Published online Aug 28, 2016. doi: 10.3748/wjg.v22.i32.7365
Peer-review started: March 18, 2016
First decision: April 14, 2016
Revised: May 11, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: August 28, 2016
To determine the prevalence of colorectal neoplasia in average risk persons 40-59 years of age in Israel and to compare the results with other populations.
We reviewed the results of asymptomatic average-risk subjects, aged 40 to 59 years, undergoing their first screening colonoscopy between April 1994 and January 2014. The detection rates of adenoma, advanced adenoma (AA) and colorectal cancer (CRC) were determined in the 40’s and 50’s age groups by gender. The prevalence of lesions was compared between age groups. After meticulous review of the literature, these results were compared to published studies addressing the prevalence of colorectal neoplasia in similar patient groups, in a variety of geographical locations.
We included first screening colonoscopy results of 1750 individuals. The prevalence of adenomas, AA and CRC was 8.3%, 1.0% and 0.2% in the 40-49 age group and 13.7%, 2.4% and 0.2% in the 50-59 age group, respectively. Age-dependent differences in adenoma and AA rates were significant only among men (P < 0.005). Literature review disclosed 17 relevant studies. As expected, in both Asian and Western populations, the risks for overall adenoma and advanced adenoma was significantly higher in the 50's age group as compared to the 40's age group in a similar fashion. The result of the current study were similar to previous studies on Western populations. A substantially higher rate of adenoma, was observed in studies conducted among Asian populations in both age groups.
The higher rate of colorectal neoplasia in Asian populations requires further investigation and reconsideration as to the starting age of screening in that population.
Core tip: This research focuses on evaluating detection rates of colorectal neoplasia among average risk individuals aged 40-59 years and comparing the detection rates between the fifth and sixth decades. In this prospective study of first screening colonoscopy from 1750 consecutive average risk subjects aged 40-59, we found that the prevalence of colorectal neoplasia is age and gender dependent. In addition we did an extensive search of the literature that revealed a markedly higher adenoma detection rate among Asians, and in particular Koreans compares to Western populations.
- Citation: Leshno A, Moshkowitz M, David M, Galazan L, Neugut AI, Arber N, Santo E. Prevalence of colorectal neoplasms in young, average risk individuals: A turning tide between East and West. World J Gastroenterol 2016; 22(32): 7365-7372
- URL: https://www.wjgnet.com/1007-9327/full/v22/i32/7365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i32.7365
Colorectal cancer (CRC) is the third leading cause of cancer mortality in the Western world[1,2], with an estimated lifetime risk of 5%-6%. Approximately 70% of patients who develop CRC are considered “average risk”[3]. The incidence and mortality rates of CRC increase after 50 years of age, the time that screening should be initiated. Only a small number of studies focusing on the prevalence of adenomas among persons younger than 50 years have been published and revealed variable results.
The aim of this study was to determine the prevalence of colorectal neoplasia in average risk persons in the fifth and sixth decades and to compare it to other populations from various geographical areas.
Between April 1994 and June 2014, we have been prospectively collecting and documenting the results of screening colonoscopies carried out by several senior gastroenterologists from the Division of Gastroenterology at the Tel-Aviv Medical Center (TASMC). In addition to endoscopic reports, all participants were interviewed by the physician regarding symptoms, details of recent laboratory tests, and any personal or family history. The collected data were entered into a computerized database. Consecutive first screening colonoscopy of asymptomatic average-risk (i.e., no personal or family history of colorectal CRC or CRC related syndrome) subjects aged 40-59 years old at the time of colonoscopy were included.
Individuals were excluded if they reported a personal history of colorectal adenoma or carcinoma at any time; if they had a family history of colorectal adenoma or carcinoma (one first-degree relative aged < 70, or two or more family relatives at any age); if they reported symptoms suggestive of neoplasia (rectal bleeding, change in bowel habit, abdominal pain, or unexplained weight loss during the previous 6 mo); or if they had a positive fecal occult blood test (FOBT), or laboratory abnormalities, such as iron-deficiency anemia. Other exclusion criteria were inflammatory bowel disease (IBD) or severe co-morbidity (e.g., malignancy, significant cardio-pulmonary, renal or hepatic diseases). Mild complaints (such as chronic constipation without a recent change in bowel habit, or minor anal or abdominal discomfort, such as flatulence or bloating) were not regarded as neoplasia-related symptoms and were not considered to be exclusion criteria. Written informed consent was obtained from all participants. The study was approved by the TASMC Helsinki Committee. Colonoscopy was performed according to the usual protocol in our institute, as previously described[4].
English language medical literature searches for human studies were performed through June 2015 using “screening”, “colonoscopy”, and “average risk” as keywords. Articles describing the prevalence of colorectal neoplasia during screening colonoscopy among asymptomatic persons in their fifth and sixth decades were used to compare the current report.
Statistical analyses were performed using IBM SPSS 21.0 software. The statistical significance level was set to 0.05. The prevalence of lesions was presented as proportions with 95%CI. Age was considered as a categorical variable, divided into two groups (40-49, 50-59 years). Pearson's χ2 tests and student's t-test were used for comparison of lesion prevalence according to sex and age group.
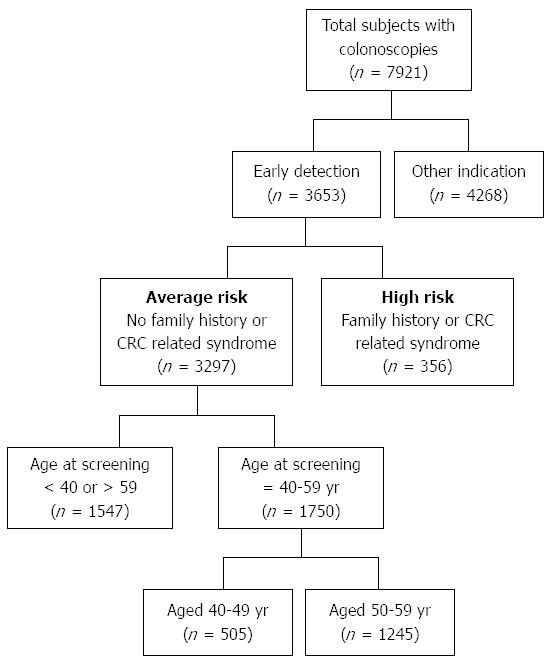
Data were collected from a total of 7921 subjects who underwent colonoscopy of which 3653 were primary screening procedures. After exclusion of subjects aged above 59 or below 40 years at the time of the procedure, there were 1750 eligible subjects for final analysis (Figure 1).
The mean age of the subjects was 51.9 ± 5.1 years and men comprised 52.3% of the study population. There were 505 subjects aged 40-49 (mean age 45.3 ± 2.8 years) of whom 278 (55.0%) were men. In the 50-59 age group (mean age 51.9 ± 5.09), there were 1245 screenees, of whom 916 (51.2%) were men. Demographic features are shown in Table 1.
| Age study group | 40-49 yr | 50-59 yr | Total |
| Number of subjects | 505 | 1245 | 1750 |
| Mean age (yr) at colonoscopy (SD) | 45.30 (2.81) | 54.64 (2.83) | 51.94 (5.09) |
| Male percentage (%) | 55 | 51.2 | 52.3 |
All lesions were sampled and sent for pathological examination. If more than one lesion was detected, the colonoscopic findings were classified according to the most advanced lesion, e.g., adenocarcinoma, adenoma, hyperplastic or inflammatory polyp. Advanced neoplasia was defined as any adenoma measuring more than 10 mm, or with villous or tubulovillous histology, adenoma with high-grade dysplasia, or cancer[5,6]. Of the 1750 study subjects, 212 (12.1%) were found to have one or more colorectal neoplasia (non-advanced adenoma 10.4%; advanced 2.0%; invasive cancer 0.2%). The prevalence of colorectal neoplasia in the two age brackets according to gender is illustrated in Table 2. Among the 40-49 year age group, 43 (8.5%) individuals had adenomas (7.5% non-advanced, 1% advanced). Among the 50-59 year age group, 174 (13.7%) individuals had adenomas (11.6% non-advanced, 2.4% advanced, P = 0.01 and 0.06 respectively). A total of four (0.2%) cases of adenomas with high-grade dysplasia were detected all of which were in the 50's age group. Four (0.2%) CRC cases were detected (three in the 40's and one in the 50's year groups). The increased prevalence of neoplastic findings among the 50's age group was significant only among men for both adenomatous polyps and advanced adenoma (P = 0.001 and 0.005, respectively). No differences were observed for women (P = 0.435 and 0.744, respectively). The difference in CRC prevalence rate between the age and gender groups didn't reach statistical significance most probably due to the small sample size.
| Gender finding type | 40-49 yr | 50-59 yr | Total | P value1 | OR (95%CI) |
| All | |||||
| Total colonoscopies | 505 | 1245 | 1750 | ||
| Adenomatous polyps | 42 (8.3) | 170 (13.7) | 212 (12.1) | 0.002 | 1.74 (1.22-2.49) |
| Advanced adenoma | 5 (1.0) | 30 (2.4) | 35 (2.0) | 0.060 | 2.47 (0.95-6.40) |
| Cancer | 1 (0.2) | 3 (0.2) | 4 (0.2) | 1.000 | 1.22 (0.13-11.73) |
| Men | |||||
| Total colonoscopies | 278 | 638 | 916 | ||
| Adenomatous polyps | 23 (8.3) | 106 (16.6) | 129 (14.1) | 0.001 | 2.21 (1.37-3.55) |
| Advanced adenoma | 1 (0.4) | 22 (3.4) | 23 (2.5) | 0.005 | 9.89 (1.33-73.76) |
| Cancer | 1 (0.4) | 2 (0.3) | 3 (0.3) | 1.000 | 0.87 (0.08-9.65) |
| Women | |||||
| Total colonoscopies | 227 | 607 | 834 | ||
| Adenomatous polyps | 19 (8.4) | 64 (10.5) | 83 (10.0) | 0.435 | 1.29 (0.76-2.21) |
| Advanced adenoma | 4 (1.8) | 8 (1.3) | 12 (1.4) | 0.744 | 0.75 (0.22-2.50) |
| Cancer | 0 (0.0) | 1 (0.2) | 1 (0.1) | 1.000 | 1 |
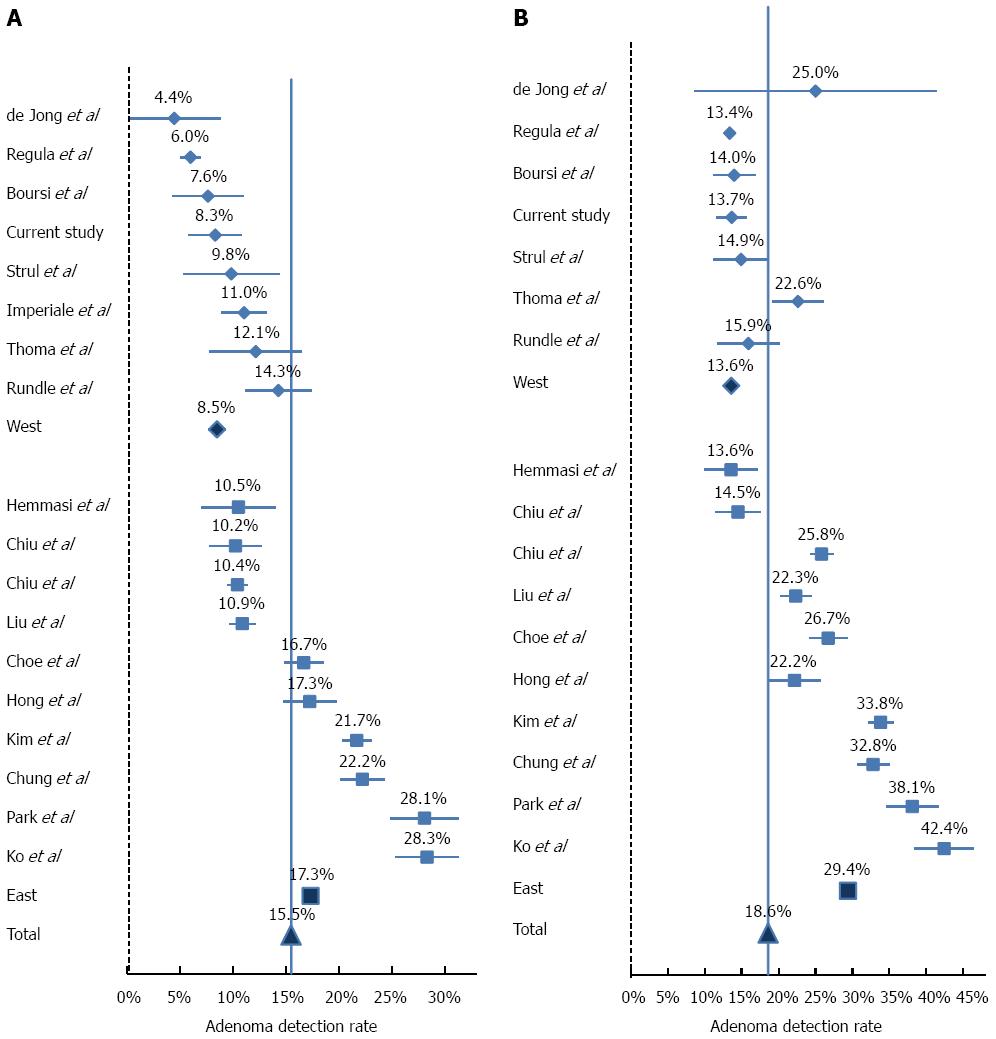
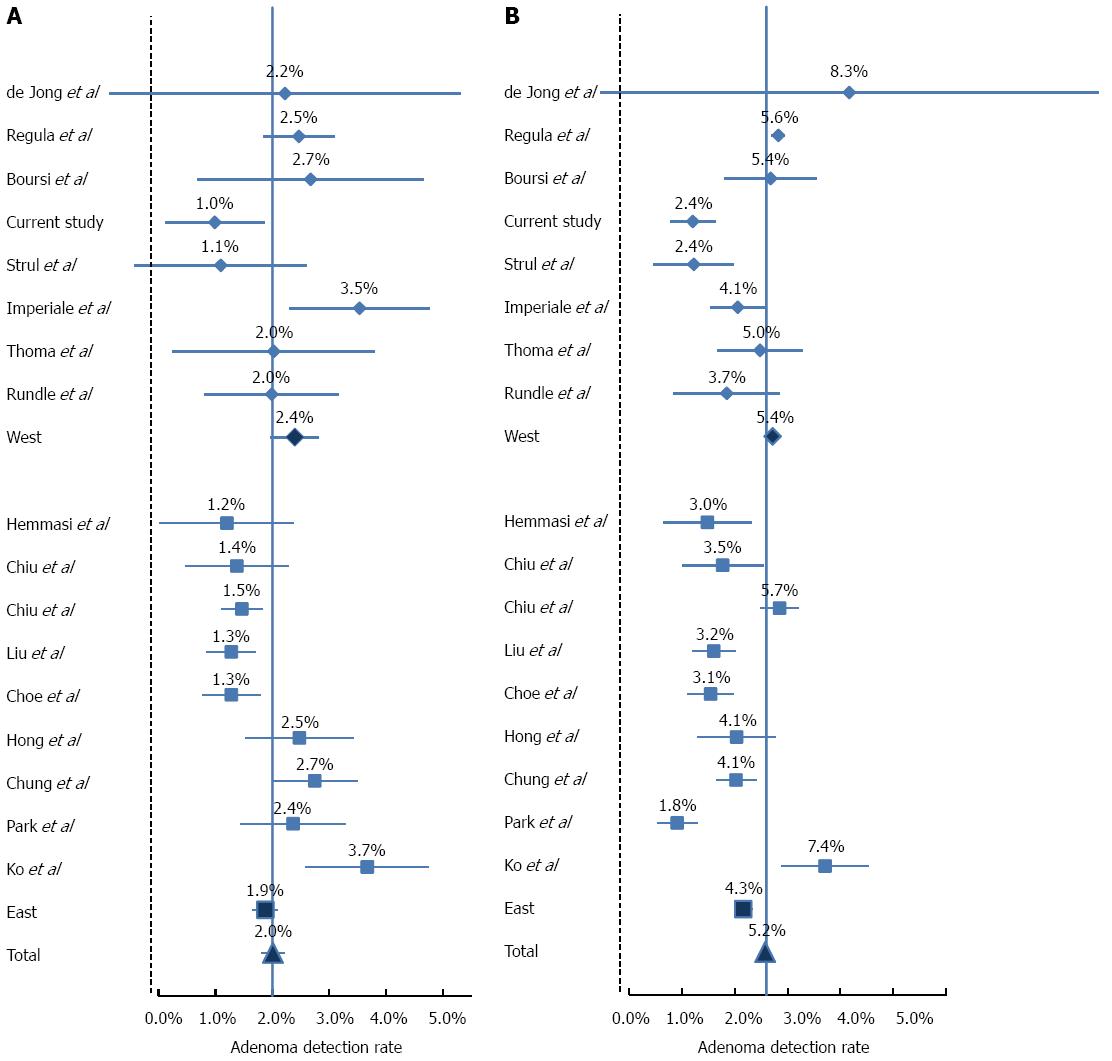
Literature review disclosed 17 relevant studies (Table 3). The adenoma detection rate (ADR) and advanced lesion in each study is depicted in Figures 2 and 3, respectively. As expected, in both Asian and Western populations, the risks for overall adenoma and advanced adenoma was significantly higher in the 50’s age group as compared to the 40’s age group in a similar fashion (Table 4).
| Ref. | Country of origin | Year published | Number of subjects in age group | |
| 40-49 yr | 50-59 yr | |||
| Western origin | ||||
| de Jong et al[17] | the Netherlands | 2005 | 90 | 36 |
| Regula et al[8] | Poland | 2006 | 2392 | 37313 |
| Boursi et al[18] | Israel | 2009 | 262 | 672 |
| Current study | Israel | - | 505 | 1245 |
| Strul et al[4] | Israel | 2006 | 183 | 409 |
| Imperiale et al[6] | United States | 2002 | 906 | 1533 |
| Thoma et al[19] | United States | 2010 | 247 | 747 |
| Rundle et al[20] | United States | 2008 | 553 | 352 |
| Studies of Western origin (8) | 5138 | 40774 | ||
| Asian origin | ||||
| Hemmasi et al[21] | Iran | 2014 | 333 | 407 |
| Chiu et al[22] | Taiwan (China) | 2005 | 654 | 592 |
| Chiu et al[23] | Taiwan (China) | 2008 | 4161 | 4211 |
| Liu et al[24] | Taiwan (China) | 2005 | 2656 | 1903 |
| Choe et al[25] | South Korea | 2006 | 1875 | 1587 |
| Hong et al[26] | South Korea | 2010 | 1049 | 712 |
| Kim et al[27] | South Korea | 2013 | 4550 | 4436 |
| Chung et al[28] | South Korea | 2009 | 1930 | 2716 |
| Park et al[29] | South Korea | 2009 | 1057 | 1207 |
| Ko et al[30] | South Korea | 2012 | 1200 | 1038 |
| Studies of Asian origin (9) | 19465 | 18807 | ||
| All studies (17) | 24603 | 59581 | ||
| Finding | OR (95%CI) | P value1 |
| Studies of Western origin | ||
| Overall adenomas | 1.7 (1.53-1.88) | < 0.0001 |
| Advanced adenoma | 2.34 (1.95-2.81) | < 0.0001 |
| Cancer | 2.33 (1.45-3.75) | < 0.0001 |
| Studies of Asian origin | ||
| Overall adenomas | 1.99 (1.89-2.09) | < 0.0001 |
| Advanced adenoma | 2.36 (2.05-2.72) | < 0.0001 |
| Cancer | 3.88 (2.41-6.23) | < 0.0001 |
| Total | ||
| Overall adenomas | 1.25 (1.20-1.30) | < 0.0001 |
| Advanced adenoma | 2.65 (2.38-2.94) | < 0.0001 |
| Cancer | 3.39 (2.45-4.68) | < 0.0001 |
The most prominent observation is the markedly higher ADR among Asians, and in particular Koreans for both age groups (OR = 2.56, 95%CI: 2.04-2.51; and OR = 2.64, 95%CI: 2.09-2.23, in the 40's and 50's age groups, respectively, P < 0.0001).This difference between Western and Asian studies is significantly diminished in both age groups with regard to advanced adenoma and CRC rates. The prevalence of advanced lesions had a narrower range between studies (range between 1.1% to 3.7% in the 40's group and between 1.5% to 7.5% in the 50's age group), and in contrast to ADR, the detection rate of advanced lesions was significantly lower in the Asian population (OR = 0.79, 95%CI: 0.72-0.86, P < 0.0001; and OR = 0.78, 95%CI: 0.63-0.97, P = 0.027). The rate of CRC ranged from 0% to 0.7% in the 40’s age group and 0.1% to 0.9% in the 50’s age group. The differences between Asian and Western populations in CRC rate were not statistically significant.
Overall, the 50 year age group had higher rate of colorectal neoplasia as compared to the 40 year age group, similar to all studies across the world. There is a significant increased risk for colorectal neoplasia, in both age groups, between Western and Asian origins.
The differences between the two age brackets are statistically significant among men only. It is consistent with previous studies which found that women have a lower risk for colorectal neoplasia across all age groups[7]. Regula et al[8] and Ferlitsch et al[9] observed that men tend to develop advanced adenomas a decade earlier than women. The current study is limited to subjects younger than 60 years which can explain the lack of difference among women.
The findings in an Israeli population are comparable with observations from previous studies conducted in the West, indicating that age and male gender are the most important risk factors for the development of colorectal adenomas. A review of studies from Western countries, including Israel, revealed heterogeneity in the prevalence rates of adenomas and advanced adenomas. ADR and the detection of advanced adenomas were slightly lower in Israel than in other countries. Various factors might explain these discrepancies, mainly differences in genetic and environmental backgrounds. The use of different study design is another possible explanation.
The current study included real average population as they were all asymptomatic subjects with no family history of CRC. Other studies, like the landmark study of Regula et al[8], included patients with a family history of CRC.
Herein, it can be confirmed that CRC screening in the West should begin earlier than the age of 50.
During our review of the literature we detected a secondary important finding regarding a significantly increased ADR among Asians, and in particular from the Far East, as compared to those from Western countries. This observation is consistent with reports showing an increase in CRC in eastern countries[10] which is considered the result of rapid economic development and life style modification. Shin et al[11] reported a rapid increase in CRC incidence in Korea between 1999-2009 in both men and women. Although in part attributed to the introduction of colorectal cancer screening, they concluded that transition in risk factors (e.g., alcohol consumption, obesity and increased meat consumption) also has a significant role in the increase of CRC incidence.
Interestingly, despite the increased overall adenoma rate in Asian countries, the prevalence of advanced adenomas did not increase in similar proportions, and were even lower than in the West. This observation suggests a slower rate of adenoma progression in Eastern Asia which may be due to genetic and/or environmental factors.
The high rates of colorectal neoplasia in East Asia population, is of great significance regarding immigrants from these countries to the West and specifically the United States. Ladabaum and colleagues[12] calculated CRC incidence rates in foreign-born Asian ethnic subgroups in California and found significant impacts of nativity and residence in an ethnic enclave on CRC incidence, suggesting a substantial effect of acquired environmental factors. In contrast to other minorities, such as African Americans, studies regarding the rate of polyps among East Asian immigrants aged 40-59 in the United States is limited, while there is a lot of information about the poor adherence of this population to CRC screening programs. In fact, Asian Americans have been described as one of the underrepresented and hard-to-reach populations[13]. Several studies have found that Asian Americans have lower CRC screening rates than non-Hispanic white Americans[14,15]. Moreover, Oh et al[16] described lower rates of CRC screening knowledge and uptake among Korean Americans compared to Asian Americans as a whole.
In conclusion, the detection rates of colorectal neoplasms among asymptomatic average-risk subjects in this study were compatible with previous results of Western origin, confirming the current guidelines to begin screening for CRC at the age of 50. The substantial differences between Western and East Asian populations in terms of overall adenoma and advanced adenoma detection rates mandate further investigation and imply the need for specific screening recommendation for this population.
Colorectal cancer (CRC) is the third leading cause of cancer mortality in the Western world and approximately 70% of patients who develop CRC are considered “average risk”. According to previous studies, the incidence and mortality rates of CRC increase after 50 years of age, and therefore screening should be initiated at this age. However, only few studies focused on the prevalence of adenomas among persons younger than 50 years and those that have been published revealed variable results.
As the rate of CRC increases over time, it is important to re-evaluate and examine the detection rates of screening colonoscopy among average-risk individuals to better determine the the recommended age to begin screening. It is of no less importance to search for CRC risk factors including gender and origin.
The present study confirms the current guidelines to begin screening for CRC at the age of 50. Environmental factors such as diet and lifestyle are known to have a significant effect on risk for CRC. The substantial differences between Western and East Asian populations in terms of overall adenoma and advanced adenoma detection rates observed in this study is a clear proof that such factors should be taken into consideration.
The results of this study mandate further investigation and imply the need for specific screening recommendation for the Asian population especially among immigrants to western countries.
CRC is the third leading cause of cancer mortality in the Western world. As in most cancers the best treatment is detection and removal of premalignant lesions by colonoscopy. Currently the recommended age for screening is 50 years in which these lesion start to develop.
This is an interesting and well-designed study, addressing the issue of CRC screening in two year groups, 40-50 and 50-59, in an Israeli population. The results are comparable with studies conducted in the West, but interestingly with a slightly lower prevalence rate of adenomas and advanced adenomas in Israel.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cremers MI S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9172] [Cited by in F6Publishing: 9815] [Article Influence: 1090.6] [Reference Citation Analysis (0)] |
| 2. | Peeters M, Haller DG. Therapy for early-stage colorectal cancer. Oncology (Williston Park). 1999;13:307-15; discussion 315-7, 320-1. [PubMed] [Cited in This Article: ] |
| 3. | Lieberman D. Screening for colorectal cancer in average-risk populations. Am J Med. 2006;119:728-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Strul H, Kariv R, Leshno M, Halak A, Jakubowicz M, Santo M, Umansky M, Shirin H, Degani Y, Revivo M. The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average-risk individuals aged 40-80 years. Am J Gastroenterol. 2006;101:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346:1781-1785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 7. | Lieberman DA, Williams JL, Holub JL, Morris CD, Logan JR, Eisen GM, Carney P. Race, ethnicity, and sex affect risk for polyps >9 mm in average-risk individuals. Gastroenterology. 2014;147:351-38; quiz e14-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Ferlitsch M, Heinze G, Salzl P, Britto-Arias M, Waldmann E, Reinhart K, Bannert C, Fasching E, Knoflach P, Weiss W. Sex is a stronger predictor of colorectal adenoma and advanced adenoma than fecal occult blood test. Med Oncol. 2014;31:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 11. | Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, Kim DY, Oh JH. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat. 2012;44:219-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Ladabaum U, Clarke CA, Press DJ, Mannalithara A, Myer PA, Cheng I, Gomez SL. Colorectal cancer incidence in Asian populations in California: effect of nativity and neighborhood-level factors. Am J Gastroenterol. 2014;109:579-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Howerton MW, Gibbons MC, Baffi CR, Gary TL, Lai GY, Bolen S, Tilburt J, Tanpitukpongse TP, Wilson RF, Powe NR. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Homayoon B, Shahidi NC, Cheung WY. Impact of asian ethnicity on colorectal cancer screening: a population-based analysis. Am J Clin Oncol. 2013;36:167-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Centers for Disease Control and Prevention (CDC). Cancer screening - United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41-45. [PubMed] [Cited in This Article: ] |
| 16. | Oh KM, Jacobsen KH. Colorectal cancer screening among Korean Americans: a systematic review. J Community Health. 2014;39:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | de Jong AE, Morreau H, Nagengast FM, Mathus-Vliegen EM, Kleibeuker JH, Griffioen G, Cats A, Vasen HF. Prevalence of adenomas among young individuals at average risk for colorectal cancer. Am J Gastroenterol. 2005;100:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Boursi B, Halak A, Umansky M, Galzan L, Guzner-Gur H, Arber N. Colonoscopic screening of an average-risk population for colorectal neoplasia. Endoscopy. 2009;41:516-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Thoma MN, Castro F, Golawala M, Chen R. Detection of colorectal neoplasia by colonoscopy in average-risk patients age 40-49 versus 50-59 years. Dig Dis Sci. 2011;56:1503-1508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Rundle AG, Lebwohl B, Vogel R, Levine S, Neugut AI. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology. 2008;134:1311-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Hemmasi G, Sohrabi M, Zamani F, Ajdarkosh H, Rakhshani N, Khoonsari M, Ameli M, Hatami K. Prevalence of colorectal adenoma in an average-risk population aged 40-50 versus 50-60 years. Eur J Cancer Prev. 2015;24:386-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Chiu HM, Wang HP, Lee YC, Huang SP, Lai YP, Shun CT, Chen MF, Wu MS, Lin JT. A prospective study of the frequency and the topographical distribution of colon neoplasia in asymptomatic average-risk Chinese adults as determined by colonoscopic screening. Gastrointest Endosc. 2005;61:547-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Chiu HM, Lin JT, Chen CC, Lee YC, Liao WC, Liang JT, Shun CT, Wang HP, Wu MS. Prevalence and characteristics of nonpolypoid colorectal neoplasm in an asymptomatic and average-risk Chinese population. Clin Gastroenterol Hepatol. 2009;7:463-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Liu HH, Wu MC, Peng Y, Wu MS. Prevalence of advanced colonic polyps in asymptomatic Chinese. World J Gastroenterol. 2005;11:4731-4734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Choe JW, Chang HS, Yang SK, Myung SJ, Byeon JS, Lee D, Song HK, Lee HJ, Chung EJ, Kim SY. Screening colonoscopy in asymptomatic average-risk Koreans: analysis in relation to age and sex. J Gastroenterol Hepatol. 2007;22:1003-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Hong SN, Kim JH, Choe WH, Han HS, Sung IK, Park HS, Shim CS. Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointest Endosc. 2010;72:480-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Kim HS, Baik SJ, Kim KH, Oh CR, Lee SI. [Prevalence and risk factors of colorectal adenoma in 14,932 koreans undergoing screening colonoscopy]. Korean J Gastroenterol. 2013;62:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Chung SJ, Kim YS, Yang SY, Song JH, Park MJ, Kim JS, Jung HC, Song IS. Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40-49 years undergoing screening colonoscopy. J Gastroenterol Hepatol. 2010;25:519-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 29. | Park HW, Byeon JS, Yang SK, Kim HS, Kim WH, Kim TI, Park DI, Kim YH, Kim HJ, Lee MS. Colorectal Neoplasm in Asymptomatic Average-risk Koreans: The KASID Prospective Multicenter Colonoscopy Survey. Gut Liver. 2009;3:35-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Ko HJ, Youn CH. Determination of the beginning age for colonoscopic screening among colonoscopy-naïve individuals. Clin Res Hepatol Gastroenterol. 2012;36:384-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |