INTRODUCTION
In the United States, Barrett’s esophagus (BE) is currently defined as the presence of endoscopically recognizable columnar mucosa in the esophagus, which is confirmed to have intestinal metaplasia in mucosal biopsy specimens that should be designated as at or above the gastroesophageal junction (GEJ). This definition has evolved in the guidelines over time as more data regarding risk for cancer progression has been discovered[1-5]. It is a condition that affects 5%-15% of patients with gastroesophageal reflux disease and approximately 2% of the total population[5,6]. It is believed to develop partly in response to acid exposure in the distal esophagus causing cellular changes that have the potential to form dysplasia or even cancer. Non-dysplastic BE progresses to cancer at a rate of 0.18%-0.3% per person per year based on robust population and cohort studies[7-10]. In the presence of high grade dysplasia (HGD), this risk of cancer is increased to the order of 15% per year[3,6,11]. While it has been demonstrated that non-dysplastic lesions can be safely monitored with surveillance endoscopy, the presence of neoplastic lesions hastens the need for interventions that eradicate dysplasia and lower both mortality and progression to cancer[4,11-14]. The rise in the incidence of esophageal adenocarcinoma in recent decades without significant improvement in patient outcomes has prompted the development of an array of endoscopic therapies for neoplastic BE. Following endoscopic therapy however, a paucity of guidelines exist for post-treatment surveillance and the optimal follow up interval is unknown[1-5]. The purpose of this paper is to review the literature regarding endoscopic surveillance following current endotherapy strategies for neoplastic BE, discuss the deficiencies of current surveillance protocols, as well as to comment on the potential role of emerging modalities for monitoring disease progression in the post treatment setting.
DYSPLASIA, ENDOTHERAPY, AND RECURRENCE
Dysplasia in BE is classified into non-dysplastic BE, low grade dysplasia (LGD), indeterminate for dysplasia, HGD, and intra-mucosal cancer (IMC). Often times HGD and IMC are combined into a category of neoplastic BE, with further sub-classifications based on depth of invasion. Recent analyses have concluded that endotherapy is equally as effective at achieving remission in high grade mucosal lesions as esophagectomy, but with less morbidity and fewer complications[4,14,15]. In the realm of endotherapy, both tissue acquiring and tissue damaging modalities exist and are often used in concert. Visible lesions are an indication for endoscopic mucosal resection (EMR) which can be used to confirm a diagnosis and allow appropriate staging[1-2,16-18]. Radical EMR has also been associated with higher complication rates, with strictures occurring in up to 37% in one cohort[16,18]. Photodynamic therapy was initially utilized to ablate Barrett’s neoplasia based on early randomized trials demonstrating a reduction in cancer progression and elimination of dysplasia vs proton pump inhibitor alone[1,19]. However, this technique has also been shown to yield higher rates of residual buried metaplasia, recurrence of dysplasia, inability to ablate non-dysplastic lesions, and more frequent adverse effects[3,15,20]. Cryotherapy is a technique that has been used in smaller cohorts, however longitudinal randomized controlled data regarding outcomes are still needed[1-4,21]. In the last decade, circumferential radiofrequency ablation (RFA) has become one of the most commonly used endoscopic ablative therapies[4]. RFA is typically combined with focal EMR of visible lesions, and this hybrid method has produced high (> 90%) rates of eradication and a durable response up to 5 years post-treatment[12,22-25]. There is also emerging data that hybrid therapy in patients with LGD can decrease rates of progression to HGD and IMC by up to 25% with an acceptable safety profile when compared to optimal surveillance alone[13]. Proton pump inhibitors remain the standard of care in medical management regardless of whether endotherapy is pursued[1-4]. Their use decreases acid exposure in the distal esophagus and is thought to prevent the cellular changes that lead to the development of dysplasia and cancer, although this relationship has never been definitively proven[5,26,27].
Despite its high rate of eradication of dysplasia, there remain concerns about durability of response and recurrence patterns following hybrid endotherapy[20,23,25,28,29]. Multiple studies have demonstrated that recurrence of intestinal metaplasia and progression to cancer still happen in the post-treatment period[30-33]. One meta-analysis quotes recurrence rates of 11% following endotherapy with complete eradication of neoplastic lesions[14]. Most gastroenterologists agree with continued surveillance, but there still remains significant variability among endoscopic follow up in practice due to both patient and physician factors[4,15,34]. Part of this has been due to rapid advances in technology causing a shifting landscape of ablative therapies, and the lack of large, high quality randomized controlled trials. The adequacy of current surveillance methods has also been called into question on numerous fronts. Sampling error, inter-observer variability, biopsy depth, properties of neosquamous epithelium and buried metaplasia, and metachronous lesions all provide challenges to the standard of targeted and four quadrant biopsies. The cost-effectiveness of post-ablation surveillance and new imaging technologies to detect buried intestinal metaplasia are also items gaining attention in the literature, as the financial burden of healthcare continues to grow[6,12,35]. All of these reasons highlight the need for evidence based protocols to guide surveillance in the post-treatment period.
CURRENT GUIDELINES FOR DYSPLASIA SURVEILLANCE AND PRACTICE TRENDS IN THE POST-ABLATION PERIOD
Endoscopic surveillance with four quadrant biopsies and targeted sampling of visible irregularities is the current standard of practice for patients diagnosed with BE. This technique has been implemented largely based on the assumption that earlier detection of dysplasia and treatable cancers will reduce deaths from esophageal adenocarcinoma and prolong survival[2,5]. A majority of the available evidence suggesting decreased mortality from surveillance has been retrospective to date[1-3]. Guidelines suggest that non-dysplastic BE can be followed with surveillance endoscopy every 3-5 years and targeted four quadrant biopsies every two centimeters (low quality of evidence). Most professional organizations recommend more aggressive surveillance intervals immediately following a diagnosis of dysplasia, based on the accelerated risk of developing esophageal adenocarcinoma. The presence of indeterminate for dysplasia or LGD calls for repeat endoscopy after 6 mo and, if confirmed, surveillance annually with biopsies every 1-2 centimeters (moderate quality of evidence). HGD/IMC requires targeted four quadrant biopsies every centimeter (low quality of evidence) with repeat endoscopy at 3 mo provided no ablative therapies or resection are initially pursued, in concert with consideration of further imaging and possible surgical consultation[1-4].
However, the frequency and duration of optimal surveillance following endoscopic therapy is less clear. Post-ablation surveillance is not discussed in the most recent position statement from the American Gastroenterological Association on the management of BE[2]. The American College of Gastroenterology guidelines suggest (grade D recommendation) that patients should be followed with biopsies in the area of prior Barrett’s mucosa at intervals appropriate for their prior grade of dysplasia until there is “reasonable certainty of complete ablation” on at least three consecutive endoscopies. They go on to say that periodic surveillance is then recommended but there is insufficient evidence to offer specific time intervals[1]. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines state that optimal surveillance intervals after ablation are unknown, however their authors recommend endoscopy every 3 mo for the first year following ablation, every 6 months in the second year, and annually thereafter (no associated level of evidence)[3]. One recent survey of 42 expert endoscopists found that all are performing post-ablation surveillance, and most are following the intervals suggested by the ASGE guidelines, with only a minority routinely ordering other imaging studies such as endoscopic ultrasound (EUS) and computed tomography for further staging[15].
DEFICIENCIES OF CURRENT SURVEILLANCE PROTOCOLS
Defining recurrence
Even though most experts agree that surveillance is beneficial following endoscopic treatment of neoplastic BE, there are deficiencies in the current surveillance process that cast doubt on our ability to reliably detect recurrence and progression of disease. First there is a lack of standardization in terminology when discussing disease recurrence. Most studies on RFA acknowledge that it often takes multiple sessions to achieve complete eradication of dysplastic lesions, but there is variability in the definition of complete eradication. Some studies count a single endoscopy free of visible and histopathologic findings of dysplasia adequate for achieving remission, whereas others require multiple consecutive endoscopies[1,22,25,36]. Often times, intestinal metaplasia is found incidentally on random biopsy of neosquamous epithelium following circumferential RFA of high grade lesions, and its implication on prognosis and the need for maintenance RFA treatment is uncertain[12,29,37-39]. When such areas are found and touched up, it is unclear if this constitutes residual metaplasia that was insufficiently treated, if it represents true recurrence of the parent lesion, or if it is a metachronous lesion that may be genetically independent with unknown malignant potential.
Sampling error and white light endoscopy
Furthering the difficulty in characterizing recurrence, is the fact that studies to date have utilized traditional white light endoscopy to identify areas with visible changes for targeted forceps biopsy, along with random four quadrant biopsies most commonly following the Seattle protocol[3]. Unfortunately, a random biopsy approach can be tedious, and does not provide information from a significant portion of the esophageal mucosa. Visible detection of the recurrence of intestinal metaplasia following ablation may also be insufficient with current imaging techniques. It has been shown that endoscopic evaluation of the neo-squamocolumnar junction, even with the use of narrow band imaging, has limited sensitivity and specificity (sensitivity 65%-71%, specificity 37%-46%) for detecting biopsy confirmed intestinal metaplasia[40]. In addition, endoscopy and histology do not always tell the full picture of genetic changes that may be present in normal appearing neosquamous tissue, predisposing certain areas to tumorigenesis[33]. The heavy reliance upon visual inspection and random biopsies in our literature to date introduces inherent sampling bias, and has the potential for missing lesions not evident to the endoscopist, altering true rates of remission and recurrence[30]. The methodological weakness in this surveillance technique may in part limit our ability draw accurate conclusions on recurrence rates.
Buried metaplasia and adequacy of pinch biopsies
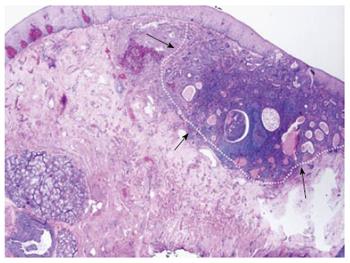
Buried intestinal metaplasia following ablation techniques has become recognized as an increasingly common phenomenon that may be underestimated by studies using pinch biopsies for surveillance. EMR and biopsy specimens can vary significantly in their final diagnosis, raising concern that surface biopsy findings do not accurately reflect the nature of a given lesion[18,32,37]. Unfortunately, EMR is not feasible in all areas where metaplasia is found on random sampling of the overlying neosquamous epithelium. Numerous studies have confirmed the existence of so-called subsquamous islands of intestinal metaplasia and dysplasia buried beneath normal and post-ablation epithelium[20,31-32,38,41]. Esophagectomy specimens have yielded rates of buried intestinal metaplasia as high as 71% confirmed by optical coherence tomography (OCT) and surgical pathology[42]. One study performing complete EMR for eradication of BE revealed subsquamous lesions with HGD or IMC in 21% of specimens prior to any tissue damaging therapy[32]. A systematic review of buried metaplasia following RFA yielded positive findings in only 0.9% of specimens on follow up biopsy, a rate much lower than for prior ablative techniques[20]. This is an encouraging finding for the use of RFA, but also raises concern over the adequacy of biopsy depth in post-RFA neosquamous epithelium. While it is known that the presence of lamina propria in a biopsy indicates adequate tissue purchase to sample the majority of mucosal lesions, this can often be difficult in the esophagus due to technical limitations[39]. Neosquamous epithelium may also be more fibrous, and limit the ability to achieve adequate biopsy depth. Three major studies looking at the depth of biopsies following photodynamic therapy or RFA ablation showed no difference between the number of pre-treatment and post-treatment biopsies containing lamina propria, despite their absolute percentages varying widely[29,43,44]. The clinical relevance of buried metaplasia remains in question due to a high prevalence and limited research into its malignant potential[38]. However, at least 8 cases of buried neoplasia have been reported in the literature to date, making the concept of subsquamous progression (Figure 1) a legitimate concern[31,38,42,45]. The possibility that endoscopically normal appearing mucosa may be harboring synchronous or metachronous lesions with cancerous potential beyond the reach of random biopsies creates a need for more precise methods of detection.
Figure 1 Subsquamous progression on endoscopic mucosal resection specimen.
Outlined is an area of adenocarcinoma buried underneath squamous epithelium found on surveillance endoscopy, highlighting the potential danger of buried intestinal metaplasia.
Histologic preparation and inter-observer variation
Appropriate post-ablation surveillance also relies on the proper handling and interpretation of biopsy specimens. The histopathological diagnosis of BE varies between American and British societies, with the American Gastroenterological Association still requiring goblet cell identification for confirming the diagnosis[2,37]. In practice, these stringent guidelines can be difficult to meet due to the small amount of tissue obtained and inability to sample the entire area of suspected Barrett’s mucosa. Every step in biopsy processing is important to give the pathologist and endoscopist an accurate picture of the tissue they are sampling. The angle of the biopsy and how it is presented on the slide can impact whether glands appear buried or exposed to the surface, which has implications for their degree of acid exposure and malignant potential[29,37,38]. There is also a spectrum of dysplasia inherent in all biopsies that must be condensed into a single categorization, and this labeling has a large impact on surveillance intervals and therapy recommendations[37]. In the post-ablation population, regenerating tissue has a tendency to appear dysplastic due to inflammation and mesenchymal changes, and differentiating the level of dysplasia may be more difficult[37]. This can lead to over-calling of dysplasia, particularly in the community setting, to decrease the number of false negatives and prevent missing a cancer. Significant inter-observer variability also exists between pathologists, so confirmation with at least one expert gastrointestinal pathologist is recommended[1-4]. Research is ongoing to find supplemental genetic markers that can reliably identify dysplastic changes that predispose an individual to developing cancer[33,46,47].
Durability of response and recurrence patterns
Effective eradication of intestinal metaplasia and IMC is believed to be possible by both tissue acquiring and tissue damaging methods[18,24,48-50]. Patient factors such as age and gender, characteristics of Barrett’s lesions themselves, and the type and quality of endotherapy have been implicated in the ease of eradication and durability of response[27,36,51-53]. Effective post-therapy surveillance guidelines would require accurate information about durability of response, rates of recurrence, and patterns of recurrence for each available treatment option. Regarding hybrid EMR/RFA therapy, Shaheen et al[12] prospectively evaluated 127 patients with dysplastic BE demonstrating rates of eradication above 90% at 3 years based on white light endoscopy with targeted and surveillance biopsies. Even when factoring in patients lost to follow up, intestinal metaplasia was eradicated in 83% and dysplasia in 85% of cases. Phoa et al[23] looked at remission of neoplastic lesions 5 years following focal EMR and serial RFA in a 54 patient cohort using EUS and neosquamous resection to detect recurrence. They showed that 90% sustained complete eradication of neoplasia and intestinal metaplasia, with both neoplastic recurrences occurring near the 5 year cut off. In a multi-center review, Gupta et al[25] reported rates of recurrence using surveillance biopsies at 1 and 2 years as 20% and 33% respectively, with a majority being non-dysplastic. In general the literature supports a rate of recurrence between 5% and 30%, with high variability owing to differing methods for detecting recurrence, inclusion or exclusion of the GEJ, and different periods of follow up. Durability studies to date are limited by the methods used to evaluate for recurrence (i.e., random biopsies) and likely underestimate its true prevalence, however the clinical relevance of buried intestinal metaplasia remains of some debate due to altered microenvironment and tissue properties[20,26,27,29,31].
Patterns and location of recurrence are another important factor surrounding the durability of endoscopic therapies that affects the need for surveillance. Studies have observed that synchronous and metachronous lesions are often present at various depths in the esophageal mucosa, and may not be adequately detected or treated by current techniques[6,28,32,37,41]. One study of EMR specimens found synchronous or metachronous lesions in as many as 28% of samples, and another using OCT found subsquamous metaplasia in 72% pre-RFA and 63% post-RFA[32,54]. The GEJ is a common culprit for harboring dysplastic lesions in patients with pre-existing BE. It has been speculated that this could be due to repeated acid exposure, difficulty in distinguishing the true location of the Z line on biopsy, and some feel that intestinal metaplasia represents a migration of cells from their origin at the GEJ. In one review of over 400 cases of BE following hybrid therapy with 37 recurrences, 19 involved the GEJ[25]. Vaccaro et al[6] also found that all cases of recurrent dysplasia in their cohort occurred at the GEJ in the absence of visible mucosal changes. For this reason, routine ablation of the GEJ has become a common practice and some also advocate surveillance and treatment of the superior gastric folds[40].
OTHER CONSIDERATIONS
Tissue acquiring vs non-tissue acquiring therapy
There is growing concern that tissue damaging therapies may select for dysplastic properties in the residual tissue and thereby predispose treated segments to the development of dysplasia over time. This may be related to certain clonal mutations being refractory to ablative therapy, and tissues containing these mutations would thereby be allowed to proliferate post-treatment creating a new, resistant dysplastic lesion[33,41]. Others have proposed that ablative therapies lead to de novo mutations in tumor suppressor genes p16 and p53 that can cause increased tumorigenesis[33]. Shaheen et al[48] showed that nearly 80% of post-RFA treated areas reverted to a neosquamous phenotype, however this does not account for genetic variants that may persist and predispose to neoplastic lesions buried under normal mucosa[31,41,45]. There has been some interest in more aggressive tissue acquiring therapies such as circumferential EMR and stepwise radical EMR to prevent these potential changes from occurring and propagating[18,50]. Epigenetic alterations are another area of evolving research into elucidating the genetic mechanism of BE progression and identifying at risk lesions. Loss of protection against hypermethylation of promoter regions around tumor suppressor genes is thought to play a role at multiple stages of tumorigenesis. These local hypermethylation events can vary in frequency across a neoplastic lesion, and less is known about their direct cause. It is unclear how various treatment strategies affect epigenetic modifications, but this area may evolve into one that can be applied clinically to identify patients at risk of recurrence after hybrid endotherapy[55-58]. Both genetic and phenotypic targets will likely be required in the future to truly understand a patient’s risk profile[33,47]. Future research advances in the area of clonal mutations and epigenetics may support alterations in surveillance guidelines based on what type of therapy was rendered, however this remains controversial and requires additional research.
Complications of therapy and surveillance
As with any invasive procedure, complications of endoscopic surveillance and therapies must be considered when weighing risks and benefits in discussion with patients. A recent meta-analysis comparing esophagectomy with endotherapy showed no difference in overall remission rate and mortality, with fewer major adverse events in the endotherapy group[14]. However, endotherapy also requires that the patient be willing to undergo some form of surveillance procedure(s), with the potential for additional therapies to be rendered on an as needed basis. Studies of decision-making have shown that patients’ perceived risk of a procedure can vary widely based on their values, past experiences, personal relationships, baseline risk perception, mood, etc. When structuring the conversation of whether to treat endoscopically and how frequently to perform surveillance, providers must take into account the patient’s subjective and objective risk perception, as some patients are willing to accept exceedingly high complication rates in order to have their disorder treated[34]. Alternatively, if a patient has an adverse event when therapy is rendered, they may be less amenable to post-treatment surveillance recommendations. Data on endoscopic complication rates and recommended surveillance intervals must be conveyed accurately to the patient using language they can easily comprehend. This can be difficult as these rates vary widely by institution, expertise of the endoscopist, and type of procedure. Pooled complication estimates for RFA or focal EMR followed by RFA vary from 5%-12% with the most common being esophageal stricture with or without dysphagia, bleeding, mucosal tears and dysrhythmias[13,25,48,49]. Some factors that have been associated with a higher risk of complications include length of BE segment, use of EMR in conjunction with RFA, and older age[25]. Reports on complete EMR also demonstrate rates of symptomatic strictures on the order of 37.8% and perforations around 1.9%[18]. Most adverse outcomes regardless of procedure type were easily treated endoscopically upon follow-up[13,25,48-50].
Cost effectiveness
In today’s ever changing healthcare landscape, quality and cost control have become major considerations in population disease management. Endoscopic therapies have been found to be cost effective in patients with HGD compared to esophagectomy, and could add three quality-adjusted life years (QALY) at minimal cost[35,59]. In non-dysplastic BE or LGD, the cost-utility depends on the ability of endotherapy to durably eradicate the lesion. If an initial ablation could be definitive for these patients and obviate the need for further surveillance, then that could be considered cost-effective[25,35]. One analysis by Hur et al[59] compared different management strategies for patients with BE ranging from non-dysplastic to HGD. The model was based on a 50 year old individual being followed until age 80 or death, and compared surveillance with RFA once HGD developed vs initial RFA followed by surveillance endoscopy. For patients with no dysplasia, the incremental cost-effectiveness ratio for initial RFA vs surveillance was $205500 per QALY, assuming rate of progression of 0.12% per year. This was well above the study’s willingness to pay threshold of $100000 per QALY. In patients with LGD, the incremental cost-effectiveness ratio for initial RFA vs a surveillance first strategy was $18231 per QALY assuming a rate of progression of 0.5% per year. Such complicated population modeling can be difficult to apply to individual patients, and there is inherent variation in the natural history of many LGD lesions[59]. Given the low rates of non-dysplastic progression, extended interval surveillance remains the recommended management strategy along with acid suppression at this time[1-4,11]. Unfortunately, over-surveillance is currently present in up to 2/3 of patients with non-dysplastic BE and presents a major area for improved health resource utilization[11]. Until further long term data are available, definitive cost-effective recommendations will remain difficult for the cohort of patients with indefinite for and LGD[11,13,35,59,60].
EMERGING SURVEILLANCE MODALITIES
Current surveillance recommendations remain dependent on biopsies of neosquamous epithelium as well as random mucosal sampling[1-4]. However, numerous advanced imaging modalities are now being applied to endoscopic techniques that have the potential for improving detection of recurrence and reducing sampling bias. Certain technologies show more progress than others in accomplishing this feat. In 2008, Savoy et al[61] showed that EUS provided little to no additional diagnostic value for patients with normal endoscopic biopsies and cross sectional imaging. It was primarily useful when abnormalities such as deeply invading tumors or extra-esophageal lymphadenopathy were found, and cannot differentiate between dysplastic and non-dysplastic mucosal lesions due to limited resolution[61,62]. Confocal laser microscopy (CLM), an endoscopic technique that allows real time microscopic analysis of surface features using fluorescent staining agents, has also been invoked to offer improvements in targeted biopsies during surveillance endoscopy[41,62-65]. It’s diagnostic yield is limited to superficial lesions as deep as 250 μm, a depth insufficient to detect many sites of buried intestinal metaplasia, making its use in surveillance still incomplete[41,64,66]. Of note, one major randomized controlled trial adding CLM to standard white light imaging was stopped early due to a lack of difference between the experimental and control groups in detection of residual intestinal metaplasia, concluding that CLM did not add any additional diagnostic information[63]. Narrow band imaging (NBI) has also been touted as having the potential to detect patterns of intestinal metaplasia with reasonable accuracy[67-69]. Many of the studies on NBI were un-blinded and involved patients with long segment BE that was endoscopically easy to visualize. In patients with a normal appearing mucosa, particularly at the neo-squamocolumnar junction following RFA ablation, NBI showed a sensitivity of 71% and specificity of 37% for detecting residual intestinal metaplasia in one study. This same article also revealed that increasing confidence of the endoscopist in the diagnosis did not change the sensitivity and specificity values[40].
Of the emerging endoscopic techniques, OCT has shown promise for future diagnostic advancement. This ultra-high resolution device encompasses a fiber-optic probe that can be inserted into the accessory port of the endoscope with the ability to provide high quality volumetric images of the esophageal wall in real time using near infrared low coherence light[42,54,66,70]. It has the capacity to image to a depth of 1-3 mm with resolution on the scale of 3-5 μm[38]. One study by Cobb et al[42], utilized ultra high resolution OCT in fresh esophagectomy specimens of patients with HGD or esophageal adenocarcinoma. OCT was able to detect histologically confirmed subsquamous intestinal metaplasia, as well as differentiate between dysplasia and adenocarcinoma by imaging alone[42]. In another application of this technology, Tsai et al[66] performed OCT at the GEJ before and after RFA treatment and demonstrated that thinner lesions predicted higher success rates of ablative therapy. A depth of 333 μm or less was associated with a 92% sensitivity, 85% specificity, and 88% accuracy in predicting the absence of residual metaplasia at follow up endoscopy[38,66]. OCT has also been used to identify buried glands in pre and post-RFA specimens, demonstrating responses to treatment in real time[53]. It offers the potential for improved depth, a larger field of view, 3-D imaging, and reliable detection and differentiation of mucosal and submucosal abnormalities when compared to white light endoscopy with random biopsies[38,39,42,54,66]. Akin to other advanced imaging technologies, it is not immune to criticisms, including lack of standardized and validated criteria, added time to procedure, expense of the probes, variable endoscopic expertise, and limited speed of image processing[16,39,42,66].
Despite the fascination with endoscopic regression, many geneticists have argued for years that phenotype is only part of the story. Endoscopic improvement in the degree of visible abnormalities is no doubt important, but the genetic changes that underlie progression to cancer can persist in normal appearing mucosa[31,33,38,41,45]. The proportion of clonal abnormalities involving p16, p53 and chromosomal ploidy in a given lesion has been implicated in the genetic instability that causes progression to adenocarcinoma[47]. Some even feel these changes make mucosal segments inherently resistant to, and therefore clonally enhanced by, tissue damaging therapies[33,46,47]. Changes in such pro-tumorigenic loci have been implicated in both neosquamous epithelium as well as buried esophageal glands that may proliferate despite histologic normalization on biopsy in some cases[33,71]. We currently have limited ability to achieve real time molecular profiling that reliably detects mucosal genetic abnormalities during endoscopic intervention. It is hoped that with further study, novel genetic markers may become easier to detect in endoscopically normal mucosa, such that a hybrid genotypic and phenotypic approach to targeted surveillance and endotherapy might be achieved.
CONCLUSION
As we are now able to achieve organ sparing eradication of superficial neoplasia in BE, we need to also then focus our attention on how best to manage these patients after eradication is achieved. Implementing optimal surveillance practices requires additional understanding of the biology of the disease, appreciation of the limits of current tools and treatments, and exploration of the role of adjunctive technologies. Novel molecular targets combined with improvements in real time imaging of the epithelium and submucosal structures will likely continue to further our recognition of disease patterns and refine our understanding of recurrence. Patient centric models of surveillance and therapy may emerge as we learn more about the disease and inherent features that lead to increased morbidity and mortality. All of these advances must also be undertaken in a cost conscious way that will promote patient autonomy within the shared-decision making model. As we strive to reach a consensus on post-therapy surveillance guidelines, continued endoscopy with biopsies and vigilance of the endoscopist after eradication is paramount to achieving long term success of endotherapy in BE.
P- Reviewer: Mullin JM, Watari J S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH