Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.9871
Peer-review started: August 2, 2016
First decision: September 28, 2016
Revised: October 13, 2016
Accepted: November 14, 2016
Article in press: November 16, 2016
Published online: December 7, 2016
Over the last few years, the importance of the resident intestinal microbiota in the pathogenesis of several gastro-intestinal diseases has been largely investigated. Growing evidence suggest that microbiota can influence gastro-intestinal motility. The current working hypothesis is that dysbiosis-driven mucosal alterations induce the production of several inflammatory/immune mediators which affect gut neuro-muscular functions. Besides these indirect mucosal-mediated effects, the present review highlights that recent evidence suggests that microbiota can directly affect enteric nerves and smooth muscle cells functions through its metabolic products or bacterial molecular components translocated from the intestinal lumen. Toll-like receptors, the bacterial recognition receptors, are expressed both on enteric nerves and smooth muscle and are emerging as potential mediators between microbiota and the enteric neuromuscular apparatus. Furthermore, the ongoing studies on probiotics support the hypothesis that the neuromuscular apparatus may represent a target of intervention, thus opening new physiopathological and therapeutic scenarios.
Core tip: This article reviews the current evidence of gut microbiota and neuromuscular apparatus connection that results to be both direct and indirect. Besides dysbiosis-driven mucosal inflammatory mediators, recent evidence suggests that gut neuromuscular apparatus can be modulated directly by microbiota metabolic products or circulating bacterial molecular components translocated from the intestinal lumen.
- Citation: Guarino MPL, Cicala M, Putignani L, Severi C. Gastrointestinal neuromuscular apparatus: An underestimated target of gut microbiota. World J Gastroenterol 2016; 22(45): 9871-9879
- URL: https://www.wjgnet.com/1007-9327/full/v22/i45/9871.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i45.9871
Microbiota and gut motility are clearly associated, but it’s difficult to establish what plays the major role in influencing the other. According to the classical theory, gastrointestinal (GI) motility can affect the microbiota in terms of amount, location and diversity. This concept is mainly supported by the association between different GI motility disorders and small intestinal bacterial overgrowth (SIBO)[1,2]. GI motility disorders and alterations of migrating motor complex (MMC), that eliminates residual content through the GI tract during periods of fasting, predispose to SIBO because bacteria are not swept from the small bowel into the colon, as reported in experimental models and specific clinical conditions[3-5]. Neuropathic and myopathic diseases, such as scleroderma and polymyositis, seem to be associated with SIBO[1,6] as well as conditions associated to long-standing diabetes, such as gastroparesis[7].
On the other hand, both in vivo and in vitro evidence highlights that microbiota can affect GI motility[8,9]. In studies conducted on germ-free animals, impairment of neural and motor functions of the GI tract due to reduced expression of neurotransmitters and contractile proteins, were reversed by gut colonization[10]. Moreover, probiotics have been shown to affect GI motility in vivo and in vitro. Prebiotic or probiotic therapies are associated with a significant clinical improvement in irritable bowel syndrome (IBS)[11,12] and animal studies suggest that the neuromuscular apparatus could represent a target for probiotics[13-15]. Finally, dysbiosis is associated with significant alterations in intestinal transit time[16].
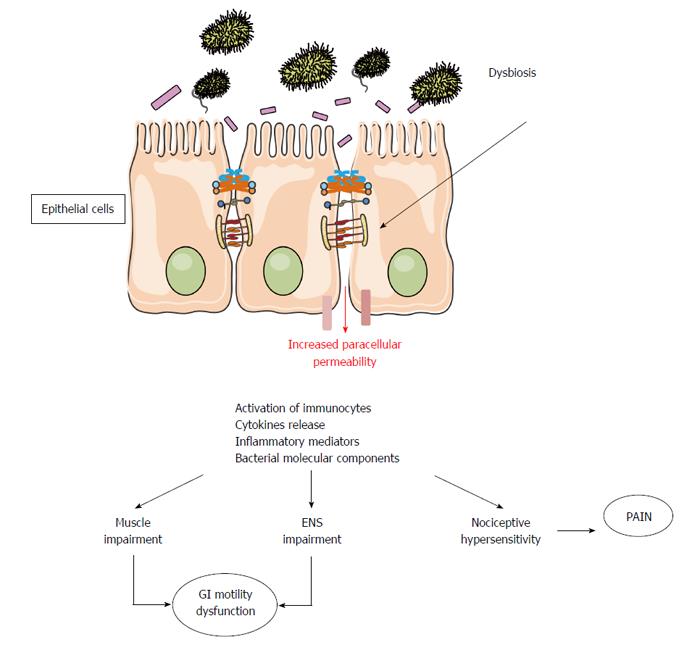
By interacting directly with mucosal environment, the microbiota impacts intestinal mucosal functions and permeability, and influences local and systemic inflammatory activity[12]. In normal conditions neuromuscular apparatus is not in contact with the luminal content and quite inaccessible by the luminal microbes. However, dysbiotic conditions cause an increase in mucosal inflammation and intestinal paracellular permeability[17,18] (Figure 1) with possible translocation of pathogens, toxins, antigens and bacteria in the circulatory system[16,19,20]. GI motility might then be affected by microbiota essentially by two mechanisms: an indirect mechanism driven by the inflammatory mediators released by the mucosal immune system and a direct mechanism driven both by the release of end products of bacterial fermentation and bacterial substances.
The potential for the microbiota to produce inflammatory alterations in the gut microenvironment deranging gastrointestinal motor function prompts to a unifying hypothesis for the role of the microbiota in the pathogenesis of IBS. To support a role of the microbiota in IBD pathophysiology is the evidence that an acute episode of gastroenteritis precedes the onset of IBS, a specific condition called post-infectious IBS (PI-IBS)[11,21,22]. PI-IBS is characterized by persistent abdominal discomfort, bloating and diarrhea, despite the elimination of the causative pathogen. In this condition, the imbalance in microbiota composition leads to low-grade inflammation followed by alteration of the sensory and motor bowel functions. An increased amount of immune cells in the colonic, ileal, and jejunal mucosa of IBS patients has been largely reported[23,24]. The persistent inflammatory state is also characterized by increased mucosal interleukin 1β levels and mast cells count, as well as activation of entero-endocrine cells (EC), mainly those producing serotonin (5-HT)[25-28]. The interesting data is that most of these mucosal alterations persist for over a year and thus could contribute to the persistence of a PI-IBS. Therefore, the mucosal inflammation resulting from an acute infection can lead to a dysfunction of intestinal motility and 5-HT could play a pivotal role as its release increases motility and secretion, features which may explain diarrheal symptoms frequent in PI-IBS patients[29]. With an experimental model of primary infection with Trichinella spiralis, that causes hypercontractility of intestinal muscle persisting for over 20 d after the infection was cleared, it was shown that chronic immune response may extend to smooth muscle layers[30]. In this model, the levels of Th2 cytokines (interleukins 4, 5, and 13) resulted increased during the acute infection but not thereafter, whereas cyclooxygenase-2 (COX-2) and relative enzymatic activity localized to muscle remained significantly increased. These effects did not occur in athymic mice, suggesting a crucial role of T cells in the impairment of intestinal muscle function in post-infective disorders[30]. The role of COX-2 in muscle impairment during inflammation has been reported both in animal and humans. During severe mucosal inflammatory conditions, it has been shown in colonic muscle cells an altered expression of contractile key-signaling molecules and an increase in nuclear factor NF-κB DNA binding, which is low or absent in normal colonic muscle cells[31-33]. In human colonic smooth muscle, NF-κB activation leads to inflammatory gene expression of COX-2 and to production of prostaglandin E, both widely considered responsible for muscle cell impairment[34-37].
Mediators released by the colonic mucosa of IBS patients are able to activate aberrant responses in the enteric nervous system[38,39] and to impair contractility of human colonic smooth muscle likely through a receptor-dependent mechanism[40]. Histamine and proteases, two soluble inflammatory products obtained from IBS biopsy supernatants, are able to excite visceral afferents neurons and to cause hyperalgesia and allodynia when introduced into the colon of mice[41,42]. Beside increased visceral sensory activation, the soluble products found in supernatants derived from the colon of IBS patients have been shown to evoke excitatory cholinergic longitudinal muscle contractions in the guinea pig ileum[43]. This effect correlates with the number of mast cells and the activation of the nerve fibers appears to be mediated by the activation of different receptors, including transient receptor potential vanilloid subfamily member 1 (TRPV1), purinergic and prostanoids receptors[43].
Many studies have been conducted in attempt to identify a specific pattern of intestinal faecal microbiota in IBS patients and, although heterogeneity of IBS patients, qualitative and quantitative alterations in intestinal microflora have been found. Differently from traditional microbial culture-based techniques, studies using DNA-based techniques showed that specific fecal and mucosal microbiota composition are associated with different subgroups of IBS patients, even if these investigations have produced non univocal results. Some studies reported increased abundance of Proteobacteria and Firmicutes and reduction in Actinobacteria and Bacteroidetes in patients with IBS[11,44] while others reported a decreased amount of Lactobacilli and Bifidobacteria[45]. A very recent meta-analysis demonstrated that composition of IBS patients microbiota vary across geographical regions. The study reported a decreased numbers of Bifidobacteria and Lactobacillus and increased numbers of Escherichia coli and Enterobacterium in Chinese IBS patients with no significant differences in the abundance of Bacteroides and Enterococcus. On the other hand, a decreased numbers of Bifidobacteria and increased numbers of Bacteroides were found in IBS patients from other regions of the world[46]. The strict relationship between dysbiosis and GI motility in IBS need to be further elucidated as one of the major challenges in IBS is the absence of an animal model that fully represent this condition.
New physiopathologic and therapeutic scenarios have arisen by the recent evidence highlighting that microbiota metabolic products or bacterial molecular components can directly affect enteric nerves and smooth muscle cells functions.
The microbiota is a formidable metabolic “organ”, not only able to capture calories from food but also to elaborate a large amount of compounds such as short-chain fatty acids (SCFAs), neurotransmitters homologs and gases that can act directly with the enteric neuromuscular apparatus[47].
SCFAs such as acetate, propionate, and butyrate are produced by bacterial fermentation of dietary fibers. SCFAs exert multiple beneficial effects and act both as signal transduction molecules, via G-protein coupled free fatty acid receptors (FFAR2, FFAR3, OLFR78, GPR109A) and regulators of gene expression[48]. Besides improving the intestinal environment, SCFAs directly affect various host peripheral tissues, generate potent motor responses and have a considerable role in regulating the propulsive activity of the gut, both in animal models and in humans. SCFAs, when administered into the human terminal ileum, have been shown to increase parietal tone and stimulate ileal propulsive contractions[49,50]. This compounds are suggested to act via either extrinsic or intrinsic afferent neurons which can ultimately stimulate myenteric cholinergic neurons[51]. Most of these responses are not observed in mucosal free preparations, suggesting that SCFAs receptors are located on mucosal EC cells. In particular, propionate acts on receptors in the mucosa causing the release of 5-HT from EC cells that activates, through 5-HT4 receptors on the endings of intrinsic primary afferent neurons, the enteric peristaltic reflex pathways[51]. In the rat distal colon, propionate causes also tonic contraction via prostaglandin release[52]. Similarly, butyrate and acetate may also affect GI motility through several mechanisms including direct effects on smooth muscle and myenteric neurons[53] and production of mucosal 5-HT[54]. SCFAs receptors have been also localized in mucosal EC cells containing peptide YY that might represent another important messenger in transducing this contractile signal[55]. However, the effect of these metabolites still remain controversial; a recent human study found no significant differences in global motility index after intracolonic infusion of SCFAs[56].
Deconjugated bile salts, another bacterial metabolite[57], have also been reported to affect gastrointestinal motility through activation of transmembrane G-protein coupled receptor (TGR5)[58]. In animals, TGR5 have been detected in inhibitory intestinal motor neurons and on gallbladder smooth muscle cells[59]. The direct activation of TGR5 causes relaxation of the smooth muscle cells and inhibition of gallbladder contractility resulting in gallbladder filling. In humans, treating normal gallbladder muscle cells with a hydrophobic bile acid, the tauro-chenodeoxycholic acid, results in impairment of contraction to cholecystokinin due to a significant reduction in receptor binding and an increase in inflammatory mediators and oxidative stress[60,61]. These latter abnormalities, observed also in gallstone patients, are prevented by treatment with the hydrophilic ursodeoxycholic acid[61,62].
Among microbiota compounds that might influence GI motility, there is tryptamine, a secondary metabolite resulting from the transformation of the aromatic amino acid tryptophan, that mimics the serotonin stimulatory effects on motility in ex vivo preparations of guinea pig ileum[63]. It is of note that most genes encoding amino-acid-metabolizing enzymes involved in the synthesis of neurotransmitters (catecholamines, serotonin/melatonin, acetylcholine) are present in the microbiota genome[64]. Commensal bacteria have also been shown to be a significant source of nitric oxide (NO), a key molecule in the control of gut motor functions[65].
Finally, fermentation by the anaerobic flora of the undigested polysaccharide fraction of certain carbohydrates generates gases, mostly hydrogen (H2) and methane (CH4). Even if clinical studies are still controversial, experimental evidence has been provided that methane is not an inert intestinal gas since it can affect the intestinal neuromuscular function[66]. In animal models, it has been shown that intestinal methane infusion slowed down small intestinal transit time and augmented ileal circular muscle contractile activity[66,67]. In turn, in an ex vivo experiment on guinea pig gut, H2 by itself has been reported to significantly shorten colonic transit times, this effect being restored by methane[68]. Finally, the resident sulfate-reducing bacteria produce hydrogen sulfide (H2S) that inhibits intestinal contractile activity acting on interstitial cells of Cajal and enteric extrinsic neurons[66]. The effects of fermentation products on GI motility are summarized in Table 1.
| Fermentation product | Effect on GI motility | Mechanism | Ref. |
| Short-chain fatty acids | Increase of ileal tone and propulsive contractions | Activation of G-protein coupled free fatty acid receptors (FFAR2, FFAR3, OLFR78, GPR109A) | [48-55] |
| Smooth muscle and myenteric neurons activation | Release of 5-HT from EC cells | ||
| Release of prostaglandins | |||
| Deconjugated bile salts | Relaxation of gallbladder smooth muscle cells | Activation of transmembrane G-protein coupled receptor | [58-61] |
| Inhibition of gallbladder contractility | Reduction in cholecystokinin receptor binding | ||
| Increase of inflammatory mediators and oxidative stress | |||
| Tryptamine | Stimulation of ileum motility | Synthesis of neurotransmitters | [63-65] |
| Gases | Decrease of small intestinal transit time | Methane (CH4) production | [66,67] |
| Augmented ileal circular muscle contractile activity | |||
| Shortening of colonic transit times | Hydrogen (H2) production | [68] | |
| Inhibition of intestinal contractile activity | Hydrogen sulfide (H2S) production | [66] |
One of the main mechanisms of bacterial recognition are toll-like receptors (TLRs) a family of pattern recognition receptors that are emerging as potential mediators between microbiota and the enteric neuromuscular apparatus. TLR-dependent signaling regulates structural integrity in both the myenteric and submucosal plexus[69,70]. The mRNA encoding for TLRs have been detected on neurons[71], glial[72] and smooth muscle cells[73]. TLR-2 activation on smooth muscle leads to the production of neurotrophins that enhance the structural and functional integrity of the enteric nervous system[74].
In acute inflammatory conditions an excessive increase of mucosal permeability leads to luminal bacteria/endotoxins translocation[75]. Bacteria or bacterial products can migrate from the intestinal lumen to mesenteric lymph nodes, or the circulation, due to the disruption of the normal host/flora equilibrium as reported in cirrhosis[76], inflammatory bowel diseases[77] and recently in diarrhea-predominant IBS patients[78].
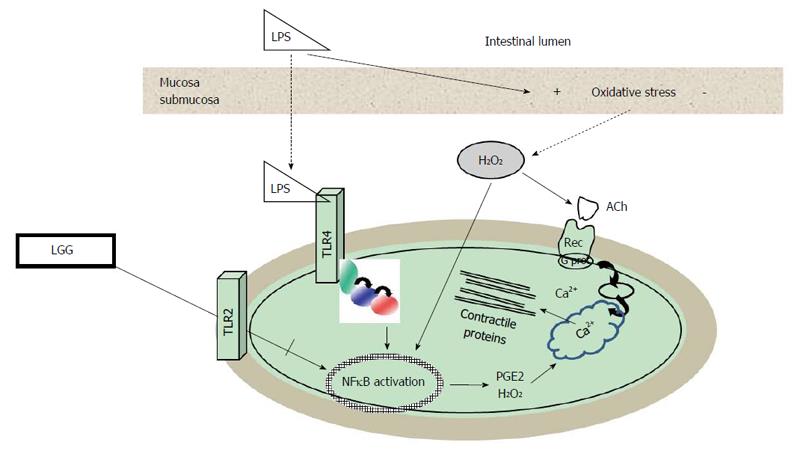
Most evidence on the effects of bacterial components on the neuromuscular apparatus derives from studies on lipopolysaccharide (LPS), the major component of the outer membrane of Gram-negative bacteria. Although the exact mechanisms whereby LPS is able to impair muscle contractility are still to be established, various targets have been demonstrated. LPS can directly activate muscular TLR4 inducing a time- and concentration-dependent impairment of contractility associated to cytoskeleton alterations, together with an intracellular oxidative imbalance as shown on human colonic smooth muscle cells[79] (Figure 2). Many of these effects persisted even after LPS withdrawn suggesting that motility dysfunction might play a pivotal role both during an acute infective process and after its resolution. In an experimental model that enables to stimulate human intestinal mucosa in a polarized fashion with LPS[37], it has been shown that LPS affects enteric contractility both through translocation from the mucosa and submucosa, with subsequent activation of TLR expressed in muscle, and through mucosal production of oxygen free radicals. LPS effects on human smooth muscle were reversed by the H2O2 scavenger catalase, by NFκB transcription inhibitors and by indomethacin, which blocks activation of COX2[37]. Besides, LPS can directly activate macrophages embedded within the intestinal muscularis externa that produce inflammatory mediators that indirectly alter smooth muscle contractility[80,81].
Interestingly, the expression of multiple TLRs receptors subtypes differentially activated by bacterial antigens on the enteric neuromuscular apparatus seems to allow a discrimination between pathogens and probiotics, as reported for both human enteric glial[72], smooth muscle cells[73,82]. The crosstalk between TLRs subtypes is emerging as an important regulatory defense mechanism also in neuromuscular apparatus[83]. On human colonic smooth muscle cells, it has been observed that the activation of TLR2, whose ligands are the components of the outer membrane of Gram-positive bacteria, prevents LPS-induced muscular alterations. By interacting with this receptor, Lactobacillus rhamnosus GG (LGG) is able to reduce LPS-induced NFκB activation and inflammatory IL6 secretion cytokine and to restore the levels of secretion of anti-inflammatory cytokine IL10[82]. These in vitro studies support the recent evidence that indicates the neuromuscular apparatus as possible target for probiotics[13-15]. Escherichia coli strain Nissle 1917 specifically modulates contractility of human colonic muscle strips[84], Lactobacillus species regulate jejunal motility[14], colonic neuron excitability[15] and attenuate post-infective muscle hypercontractility[85]. Bifidobacterium and Lactobacillus also alleviate visceral hypersensitivity and recover intestinal barrier function as well as inflammation[86]. Also in humans recent evidence further suggests that probiotics might be effective in neuro-motor disorders[87,88].
In summary, the current working hypothesis is that dysbiosis-driven mucosal alterations induce the production of several inflammatory/immune mediators which affect gut neuro-muscular functions suggesting a potential for disturbances in the microbiota to elicit directly intestinal dismotility or, if sustained, to lead to chronic sensory-motor dysfunction. The understanding in these fields would hopefully open new therapeutic scenarios in GI disease with underlying neuromuscular disorders as manipulation of gut microbiota composition could also correct the mechanisms promoting development and maintenance of symptoms.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dumitrascu DL, Plaza MA, Xu WX S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y). 2007;3:112-122. [PubMed] [Cited in This Article: ] |
| 2. | Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 597] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Nieuwenhuijs VB, Verheem A, van Duijvenbode-Beumer H, Visser MR, Verhoef J, Gooszen HG, Akkermans LM. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg. 1998;228:188-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Gunnarsdottir SA, Sadik R, Shev S, Simrén M, Sjövall H, Stotzer PO, Abrahamsson H, Olsson R, Björnsson ES. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98:1362-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Strid H, Simrén M, Stotzer PO, Ringström G, Abrahamsson H, Björnsson ES. Patients with chronic renal failure have abnormal small intestinal motility and a high prevalence of small intestinal bacterial overgrowth. Digestion. 2003;67:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Kaye SA, Lim SG, Taylor M, Patel S, Gillespie S, Black CM. Small bowel bacterial overgrowth in systemic sclerosis: detection using direct and indirect methods and treatment outcome. Br J Rheumatol. 1995;34:265-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Armbrecht U, Lundell L, Lindstedt G, Stockbruegger RW. Causes of malabsorption after total gastrectomy with Roux-en-Y reconstruction. Acta Chir Scand. 1988;154:37-41. [PubMed] [Cited in This Article: ] |
| 8. | Quigley EM. Microflora modulation of motility. J Neurogastroenterol Motil. 2011;17:140-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560-2568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1565] [Cited by in F6Publishing: 1424] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 11. | Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:497-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 12. | Foxx-Orenstein AE, Chey WD. Manipulation of the Gut Microbiota as a Novel Treatment Strategy for Gastrointestinal Disorders. Am J Gastroenterol Suppl. 2012;1:41-46. [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Massi M, Ioan P, Budriesi R, Chiarini A, Vitali B, Lammers KM, Gionchetti P, Campieri M, Lembo A, Brigidi P. Effects of probiotic bacteria on gastrointestinal motility in guinea-pig isolated tissue. World J Gastroenterol. 2006;12:5987-5994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Wang B, Mao YK, Diorio C, Pasyk M, Wu RY, Bienenstock J, Kunze WA. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. FASEB J. 2010;24:4078-4088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13:2261-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2501] [Cited by in F6Publishing: 2604] [Article Influence: 186.0] [Reference Citation Analysis (1)] |
| 17. | Barbara G, Zecchi L, Barbaro R, Cremon C, Bellacosa L, Marcellini M, De Giorgio R, Corinaldesi R, Stanghellini V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46 Suppl:S52-S55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 594] [Cited by in F6Publishing: 601] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 19. | Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21:1691-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 110] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 20. | Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol. 2015;7:425-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, Gray JJ, Letley LH, Rait G, Tompkins DS. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 376] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 23. | Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, De Giorgio R, Corinaldesi R, Stanghellini V. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol. 2014;20:2456-2469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 102] [Cited by in F6Publishing: 99] [Article Influence: 9.9] [Reference Citation Analysis (2)] |
| 25. | Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J Neurogastroenterol Motil. 2012;18:258-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 300] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 805] [Cited by in F6Publishing: 788] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 28. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 554] [Cited by in F6Publishing: 551] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 29. | Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 2004;5:55-60. [PubMed] [Cited in This Article: ] |
| 30. | Barbara G, De Giorgio R, Deng Y, Vallance B, Blennerhassett P, Collins SM. Role of immunologic factors and cyclooxygenase 2 in persistent postinfective enteric muscle dysfunction in mice. Gastroenterology. 2001;120:1729-1736. [PubMed] [Cited in This Article: ] |
| 31. | Shi XZ, Lindholm PF, Sarna SK. NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology. 2003;124:1369-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 566] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 33. | Funakoshi T, Yamashita K, Ichikawa N, Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T, Ichihara S. A novel NF-κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J Crohns Colitis. 2012;6:215-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Salinthone S, Singer CA, Gerthoffer WT. Inflammatory gene expression by human colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G627-G637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Khan I, Oriowo MA. Mechanism underlying the reversal of contractility dysfunction in experimental colitis by cyclooxygenase-2 inhibition. Inflammopharmacology. 2006;14:28-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Rebollar E, Arruebo MP, Plaza MA, Murillo MD. Effect of lipopolysaccharide on rabbit small intestine muscle contractility in vitro: role of prostaglandins. Neurogastroenterol Motil. 2002;14:633-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Guarino MP, Sessa R, Altomare A, Cocca S, Di Pietro M, Carotti S, Schiavoni G, Alloni R, Emerenziani S, Morini S. Human colonic myogenic dysfunction induced by mucosal lipopolysaccharide translocation and oxidative stress. Dig Liver Dis. 2013;45:1011-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 533] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 39. | Nasser Y, Boeckxstaens GE, Wouters MM, Schemann M, Vanner S. Using human intestinal biopsies to study the pathogenesis of irritable bowel syndrome. Neurogastroenterol Motil. 2014;26:455-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Guarino MP, Barbara G, Cicenia A, Altomare A, Barbaro MR, Cocca S, Scirocco A, Cremon C, Emerenziani S, Stanghellini V. Supernatants of irritable bowel syndrome mucosal biopsies impair human colonic smooth muscle contractility. Neurogastroenterol Motil. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 432] [Cited by in F6Publishing: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 42. | Buhner S, Li Q, Berger T, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Schemann M. Submucous rather than myenteric neurons are activated by mucosal biopsy supernatants from irritable bowel syndrome patients. Neurogastroenterol Motil. 2012;24:1134-e572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Balestra B, Vicini R, Cremon C, Zecchi L, Dothel G, Vasina V, De Giorgio R, Paccapelo A, Pastoris O, Stanghellini V. Colonic mucosal mediators from patients with irritable bowel syndrome excite enteric cholinergic motor neurons. Neurogastroenterol Motil. 2012;24:1118-e570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 45. | Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J Gastroenterol. 2016;22:2219-2241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 210] [Cited by in F6Publishing: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 46. | Zhuang X, Xiong L, Li L, Li M, Chen MH. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 47. | Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 774] [Cited by in F6Publishing: 831] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 48. | Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839-2849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 49. | Coffin B, Lémann M, Flourié B, Jouet P, Rambaud JC, Jian R. Local regulation of ileal tone in healthy humans. Am J Physiol. 1997;272:G147-G153. [PubMed] [Cited in This Article: ] |
| 50. | Kamath PS, Phillips SF, Zinsmeister AR. Short-chain fatty acids stimulate ileal motility in humans. Gastroenterology. 1988;95:1496-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 107] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292:G429-G437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Haschke G, Schafer H, Diener M. Effect of butyrate on membrane potential, ionic currents and intracellular Ca2+ concentration in cultured rat myenteric neurones. Neurogastroenterol Motil. 2002;14:133-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269-R1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 55. | Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 56. | Jouët P, Moussata D, Duboc H, Boschetti G, Attar A, Gorbatchef C, Sabaté JM, Coffin B, Flourié B. Effect of short-chain fatty acids and acidification on the phasic and tonic motor activity of the human colon. Neurogastroenterol Motil. 2013;25:943-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1367] [Cited by in F6Publishing: 1460] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 58. | Penney NC, Kinross J, Newton RC, Purkayastha S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes (Lond). 2015;39:1565-1574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 59. | Lavoie B, Balemba OB, Godfrey C, Watson CA, Vassileva G, Corvera CU, Nelson MT, Mawe GM. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels. J Physiol. 2010;588:3295-3305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Parkman HP, Bogar LJ, Bartula LL, Pagano AP, Thomas RM, Myers SI. Effect of experimental acalculous cholecystitis on gallbladder smooth muscle contractility. Dig Dis Sci. 1999;44:2235-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Guarino MP, Cong P, Cicala M, Alloni R, Carotti S, Behar J. Ursodeoxycholic acid improves muscle contractility and inflammation in symptomatic gallbladders with cholesterol gallstones. Gut. 2007;56:815-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 62. | Guarino MP, Carotti S, Morini S, Perrone G, Behar J, Altomare A, Alloni R, Caviglia R, Emerenziani S, Rabitti C. Decreased number of activated macrophages in gallbladder muscle layer of cholesterol gallstone patients following ursodeoxycholic acid. Gut. 2008;57:1740-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Takaki M, Mawe GM, Barasch JM, Gershon MD, Gershon MD. Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience. 1985;16:223-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004;20:292-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Sobko T, Huang L, Midtvedt T, Norin E, Gustafsson LE, Norman M, Jansson EA, Lundberg JO. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic Biol Med. 2006;41:985-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil. 2014;20:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 67. | Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, Park S, Kong Y, Conklin J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089-G1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 284] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 68. | Jahng J, Jung IS, Choi EJ, Conklin JL, Park H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol Motil. 2012;24:185-190, e92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Kamada N, Kao JY. The tuning of the gut nervous system by commensal microbiota. Gastroenterology. 2013;145:1193-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006-1016.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 71. | Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57:1013-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 72. | Turco F, Sarnelli G, Cirillo C, Palumbo I, De Giorgi F, D’Alessandro A, Cammarota M, Giuliano M, Cuomo R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut. 2014;63:105-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 73. | Tattoli I, Petitta C, Scirocco A, Ammoscato F, Cicenia A, Severi C. Microbiota, innate immune system, and gastrointestinal muscle: ongoing studies. J Clin Gastroenterol. 2012;46 Suppl:S6-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Brun P, Gobbo S, Caputi V, Spagnol L, Schirato G, Pasqualin M, Levorato E, Palù G, Giron MC, Castagliuolo I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci. 2015;68:24-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol. 1999;473:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 278] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 76. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 484] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 77. | Pastor Rojo O, López San Román A, Albéniz Arbizu E, de la Hera Martínez A, Ripoll Sevillano E, Albillos Martínez A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:269-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Dlugosz A, Nowak P, D’Amato M, Mohammadian Kermani G, Nyström J, Abdurahman S, Lindberg G. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1747-1754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 79. | Matarrese P, Petitta C, Scirocco A, Ascione B, Ammoscato F, Di Natale G, Anastasi E, Marconi M, Chirletti P, Malorni W. Antioxidants counteract lipopolysaccharide-triggered alterations of human colonic smooth muscle cells. Free Radic Biol Med. 2012;53:2102-2111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Eskandari MK, Kalff JC, Billiar TR, Lee KK, Bauer AJ. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Physiol. 1999;277:G478-G486. [PubMed] [Cited in This Article: ] |
| 81. | Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 422] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 82. | Ammoscato F, Scirocco A, Altomare A, Matarrese P, Petitta C, Ascione B, Caronna R, Guarino M, Marignani M, Cicala M. Lactobacillus rhamnosus protects human colonic muscle from pathogen lipopolysaccharide-induced damage. Neurogastroenterol Motil. 2013;25:984-e777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Kotaki R, Wajima S, Shiokawa A, Hachimura S. Toll-like receptor 2 suppresses Toll-like receptor 9 responses in Peyer’s patch dendritic cells. Immunobiology. 2015;220:734-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Bär F, Von Koschitzky H, Roblick U, Bruch HP, Schulze L, Sonnenborn U, Böttner M, Wedel T. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil. 2009;21:559-66, e16-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Verdú EF, Bercík P, Bergonzelli GE, Huang XX, Blennerhasset P, Rochat F, Fiaux M, Mansourian R, Corthésy-Theulaz I, Collins SM. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004;127:826-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 86. | Wang H, Gong J, Wang W, Long Y, Fu X, Fu Y, Qian W, Hou X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS One. 2014;9:e90153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 87. | Kim SE, Choi SC, Park KS, Park MI, Shin JE, Lee TH, Jung KW, Koo HS, Myung SJ. Change of Fecal Flora and Effectiveness of the Short-term VSL#3 Probiotic Treatment in Patients With Functional Constipation. J Neurogastroenterol Motil. 2015;21:111-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 88. | Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072-3084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 232] [Cited by in F6Publishing: 205] [Article Influence: 22.8] [Reference Citation Analysis (3)] |