed] [Cited in This Article: ] |
| 68. | Kim KE, Kim H, Heo RW, Shin HJ, Yi CO, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS. Myeloid-specific SIRT1 Deletion Aggravates Hepatic Inflammation and Steatosis in High-fat Diet-fed Mice. Korean J Physiol Pharmacol. 2015;19:451-460. [PubMed] [Cited in This Article: ] |
| 69. | Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708-715. [PubMed] [Cited in This Article: ] |
| 70. | Andrade JM, Paraíso AF, de Oliveira MV, Martins AM, Neto JF, Guimarães AL, de Paula AM, Qureshi M, Santos SH. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915-919. [PubMed] [Cited in This Article: ] |
| 71. | Mariani S, Fiore D, Basciani S, Persichetti A, Contini S, Lubrano C, Salvatori L, Lenzi A, Gnessi L. Plasma levels of SIRT1 associate with non-alcoholic fatty liver disease in obese patients. Endocrine. 2015;49:711-716. [PubMed] [Cited in This Article: ] |
| 72. | Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664-1679. [PubMed] [Cited in This Article: ] |
| 73. | Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807-8814. [PubMed] [Cited in This Article: ] |
| 74. | Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654-661. [PubMed] [Cited in This Article: ] |
| 75. | Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121-125. [PubMed] [Cited in This Article: ] |
| 76. | Osborne B, Cooney GJ, Turner N. Are sirtuin deacylase enzymes important modulators of mitochondrial energy metabolism? Biochim Biophys Acta. 2014;1840:1295-1302. [PubMed] [Cited in This Article: ] |
| 77. | Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105:14447-14452. [PubMed] [Cited in This Article: ] |
| 78. | Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158:647-657. [PubMed] [Cited in This Article: ] |
| 79. | Osborne B, Montgomery M, Reznick J, Cooney GJ, Turner N. Effect of acute hepatic overexpression of SIRT3 on metabolic parameters in short-term high fat fed mice. Diabetologia. 2012;55:S263. [Cited in This Article: ] |
| 80. | Sebastian C, Mostoslavsky R. SIRT3 in calorie restriction: can you hear me now? Cell. 2010;143:667-668. [PubMed] [Cited in This Article: ] |
| 81. | Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802-812. [PubMed] [Cited in This Article: ] |
| 82. | Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41:139-149. [PubMed] [Cited in This Article: ] |
| 83. | Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662-667. [PubMed] [Cited in This Article: ] |
| 84. | Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci USA. 2006;103:10224-10229. [PubMed] [Cited in This Article: ] |
| 85. | Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790-801. [PubMed] [Cited in This Article: ] |
| 86. | Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49:304-311. [PubMed] [Cited in This Article: ] |
| 87. | Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, Haigis MC. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One. 2011;6:e23295. [PubMed] [Cited in This Article: ] |
| 88. | Li YH, Choi DH, Lee EH, Seo SR, Lee S, Cho EH. Sirtuin 3 (SIRT3) Regulates α-Smooth Muscle Actin (α-SMA) Production through the Succinate Dehydrogenase-G Protein-coupled Receptor 91 (GPR91) Pathway in Hepatic Stellate Cells. J Biol Chem. 2016;291:10277-10292. [PubMed] [Cited in This Article: ] |
| 89. | Fernandez-Marcos PJ, Jeninga EH, Canto C, Harach T, de Boer VC, Andreux P, Moullan N, Pirinen E, Yamamoto H, Houten SM. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep. 2012;2:425. [PubMed] [Cited in This Article: ] |
| 90. | Choudhury M, Jonscher KR, Friedman JE. Reduced mitochondrial function in obesity-associated fatty liver: SIRT3 takes on the fat. Aging (Albany NY). 2011;3:175-178. [PubMed] [Cited in This Article: ] |
| 91. | Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53:454-457. [PubMed] [Cited in This Article: ] |
| 92. | Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffé-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645-651. [PubMed] [Cited in This Article: ] |
| 93. | Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003;52:1958-1966. [PubMed] [Cited in This Article: ] |
| 94. | Nassir F, Arndt J. J, Ibdah, . Hepatic Overexpression of SIRT3 in Mice Heterozygous for Mitochondrial Trifunctional Protein Rescues Hepatic Steatosis and Improves Insulin Sensitivity. Gastroenterology. 2015;148:S973. [Cited in This Article: ] |
| 95. | He J, Hu B, Shi X, Weidert ER, Lu P, Xu M, Huang M, Kelley EE, Xie W. Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3. Mol Cell Biol. 2013;33:2047-2055. [PubMed] [Cited in This Article: ] |
| 96. | Bao J, Scott I, Lu Z, Pang L, Dimond CC, Gius D, Sack MN. SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med. 2010;49:1230-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 97. | Cheng Y, Mai J, Hou T, Ping J. MicroRNA-421 induces hepatic mitochondrial dysfunction in non-alcoholic fatty liver disease mice by inhibiting sirtuin 3. Biochem Biophys Res Commun. 2016;474:57-63. [PubMed] [Cited in This Article: ] |
| 98. | Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJ, Badger TM, Shankar K. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One. 2011;6:e24068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 99. | Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349-359. [PubMed] [Cited in This Article: ] |
| 100. | Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658-664. [PubMed] [Cited in This Article: ] |
| 101. | Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, Tsuneyama K, Fujisaka S, Bukhari A, Suzuki H, Senda S, Imanishi S. Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am J Physiol Endocrinol Metab. 2009;297:E1179-E1186. [PubMed] [Cited in This Article: ] |
| 102. | Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1392] [Cited by in F6Publishing: 1313] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 103. | Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Joshi NV, Mills NL, Hoffmann E, Jacobson EW, Vlasuk GP, Waterhouse BR. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc. 2013;2:e000042. [PubMed] [Cited in This Article: ] |
| 104. | Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612-622. [PubMed] [Cited in This Article: ] |
| 105. | Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 106. | Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340-8351. [PubMed] [Cited in This Article: ] |
| 107. | Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712-716. [PubMed] [Cited in This Article: ] |
| 108. | Libri V, Brown AP, Gambarota G, Haddad J, Shields GS, Dawes H, Pinato DJ, Hoffman E, Elliot PJ, Vlasuk GP. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One. 2012;7:e51395. [PubMed] [Cited in This Article: ] |
| 109. | Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191-196. [PubMed] [Cited in This Article: ] |
| 110. | Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333:593-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 111. | Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347-358. [PubMed] [Cited in This Article: ] |
| 112. | Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore-Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson M. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2092-2103.e1-6. [PubMed] [Cited in This Article: ] |
| 113. | Amiot MJ, Romier B, Dao TM, Fanciullino R, Ciccolini J, Burcelin R, Pechere L, Emond C, Savouret JF, Seree E. Optimization of trans-Resveratrol bioavailability for human therapy. Biochimie. 2013;95:1233-1238. [PubMed] [Cited in This Article: ] |
| 114. | Hoffmann E, Wald J, Lavu S, Roberts J, Beaumont C, Haddad J, Elliott P, Westphal C, Jacobson E. Pharmacokinetics and tolerability of SRT2104, a first-in-class small molecule activator of SIRT1, after single and repeated oral administration in man. Br J Clin Pharmacol. 2013;75:186-196. [PubMed] [Cited in This Article: ] |
| 115. | Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421-433. [PubMed] [Cited in This Article: ] |
| 116. | Sauve AA, Schramm VL. Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry. 2003;42:9249-9256. [PubMed] [Cited in This Article: ] |
| 117. | Guan X, Lin P, Knoll E, Chakrabarti R. Mechanism of inhibition of the human sirtuin enzyme SIRT3 by nicotinamide: computational and experimental studies. PLoS One. 2014;9:e107729. [PubMed] [Cited in This Article: ] |
| 118. | Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A, Altucci L. Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics. 2016;8:61. [PubMed] [Cited in This Article: ] |