Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9117
Peer-review started: June 29, 2016
First decision: August 8, 2016
Revised: August 25, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: November 7, 2016
To evaluate the inflammatory state in Crohn’s disease (CD) patients and correlate it with genetic background and microbial spreading.
By means of flow cytometry, production of tumor necrosis factor-alpha (TNF-α) was measured in peripheral blood monocytes from patients suffering from CD, ulcerative colitis (UC) and in healthy subjects after stimulation of the NOD2 and TLR pathways. CD patients were genotyped for the three most common NOD2 variants (R702W, G908R and L1007Pfs*2) and basal production of TNF-α was correlated to NOD2 genotype. Also, production of TNF-α was correlated to plasmatic levels of LPS Binding Protein (LBP), soluble (s) CD14 and to the activity state of the disease.
The patients with CD were characterized by a significantly higher monocyte basal expression of TNF-α compared with healthy subjects and UC patients, and after stimulation with Pam3CSK4 (ligand of TLR2/1) and MDP-L18 (ligand of NOD2) this difference was maintained, while other microbial stimuli (LPS, ligand of TLR4 and PolyI:C, ligand of TLR3) induced massive activation in CD monocytes as well as in UC and in healthy control cells. There was no significant difference in the production of TNF-α between patients who carried CD-associated heterozygous or homozygous variants in NOD2 and patients with wild type NOD2 genotype. Although serum LBP levels have been shown to correlate positively with the state of activity of the disease, TNF-α production did not show a clear correlation with either LBP or sCD14 levels in plasma. Moreover, no clear correlation was seen between TNF-α production and activity indices in either CD or UC.
Peripheral monocytes from CD express higher basal and stimulated TNF-α than controls, regardless of NOD2 genotype and without a clear correlation with disease activity.
Core tip: Crohn’s disease (CD) is characterized by an aberrant activation of the mucosal immune system in genetically susceptible subjects, who often harbor variants in genes involved in the innate immunity. To study the integrity of innate immune response, the activity of the TLR and NOD2 pathways was investigated, measuring TNF-α expression in peripheral blood monocytes. CD monocytes showed a higher production of TNF-α, which was not clearly related to disease activity, to NOD2 genotype or to the presence of translocated bacteria (indirectly measured by serum LPS-binding protein), indicating that this TNF-α hyper-production may rely on a NOD2-independent pathway and is not due to systemic exposure to LPS.
- Citation: Loganes C, Pin A, Naviglio S, Girardelli M, Bianco AM, Martelossi S, Tommasini A, Piscianz E. Altered pattern of tumor necrosis factor-alpha production in peripheral blood monocytes from Crohn's disease. World J Gastroenterol 2016; 22(41): 9117-9126
- URL: https://www.wjgnet.com/1007-9327/full/v22/i41/9117.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i41.9117
Inflammatory bowel diseases (IBD) are complex inflammatory conditions that include different chronic and relapsing intestinal diseases, such as Crohn’s disease (CD) and ulcerative colitis (UC). Both genetic and environmental factors are thought to play a role in determining the disease phenotype[1,2].
On the one hand, CD and UC both involve intestinal mucosa and share some clinical symptoms, i.e., bloody diarrhoea, abdominal pain and weight loss. On the other hand, they present peculiar clinical and pathogenic features. Indeed, major differences between the two pathologies are found in the localization of lesions: while CD interests the small intestine (ileum) in particular, UC manifestations are localized mainly in the colon and rectum. Moreover, CD intestinal lesions, differently from UC, cause transmural inflammation and are characterized by skip lesions, alternating inflamed and not inflamed regions[3].
Diagnosis is based on clinical symptoms and medical history of the patients and then confirmed by endoscopic evaluations and histology. The classification of the disease, which is useful for prognosis and management of patients, is based on index of activity. In particular, the reference scores for pediatric patients is the Pediatric Crohn’s disease Activity Index (PCDAI)[4] and the Pediatric Ulcerative Colitis Activity Index (PUCAI)[5,6].
The immunopathogenesis underlying CD involves immune cells, such as monocytes, macrophages, neutrophils, recruited and activated via inflammatory cytokines, i.e., interleukin 6, interleukin 10, Interferon-gamma and Tumor Necrosis Factor-alpha (TNF-α) as well as commensal microbiota[7]. In contrast, lymphocytic immune dysregulation seems to play an important role in UC, as shown by the increase of T cells activation markers and cytokines. Several gene variants are associated with the activity of the T cells and with the control of mucosal inflammation[8].
Although the etiopathology of CD is still unclear, several studies have linked the onset of the disease with an imbalance between mucosal immune system and microbiota[9,10]. For this reason, it has been suggested that CD inflammation may represent the compensatory response to a variety of minor defects in mucosal innate immunity. These deficiencies may account for changes in the intestinal microbiota (dysbiosis), which, together with multifactorial damages of the intestinal barrier, may facilitate bacterial translocation to the lamina propria and engagement of inflammatory cells. Microbial components from translocated bacteria can reach mesenteric lymph nodes and the bloodstream, making it possible to measure lipopolysaccharide (LPS), LPS-binding Protein (LBP) and soluble (s)CD14, as markers of bacterial spread across the intestinal mucosa[11]. Moreover, the circulating microbial components can induce excessive stimulation of toll like receptors (TLRs) followed by exacerbated activation of the immune system. It has been proposed that genetic variants in nucleotide oligomerization domain 2 (NOD2), the first gene associated with IBD[12-14], may contribute to increasing the risk of disease by interfering with the response of monocytes to bacterial compounds. NOD2 is a cytoplasmic receptor that specifically recognizes muramyl dipeptide (MDP) present on the bacterial wall. It is involved in the regulation of acute inflammation, secreting pro-inflammatory cytokines such as TNF-α[15]. However, despite the huge amount of researches on this topic, it is still not clear how CD-associated NOD2 variants may contribute to the pathogenesis of the disease. On the one hand, dysfunctional pathogen associated molecular patterns (PAMPs) sensing in CD, involving NOD2 and other TLR pathways, could lead to altered shaping of gut microbial species, or dysbiosis[16-18]. On the other hand, NOD2 deficiency could lead to impaired autophagy and inflammation, with excessive response to TLR stimulation[19].
All this considered, a first aim of this study was to evaluate NOD2 and TLR signaling pathway integrity in CD. Immune activation was evaluated in terms of production of TNF-α by peripheral monocytes, since the NOD2 pathway is particularly important in these cells. Moreover peripheral blood monocytes are good representatives of the innate immune system and can easily be obtained from patients during blood sampling performed for clinical purposes.
CD monocytes were activated with different purified microbial compounds [MDP-L18 (NOD2 ligand), LPS (TLR4 ligand), Pam3CSK4 (TLR2/1 ligand), Poly I:C (TLR3 ligand)]. Production of TNF-α was then evaluated by flow cytometry in comparison with samples from UC and healthy donors. Intracellular production of TNF-α was correlated with the activity state of the disease, with the genotype of the patients and with the levels of plasmatic LBP and sCD14.
As expected, CD patients displayed higher monocyte TNF-α production in basal condition and after stimulation of NOD2 and TLR2/1. Conversely, stimulation of TLR3 and TLR4 induced similar TNF-α production among CD, UC and healthy donors, suggesting that in CD, monocytes might be primed by mycobacterial components that can induce reduced tolerance to PAMPs. Neither the variants in NOD2 susceptibility gene nor the state of activity of the disease (PCDAI score) correlated with intracellular TNF-α. Moreover, the levels of plasmatic LPB, which have been shown to correlate positively with the activity of the disease, did not exhibit a clear correlation with monocyte activation.
Monocytes were obtained from heparinized blood of 38 CD patients, 31 UC patients (diseased controls) and 50 healthy donors (HD; negative controls) subsequent to receiving written consent. All subjects were recruited from the Gastroenterology and Clinical Nutrition Unit of the Institute for Maternal and Child Health IRCCS “Burlo Garofolo”, Trieste, Italy. The IBD patients were classified according to the disease activity evaluated by physician global assessment (based on PCDAI and PUCAI scores).
The present study was approved by the Institutional Review Board of the Institute for Maternal and Child Health “Burlo Garofolo” (RC 03/2009).
This cytometric technique allows for the detection of intracellular TNF-α in monocytes stimulated with synthetic microbial stimuli, to investigate the functionality of the innate immune pathway. The protocol was adapted from Takada et al[20].
Briefly, Peripheral Blood Mononuclear Cells (PBMCs) were isolated from whole heparinized blood by centrifugation on Ficoll separating solution (Lympholite, Cederlane, Burlington, NC, United States) at 500 ×g for 30 min at room temperature. 2 × 105 PBMCs were resuspended in 200 μL of culture medium (RPMI, EuroClone, Milano, Italy) supplemented with 10% human AB serum (Sigma Aldrich, Milano, Italy), 2 mmol/L L-glutamine (EuroClone), 100 U/mL penicillin (EuroClone) and 0.1 mg/mL streptomycin (EuroClone) and stimulated with TLR and NOD2 ligands: 100 ng/ml Lipopolysaccharide (LPS; TLR4 ligand, Sigma Aldrich), 10 μg/mL Polyinosinic-polycytidylic acid sodium salt (PolyI:C; TLR3 ligand, Sigma Aldrich), 500 ng/mL L-18 Muramyl DiPeptide (MDP-L18; NOD2 ligand, InvivoGen, San Diego, CA, United States), 500 ng/mL Pam3CSK4 (TLR2/1 ligand, InvivoGen) for an initial period of 30 min at 37 °C, in a CO2 incubator. Subsequently, 10 μg/mL of Brefeldin A (BFA, Sigma Aldrich) were added to inhibit the secretion of newly synthesized cytokines and cells were incubated for additional 3.5 h. BFA, without any stimulus, was added to the unstimulated control tube. After incubation, FITC conjugated anti-CD14 antibody (eBiosciences, San Diego, CA, United States) was added for surface staining to identify monocytes, followed by a fixation step with FACS Lysing Solution (BD Biosciences, San Jose, CA, United States). After centrifugation at 300 ×g for 8 min, cells were permeabilized using FACS Permeabilizing Solution 2 (BD Biosciences). PBMCs were washed with Wash Buffer (PBS + 1% BSA + 0.1% NaN3), and anti-TNF-α PE antibody (BD Biosciences) was added to perform the intracellular staining. A fluorescent-conjugated isotype control antibody was used to detect non-specific bindings (PE IgG Isotype control, BD Biosciences). Finally, after an additional wash, the cells were fixed with PBS + 1% paraformaldehyde for analysis on a flow cytometer. Data were acquired on a CyAn ADP flow cytometer (Beckman Coulter, Fort Collins, CO, United States) and analyzed using FlowJo software v 7.6 (TreeStar, Ashland, OR, United States). Results are expressed as percent of TNF-α positive monocytes, after gating on CD14 positive cells.
CD patients were screened using targeted gene sequencing to identify the three principal variants in NOD2 susceptibility gene [c.2104C>T;p.R702W (rs2066844), c.2722G>C;p.G908R (rs2066845) and c.3016_3017insC; p.L1007Pfs*2 (rs2066847)].
DNA samples were collected from patients suffering from CD (n = 36). Genomic DNA of each patient was extracted from 1-2 ml EDTA-anticoagulated blood using the EZ1 DNA Blood Kit (QIAGEN, Valencia, CA, United States) according to the manufacturer’s instructions. To analyze the three IBD associated variants in the NOD2 gene (NM_022162), Polymerase chain reaction (PCR) amplification was performed using the KAPA 2G Fast Hot Start Readymix (RESNOVA, Rome, Italy). Purified PCR products were directly sequenced in both directions using the amplification primers by the ABI PRISM 3130XL automated DNA Sequencer (Applied Biosystems, United States). Sequences were analyzed using Seqman II Software (DNASTAR I Lasergene 7.0, Madison, WI, United States). Primers, used for PCR and sequencing were designed using Primer Blast, based on the relative sequence deposited in GeneBank. The specific primers for each variant are listed below:
R702W_For 5’-CTTCAACCTTCTGCAGGGC-3’
R702W_Rev 5’- GGTGGCAGAGGCGAAGCT-3’
R702W_sequencing_For 5’-TGCTGATGTGCCACCAG-3’
G908R_For 5’-CTGCCCCTCTGGCTGGGACT-3’
G908R_Rev 5’-CCCAGCTCCTCCCTCTTC-3’
L1007Pfs*2_For 5’-GTAGACTGGCTAACTCCTGC-3’
L1007Pfs*2_Rev 5’-AGGAGGGCGGGAGCTGACTT-3
Samples were collected from patients suffering from CD (n = 27), UC (n = 22) and from healthy individuals (n = 36). Plasma samples were obtained by centrifuging heparinized blood at 1300 ×g for 10 min and were stored at -80 °C until LBP and sCD14 levels were measured.
Quantifications were performed using the LPS-binding Protein (Human) ELISA kit (Abnova, Taipei City, Taiwan) and the human CD14 ELISA kit (RayBio, Norcross GA, United States), following the manufacturer’s instructions.
Absorptions were measured at 450 nm with a GloMax®-Multi+ Microplate Multimode Reader (Promega Corporation, Madison, United States). The concentration of each sample was calculated from the standard curve, obtained by plotting absorbance vs known standard concentrations.
All statistical analyses were performed using GraphPad Prism software version 5 (GraphPad, SanDiego, United States).
Data on intracellular TNF-α production are expressed as mean ± standard error of mean (SEM), while values of plasmatic proteins are described as medians and interquartile ranges and are represented with box plots using Tukey’s whiskers.
Statistical significance was assessed by one-way analysis of variance (ANOVA) with Kruskal-Wallis non-parametric test and denoted by letters (aP < 0.05, bP < 0.01, cP < 0.001).
The clinical characteristics of all the patients included in the study are presented in Supplementary Table 1 and summarized in Table 1.
| Patients | Age (yr) | Male/female | Active/remission |
| (n = 69) | (mean ± SD) | ||
| CD (n = 38) | 13 ± 4.47 | 25/13 | 18/20 |
| UC (n = 31) | 13 ± 5.13 | 15/16 | 19/12 |
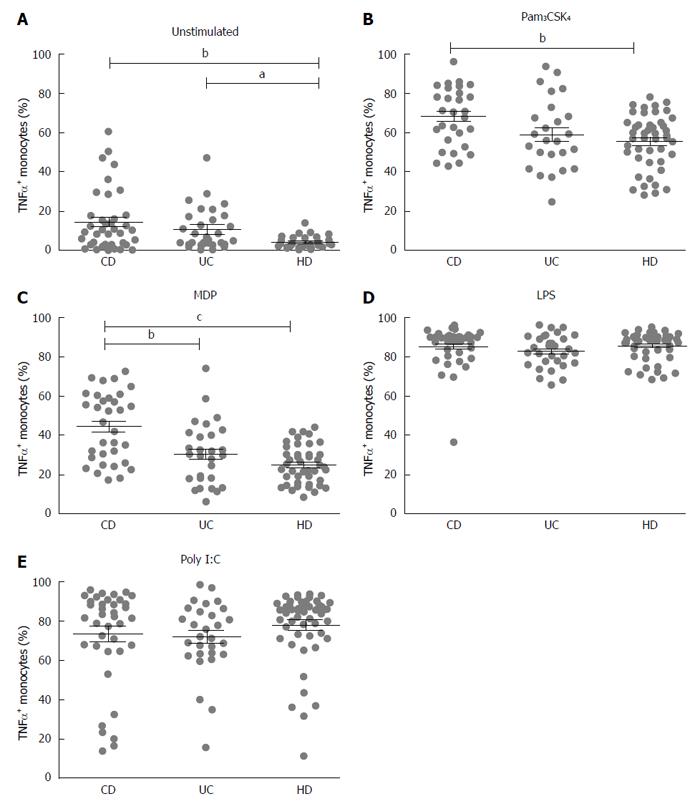
To study the production of TNF-α by monocytes, cells were isolated from peripheral blood of patients with CD and UC and of healthy donors (HD). Monocytes were stimulated with TLR and NOD2 purified stimuli: LPS (TLR4 ligand), Pam3CSK4 (TLR2/1 ligand), Poly I:C (TLR3 ligand) and MDP-L18 (NOD2 ligand). TNF-α expression in monocytes was analyzed by flow cytometry after gating on CD14 positive cells.
Both CD and UC showed increased basal expression of TNF-α (unstimulated) compared to healthy donors (HD 4.3 ± 0.4; CD 14.5 ± 2.5, P < 0.01; UC 10.7 ± 1.9, P < 0.05) (Figure 1A).
After stimulation with Pam3CSK4 (TLR2/1 ligand), a higher percentage of activated monocytes producing TNF-α was recorded in CD compared with HD (CD vs HD: 68.4 ± 2.7 vs 55.6 ± 2.1, P < 0.01) (Figure 1B), while stimulation with MDP-L18 (NOD2 ligand) induced a significant higher production of TNF-α in CD compared to both HD and UC (CD 44.2 ± 3.0; UC 30.2 ± 3.0, P < 0.01; HD 24.9 ± 1.4, P < 0.001) (Figure 1C).
Stimulation with LPS (TLR4 ligand) or Poly I:C (TLR3 ligand) did not lead to significant differences among the three groups (respectively Figure 1D and Figure 1E).
A genetic survey was carried out on 36 available patients with CD (out of 38). PCR and DNA sequencing analyses revealed that 13 patients were homozygous or heterozygous for one or more of the three CD-associated genetic variants in NOD2 (Table 2). The remaining 23 patients were found to be wild type for the three polymorphisms (Supplementary Table 1).
| CD patient | NOD2 genotypes | ||
| Ex. 4 | Ex. 8 | Ex. 11 | |
| C>T | G>C | InsC | |
| R702W | G908R | L1007Pfs*2 | |
| 3 | C/C | G/G | InsC/- |
| 4 | T/T | G/G | -/- |
| 7 | C/T | G/G | InsC/- |
| 14 | C/C | G/C | -/- |
| 18 | C/C | G/C | InsC/- |
| 24 | C/T | G/G | -/- |
| 25 | C/C | G/C | -/- |
| 44 | C/C | G/G | InsC/- |
| 45 | C/C | G/G | InsC/- |
| 47 | C/C | G/C | InsC/- |
| 53 | C/C | G/G | InsC/- |
| 54 | C/C | G/C | -/- |
| 63 | C/C | G/G | InsC/- |
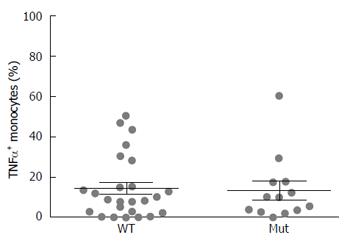
After grouping CD patients based on NOD2 genotype, no significant differences in TNF-α positive monocytes were detected between patients with wild type or mutated genotype (CD WT: 14.87 ± 3.1 vs CD Mut 13.82 ± 4.5) (Figure 2). Data regarding the single NOD2 variants are shown in Supplementary Figure 1.
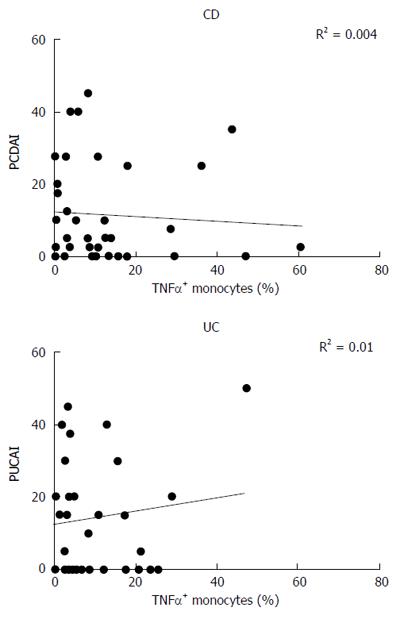
Intracellular production of TNF-α by monocytes from CD and UC patients was correlated to the disease activity score, expressed as PCDAI (Pediatric Crohn’s Disease Activity Index) or PUCAI (Pediatric Ulcerative Colitis Activity Index). No correlation between the two parameters was found, either in CD (R2 = 0.004) or in UC (R2 = 0.01) subjects (Figure 3).
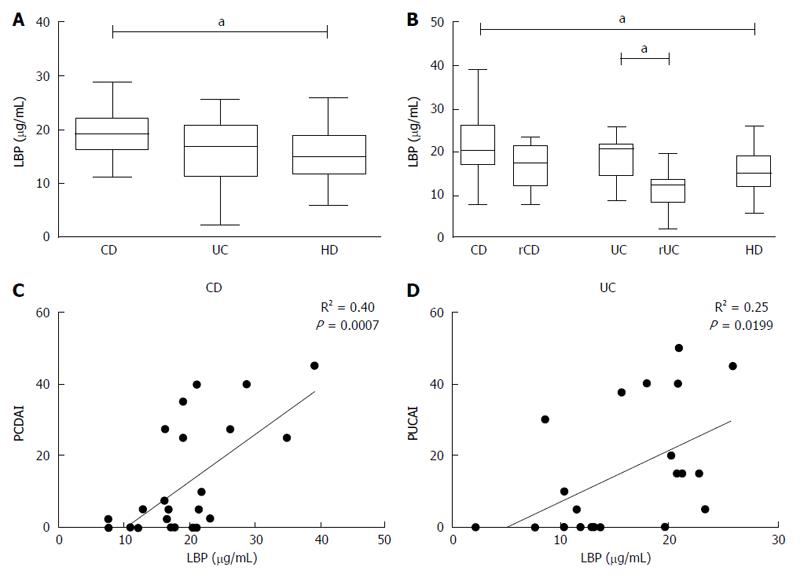
Plasmatic levels of LBP and sCD14 were measured to verify if the basal inflammatory state in CD patients could be due to exposition of monocytes to microbial components in peripheral blood.
Levels of LBP were higher in plasma from patients with CD compared to HD [(median CD 19.11 (16.35-21.89), HD 15.01 (11.85-18.84), P < 0.05)] (Figure 4A) and this condition was noticed also when patients were grouped by disease activity [(median activeCD: 20.44 (16.81- 26.31) vs HD: 15.01 (11.85-18.84); P < 0.05)] (Figure 4B); furthermore a significant difference was also found between UC in active or remissive phase [(median UC: 20.74 (14.55- 21.57) vs rUC: 12.35 (8.35-13.5); P < 0.05)] (Figure 4B).
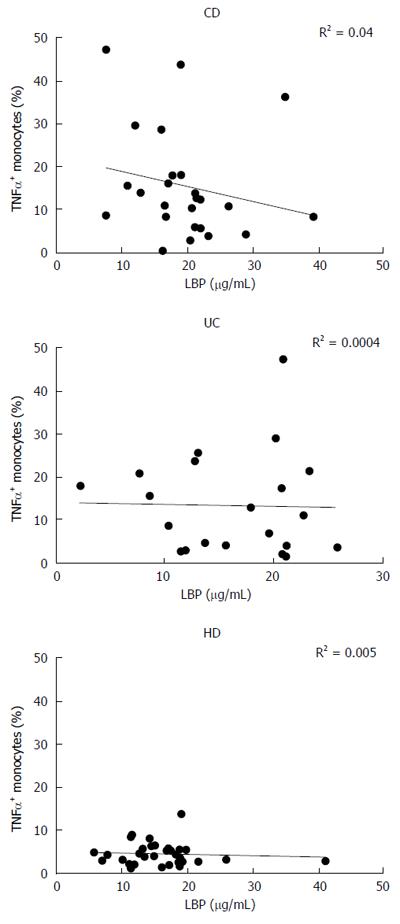
LBP values displayed a significant correlation with the disease activity index, especially for CD (R2 = 0.40, P < 0.001) (Figure 4C and D). However, there was no correlation between monocyte TNF-α expression in basal condition and plasmatic levels of LBP in any of the analyzed groups (CD: R2 = 0.04, UC: R2 = 0.0004, HD: R2 = 0.005) (Figure 5).
The quantification of sCD14 was not significantly different among the three groups and did not correlate with the degree of monocyte activation in CD (data not shown).
The activation of the innate immune system is thought to play a pivotal role in the pathogenesis of CD. Most genetic variants associated with the disease involve genes that are expressed in monocytes and that contribute to functional innate response to microbes. For example, NOD2 and ATG16L1 are involved in the response to molecular patterns associated with pathogens (PAMPs) and in autophagy, a process that can amplify the inflammatory response to phagocytized materials. Notably, there is a number of genes and processes involved in phagocytic function and innate immunity that have been shown to be responsible for rare monogenic forms of Crohn’s-like intestinal inflammation. For instance, early onset CD-like inflammatory bowel disease has been described in neutropenias and in functional deficiencies of phagocytes, such as chronic granulomatous disease and leukocyte adhesion deficiency, as well as in defects of other genes interacting with the NOD2 pathway of response to PAMPs, such as XIAP[8,21]. These findings have raised the question of whether CD should be considered more like an immunodeficiency than an autoinflammatory condition[22-24].
For this reason, several studies have investigated the function of peripheral blood monocytes, an easily available sample cell that can be, at least in part, representative of the innate immune profile of CD. The observation of impaired monocyte/macrophage function against yeast particles dates back decades[25]. However, it is still not clear whether these defects are a cause or a consequence of CD inflammation. For example, it has been hypothesized that CD-associated monocytes may display hyporesponsiveness because of massive mucosal stimulation[26]. Indeed, we and other groups have demonstrated that peripheral blood monocytes from CD show an increased expression of activation markers[27-29], whilst ex-vivo maturation of dendritic cells may be somehow impaired[30]. Overall, these results have highlighted the paradox of defective and excessive immunities in CD[31]: CD monocytes display increased activation and production of inflammatory cytokines, but they may present functional defects in terms of capacity to mature effectively and to clear bacteria efficiently[32]. Indeed, the depletion of monocytes could improve the response to anti-inflammatory treatments in CD[33]. Thus, the contribution of monocytes to the pathogenesis of CD seems to be quite complex, involving hyper-activation and defective functionality at the same time.
Beynon et al[34] demonstrated that in spite of increased basal activation, CD-associated monocytes display reduced response to MDP stimulation, in a manner that greatly depends on NOD2 genotype. The effect of NOD2 variants on the activation of monocytes has been confirmed by other studies showing that homozygous mutations can almost abrogate TNF-α production after MDP stimulation[35]. However, NOD2 defects can also impair MDP-induced tolerance, resulting in sustained monocyte activation and TNF-α production[36].
The aim of our research was the analysis of the integrity of the different pathways of the innate immune network. By means of a cytometric protocol, adapted from a clinically oriented technique already used for the screening of some primary immunodeficiency[20], we analysed the production of TNF-α triggered in monocytes by different stimuli, to depict possible alterations in different pathways of innate immunity to PAMPs.
We demonstrated that peripheral monocytes from CD show significant basal production of TNF-α and that this is not clearly related to either the disease activity or the NOD2 genotype, supporting the idea that, in these cases, hyper-production of TNF-α relies on NOD2-independent pathways. Indeed, stimulation with MDP or with Pam3CSK4 led to higher TNF-α production in CD patients than in controls. The stronger response in CD after the stimulation with the NOD2 ligand did not find a clear explanation, but it highlights the importance of NOD2 signalling in CD and not in UC. This result is coherent with the knowledge that NOD2 is the main genetic factor associated with CD, even though the mechanisms underlying the pathogenesis of the disease remain controversial.
Thus, the activation of monocytes resulting from the mucosal immunopathology of CD could play a greater role in inducing TNF-α than the NOD2 genotype, not only in the intestinal mucosa but also in peripheral blood. A possible hypothesis is that excessive activation of monocytes occurs in response to bacterial translocation across the epithelium and to minor defects in bacterial clearance or in MDP-induced tolerance. Notably, the paradoxical efficacy of GM-CSF in some cases of CD may be due to the correction of such defects in epithelial monocytes, for example by rescuing their epithelial repair function[37]. Another critical function for mucosal monocytes may concern the induction of antimicrobial peptide production by Paneth cells[38]; indeed, a defective production of defensins after bacterial translocation may result in altered shaping of gut microbiota, leading to inflammatory amplification in a vicious circle.
We investigated whether increased activation of peripheral monocytes correlated with plasma levels of LPS-binding protein (LBP). We showed that plasma concentrations of LBP correlate positively with disease activity, both in CD and in UC, in agreement with previous studies[11]. However, there was no significant correlation between LBP levels and activation of peripheral monocytes, suggesting that monocytes are not activated by systemic exposure to LPS. We cannot, however, rule out the existence of other bacterial molecular patterns.
An added value of our research is that we studied monocyte function after stimulation of different TLR pathways. Several studies have already reported that altered patterns of TLR expression can be differentially found in CD, rather than in UC[39], even though no definitive association between the pathway dysregulations and the disease phenotypes has been ever identified. We interestingly found that peripheral blood monocytes from CD patients displayed increased response to Pam3CSK4, which is a mycobacterial-like ligand for TLR2/1. The increased response to Pam3CSK4 was quite specific to CD monocytes and was not found in UC. Thus, it is unlikely that the hyper-response to TLR2/1 signaling depended on monocyte activation alone. A possible explanation is that peripheral monocytes from patients with CD are primed by mycobacterial components released in the intestinal mucosa, maybe as a result of colonization with bacteria such as mycobacterium avium paratuberculosis (MAP). The presence of MAP in CD intestinal mucosa and bloodstream has been proved by several works and accomplished by different techniques; it has been repetitively associated with the pathogenesis of CD but its role in the etiology remains to be defined[40]. Our results should prompt further investigation on the immune response to MAP in healthy population and in CD.
In conclusion, we showed that peripheral monocytes from patients with CD have higher basal production of TNF-α than controls, regardless of NOD2 genotype and without a clear correlation with disease activity. Moreover, peripheral monocyte activation does not seem to be due to plasma exposition to LPS. There is also an increased response of monocytes to MDP or Pam3CSK4 stimulation, which could reflect a reduced tolerance to bacterial PAMPs. Based on these data, it is worth investigating the possible priming of monocytes by mycobacterial colonization, for example with MAP.
The authors would like to thank Alessandra Knowles for revising carefully the manuscript.
Crohn’s disease (CD) is characterized by an aberrant activation of the mucosal immune system in genetically susceptible subjects, due to an altered crosstalk between innate immune system and environmental factors, with particular reference to gut microbiota.
CD could be considered as a paradoxical condition in which defective immune functions coexist with excessive inflammation. Indeed, peripheral blood monocytes from CD patients display increased activation and production of pro-inflammatory tumor necrosis factor-alpha (TNF-α), but they seem to present functional defects in their capacity to respond to muramyl dipeptide (MDP) and to bacteria. Several studies have investigated the function of peripheral blood monocytes as representative cells of the innate immune profile of CD.
The analysis of intracellular TNF-α allowed the evaluation of the integrity and functionality of NOD2 and TLR signaling pathways in peripheral monocytes. Unexpectedly, CD monocytes showed increased basal and MDP induced TNF-α production, irrespective of NOD2 genotype and disease activity.
Hyper-production of TNF-α by CD monocytes probably relies on a NOD2-indipendent pathway and is not due to systemic exposure to LPS, but to other bacterial molecular patterns. The fact that CD monocytes display higher production of TNF-α compared to controls, both in basal and in stimulated conditions, may be due to reduced tolerance to bacterial PAMPs. The finding of increased response to TLR2/1 stimulation may indicate a possible priming of monocytes by mycobacterial colonization, for example by MAP.
The manuscript described that peripheral monocytes from CD express higher basal and stimulated TNF-α than controls, regardless of NOD2 genotype and without a clear correlation with disease activity. In general, the results are very interesting.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen L S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. 2010;50:27-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 2. | Ponsky T, Hindle A, Sandler A. Inflammatory bowel disease in the pediatric patient. Surg Clin North Am. 2007;87:643-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1967] [Cited by in F6Publishing: 2037] [Article Influence: 135.8] [Reference Citation Analysis (5)] |
| 4. | Turner D, Levine A, Walters TD, Focht G, Otley A, López VN, Koletzko S, Baldassano R, Mack D, Hyams J. Which PCDAI Version Best Reflects Intestinal Inflammation in Pediatric Crohn’s Disease? J Pediatr Gastroenterol Nutr. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Dotson JL, Crandall WV, Zhang P, Forrest CB, Bailey LC, Colletti RB, Kappelman MD. Feasibility and validity of the pediatric ulcerative colitis activity index in routine clinical practice. J Pediatr Gastroenterol Nutr. 2015;60:200-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Turner D, Travis SP, Griffiths AM, Ruemmele FM, Levine A, Benchimol EI, Dubinsky M, Alex G, Baldassano RN, Langer JC. Consensus for managing acute severe ulcerative colitis in children: a systematic review and joint statement from ECCO, ESPGHAN, and the Porto IBD Working Group of ESPGHAN. Am J Gastroenterol. 2011;106:574-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Yadav V, Varum F, Bravo R, Furrer E, Bojic D, Basit AW. Inflammatory bowel disease: exploring gut pathophysiology for novel therapeutic targets. Transl Res. 2016;176:38-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Bianco AM, Girardelli M, Tommasini A. Genetics of inflammatory bowel disease from multifactorial to monogenic forms. World J Gastroenterol. 2015;21:12296-12310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 68] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Chandel S, Prakash A, Medhi B. Current scenario in inflammatory bowel disease: drug development prospects. Pharmacol Rep. 2015;67:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Yu LC, Wang JT, Wei SC, Ni YH. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J Gastrointest Pathophysiol. 2012;3:27-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 157] [Cited by in F6Publishing: 156] [Article Influence: 13.0] [Reference Citation Analysis (2)] |
| 11. | Lakatos PL, Kiss LS, Palatka K, Altorjay I, Antal-Szalmas P, Palyu E, Udvardy M, Molnar T, Farkas K, Veres G. Serum lipopolysaccharide-binding protein and soluble CD14 are markers of disease activity in patients with Crohn’s disease. Inflamm Bowel Dis. 2011;17:767-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016;22:1137-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 445] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 13. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3986] [Cited by in F6Publishing: 3811] [Article Influence: 165.7] [Reference Citation Analysis (0)] |
| 14. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3555] [Cited by in F6Publishing: 3393] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 15. | Sorbara MT, Philpott DJ. Peptidoglycan: a critical activator of the mammalian immune system during infection and homeostasis. Immunol Rev. 2011;243:40-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Cantó E, Ricart E, Monfort D, González-Juan D, Balanzó J, Rodríguez-Sánchez JL, Vidal S. TNF alpha production to TLR2 ligands in active IBD patients. Clin Immunol. 2006;119:156-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, Quertinmont E, Abramowicz M, Van Gossum A, Devière J. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s disease and ulcerative colitis. Gut. 2004;53:987-992. [PubMed] [Cited in This Article: ] |
| 19. | Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 419] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 20. | Takada H, Yoshikawa H, Imaizumi M, Kitamura T, Takeyama J, Kumaki S, Nomura A, Hara T. Delayed separation of the umbilical cord in two siblings with Interleukin-1 receptor-associated kinase 4 deficiency: rapid screening by flow cytometer. J Pediatr. 2006;148:546-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, Ouahed J, Wilson DC, Travis SP, Turner D. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990-1007.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 427] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 22. | Tommasini A, Pirrone A, Palla G, Taddio A, Martelossi S, Crovella S, Ventura A. The universe of immune deficiencies in Crohn’s disease: a new viewpoint for an old disease? Scand J Gastroenterol. 2010;45:1141-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kelsen JR, Dawany N, Moran CJ, Petersen BS, Sarmady M, Sasson A, Pauly-Hubbard H, Martinez A, Maurer K, Soong J. Exome sequencing analysis reveals variants in primary immunodeficiency genes in patients with very early onset inflammatory bowel disease. Gastroenterology. 2015;149:1415-1424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Casanova JL, Abel L. Revisiting Crohn’s disease as a primary immunodeficiency of macrophages. J Exp Med. 2009;206:1839-1843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Miura M, Hiwatashi N. Impaired monocyte macrophages function in patients with Crohn’s disease. J Clin Lab Immunol. 1987;24:167-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 26. | Capobianchi MR, Fais S, Mercuri F, Boirivant M, Dianzani F, Pallone F. Interferon-alpha (IFN-alpha) production by human intestinal mononuclear cells. Response to virus in control subjects and in Crohn’s disease. Gut. 1992;33:897-901. [PubMed] [Cited in This Article: ] |
| 27. | Liu ZX, Hiwatashi N, Noguchi M, Toyota T. Increased expression of costimulatory molecules on peripheral blood monocytes in patients with Crohn’s disease. Scand J Gastroenterol. 1997;32:1241-1246. [PubMed] [Cited in This Article: ] |
| 28. | Sawada-Hase N, Kiyohara T, Miyagawa J, Ueyama H, Nishibayashi H, Murayama Y, Kashihara T, Nakahara M, Miyazaki Y, Kanayama S. An increased number of CD40-high monocytes in patients with Crohn’s disease. Am J Gastroenterol. 2000;95:1516-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Marcuzzi A, Girardelli M, Bianco AM, Martelossi S, Magnolato A, Tommasini A, Crovella S. Inflammation profile of four early onset Crohn patients. Gene. 2012;493:282-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Granzotto M, Fabbro E, Maschio M, Martelossi S, Quaglia S, Tommasini A, Presani G, Ventura A. Heterozygous nucleotide-binding oligomerization domain-2 mutations affect monocyte maturation in Crohn’s disease. World J Gastroenterol. 2007;13:6191-6196. [PubMed] [Cited in This Article: ] |
| 31. | Notarangelo LD, Tommasini A. Defective and excessive immunities in pediatric diseases. Curr Pharm Des. 2012;18:5729-5734. [PubMed] [Cited in This Article: ] |
| 32. | Strisciuglio C, Duijvestein M, Verhaar AP, Vos AC, van den Brink GR, Hommes DW, Wildenberg ME. Impaired autophagy leads to abnormal dendritic cell-epithelial cell interactions. J Crohns Colitis. 2013;7:534-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Fukunaga K, Yokoyama Y, Kamikozuru K, Yoshida K, Kikuyama R, Nagase K, Nakamura S, Takei Y, Miwa H, Matsumoto T. Selective depletion of peripheral granulocyte/monocyte enhances the efficacy of scheduled maintenance infliximab in Crohn’s disease. J Clin Apher. 2010;25:226-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Beynon V, Cotofana S, Brand S, Lohse P, Mair A, Wagner S, Mussack T, Ochsenkühn T, Folwaczny M, Folwaczny C. NOD2/CARD15 genotype influences MDP-induced cytokine release and basal IL-12p40 levels in primary isolated peripheral blood monocytes. Inflamm Bowel Dis. 2008;14:1033-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Kuuliala K, Lappalainen M, Turunen U, Puolakkainen P, Kemppainen E, Siitonen S, Repo H, Mustonen H. Detection of muramyl dipeptide-sensing pathway defects in monocytes of patients with Crohn’s disease using phospho-specific whole blood flow cytometry. Scand J Clin Lab Invest. 2013;73:494-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Cantó E, Moga E, Ricart E, Garcia-Bosch O, Garcia-Planella E, Juarez C, Vidal S. MDP-Induced selective tolerance to TLR4 ligands: impairment in NOD2 mutant Crohn’s disease patients. Inflamm Bowel Dis. 2009;15:1686-1696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Bernasconi E, D’Angelo F, Michetti P, Velin D. Critical role of the GM-CSF signaling pathway in macrophage pro-repair activities. Pathobiology. 2014;81:183-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Courth LF, Ostaff MJ, Mailänder-Sánchez D, Malek NP, Stange EF, Wehkamp J. Crohn’s disease-derived monocytes fail to induce Paneth cell defensins. Proc Natl Acad Sci USA. 2015;112:14000-14005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010-7017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 917] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 40. | Timms VJ, Daskalopoulos G, Mitchell HM, Neilan BA. The Association of Mycobacterium avium subsp. paratuberculosis with Inflammatory Bowel Disease. PLoS One. 2016;11:e0148731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |