Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3381
Peer-review started: September 16, 2015
First decision: October 14, 2015
Revised: October 23, 2015
Accepted: December 12, 2015
Article in press: December 14, 2015
Published online: March 28, 2016
AIM: To assess the usefulness of contrast-enhanced harmonic endoscopic ultrasonography (CH-EUS) for lymph node metastasis in pancreatobiliary carcinoma.
METHODS: All patients suspected of pancreatobiliary carcinoma with visible lymph nodes after standard EUS between June, 2009 and January, 2012 were enrolled. In the primary analysis, patients with successful EUS-fine needle aspiration (FNA) were included. The lymph nodes were assessed by several standard EUS variables (short and long axis lengths, shape, edge characteristic and echogenicity), color Doppler EUS variable [central intranodal blood vessel (CIV) presence] and CH-EUS variable (heterogeneous/homogeneous enhancement patterns). The diagnostic accuracy relative to EUS-FNA was calculated. In the second analysis, N-stage diagnostic accuracy of CH-EUS was compared with EUS-FNA in patients who underwent surgical resection.
RESULTS: One hundred and nine patients (143 lymph nodes) fulfilled the criteria. The short axis cut-off ≥ 13 mm predicted malignancy with a sensitivity and specificity of 72% and 85%, respectively. These values were 72% and 63% for the long axis cut-off ≥ 20 mm, 62% and 75% for the round shape variable, 81% and 30% for the sharp edge variable, 66% and 61% for the hypoechogenicity variable, 70% and 72% for the CIV-absent variable, and 83% and 91% for the heterogeneous CH-EUS-enhancement variable, respectively. CH-EUS was more accurate than standard and color Doppler EUS, except the short axis cut-off. Notably, three patients excluded because of EUS-FNA failure were correctly N-staged by CH-EUS.
CONCLUSION: CH-EUS complements standard and color Doppler EUS and EUS-FNA for assessment of lymph node metastases.
Core tip: Diagnosis of malignant intra-abdominal lymph nodes is often challenging for endoscopists and radiologists. In the present study, the diagnostic accuracy for differentiating malignant from benign lymph nodes of standard endoscopic ultrasonography (EUS), color Doppler EUS, and contrast-enhanced harmonic (CH)-EUS relative to EUS-fine needle aspiration (FNA) was assessed. A secondary objective of the present study was to assess the N-stage diagnostic accuracy of CH-EUS and EUS-FNA in patients who underwent surgical resection. In conclusion, CH-EUS was more accurate than standard and color Doppler EUS, except the short axis cut-off. Notably, three patients excluded because of EUS-FNA failure were correctly N-staged by CH-EUS.
- Citation: Miyata T, Kitano M, Omoto S, Kadosaka K, Kamata K, Imai H, Sakamoto H, Nisida N, Harwani Y, Murakami T, Takeyama Y, Chiba Y, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for assessment of lymph node metastases in pancreatobiliary carcinoma. World J Gastroenterol 2016; 22(12): 3381-3391
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3381
Accurate staging by using the tumor, node and metastasis (TNM) classification system is the most important variable for determining the optimal treatment of pancreatobiliary carcinomas. In particular, since the lymph node stage relates not only to the choice of treatment but also to the prognosis, it is essential that the techniques used for N-staging are reliable[1,2]. However, diagnosis of malignant intra-abdominal lymph nodes is often challenging for endoscopists and radiologists[3]. Several studies report that although endoscopic ultrasonography (EUS) (which has good spatial resolution) is useful for the differential diagnosis of malignant and benign lymph nodes, its diagnostic accuracy remains unsatisfactory[4-6]. By contrast, a cyto-pathological diagnosis via EUS-fine needle aspiration (FNA) is highly accurate. However, an accurate noninvasive evaluating method[7] is needed for cases in which a lymph node cannot be accessed for EUS-FNA or EUS-FNA does not obtain adequate material for analysis[8]. In addition, noninvasive methods could facilitate EUS-FNA by identifying the target lymph node for EUS-FNA, namely, the lymph node that is most suspicious of malignancy and whose sampling will shape treatment decisions. One such noninvasive evaluation method is vascular imaging. Although color Doppler imaging can evaluate the vasculature in lymph nodes, it has several limitations, including blooming, overpainting and motion artifacts. It is also difficult to evaluate perfusion by using color Doppler imaging. This problem was recently overcome by a revolution in US technology, namely, the invention of US contrast agents that, when combined with contrast harmonic imaging, make it possible to depict the microvasculature in real time[9]. Recently, EUS was equipped with this novel perfusion imaging technique, thus yielding contrast-enhanced harmonic EUS (CH-EUS)[10,11].
In the present study, the diagnostic accuracy for differentiating malignant from benign lymph nodes of standard EUS, color Doppler EUS, and CH-EUS relative to EUS-FNA was assessed. For this, all patients with standard EUS-detected pancreatobiliary carcinomas with apparently visible intra-abdominal lymph nodes who underwent all four procedures during the study period were recruited prospectively and followed up. The CH-EUS variable that was analyzed was the detection of the microvasculature in visible lymph node(s); this was expressed as heterogeneous/homogeneous enhancement. A secondary objective of the present study was to assess the N-stage diagnostic accuracy of CH-EUS and EUS-FNA in patients who underwent surgical resection.
All consecutive patients who were suspected of having pancreatobiliary diseases due to CT, MRI, or transabdominal US results and who then underwent standard EUS between June, 2009 and January, 2012 in a tertiary care referral center in Japan were recruited prospectively (Figure 1). All patients also underwent color Doppler EUS, CH-EUS, and EUS-FNA immediately after the standard EUS procedure. The primary objective of this study was to compare the diagnostic accuracy of standard EUS, color Doppler EUS and CH-EUS in terms of the ability to differentiate malignant nodes from benign nodes. For this primary retrospective analysis, only the patients from whom adequate and accurate EUS-FNA samples were retrieved and who were followed up for at least 12 mo after the standard EUS were included. The patients where a diagnosis was obtained by specimen histology rather than EUS-FNA because of EUS-FNA failure (sample inadequacy or lymph node inaccessibility) were excluded from this analysis because it was sometimes difficult to ensure that the lymph nodes harvested from surgical specimens were the same as those that were identified by imaging.
The study also had a secondary aim, namely, to compare the accuracy of CH-EUS and EUS-FNA in terms of N-stage diagnosis in all of the patients in the original cohort who underwent surgical resection.
The study was approved by the Institutional Review Board of Kinki University Faculty of Medicine. All patients provided informed consent with regard to the procedures and participation in the study.
An echoendoscope developed for CH-EUS (Olympus GF-UCT260; Olympus Medical Systems, Tokyo, Japan) was used. An ALOKA ProSound SSD α-10 (Aloka Co Ltd, Tokyo, Japan) was used for US imaging. For CH-EUS, the extended pure harmonic detection mode was used. This mode selectively depicts signals from the microbubbles by simultaneously filtering the harmonic component and synthesizing the phase-shift signals. The preset variables were established for EUS and CH-EUS previously[10,11]. The transmitting frequency and mechanical indices were set at 4.7 MHz and 0.3, respectively. The frame rate was set at 10-15 frame per second. The focus point was set at the distal portion of the target lymph node.
Sonazoid (Daiichi-Sankyo, Tokyo, Japan; GE Healthcare, Milwaukee, Wis) was used as the US contrast agent. This second generation US contrast agent is composed of perfluorobutane microbubbles with a median diameter of 2-3 μm[12]. Sonazoid was reconstituted with 2 mL of sterile water for injection. A dose of 0.015 mL/kg body weight was used.
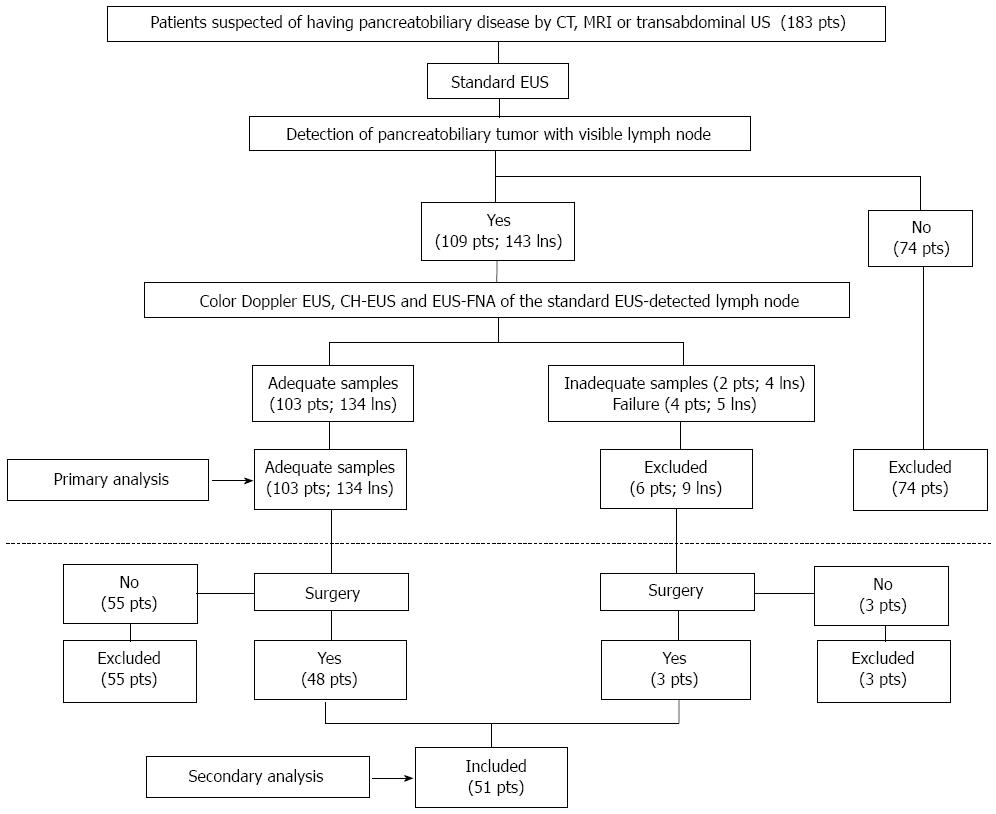
During the EUS analyses, the patients were sedated by midazolam and propofol. Standard EUS, color Doppler EUS, and CH-EUS were performed by two endosonographers (Kitano M and Sakamoto H). One was responsible for the endoscopic manipulation and scanning and the other for operating the US image scanner. Both endosonographers (who were qualified by the Japan Gastroenterological Endoscopy Society) have had experience with CH-EUS for more than 10 years: both have performed more than 1000 CH-EUS procedures. Each examination was performed by using the same protocol. Thus, after a pancreatobiliary carcinoma was observed, the trans-gastric or trans-duodenal approach was used to search for intra-abdominal lymph node(s). If an apparently visible lymph node was detected, standard EUS was used to evaluate the size (i.e., the short and long axis lengths), shape (round or oval), edge characteristics (sharp or fuzzy), and echogenicity (hypo or hyper) of the lymph node. Thereafter, the imaging modality was changed to color Doppler EUS, which was used to determine whether a central intranodal blood vessel (CIV) was present in the lymph node.
Subsequently, the specific mode for CH-EUS (extended pure harmonic detection mode) was selected and a bolus injection of Sonazoid was administered at a speed of 1 mL/s through a 22-gauge cannula that was placed in the antecubital vein. This was followed by a 10-mL saline solution flush to ensure that all contrast was administered into the circulation system. If there were multiple apparently visible lymph nodes, each was separately assessed by injecting US contrast agent, performing CH-EUS, and then conducting EUS-FNA. These multiple CH-EUS procedures were performed at intervals of at least 10 min, which was found to be sufficient for the US contrast from the preceding CH-EUS procedure to be washed out from all lymph nodes. All movie clips were stored on the hard disk of the scanner for offline analysis.
All standard EUS, color Doppler EUS and CH-EUS variables were measured independently in a blinded fashion by two readers (Kudo M and Imai H). Both have had experience with CH-EUS for more than 8 years: both have read the data of more than 500 CH-EUS procedures. The two readers evaluated the movie clips of the lymph nodes. They were told that the movie clips that they were evaluating were standard EUS/color Doppler/CH-EUS analyses of lymph nodes. However, they were blinded to all CT, MRI, transabdominal US, and standard EUS findings of the primary lesions.
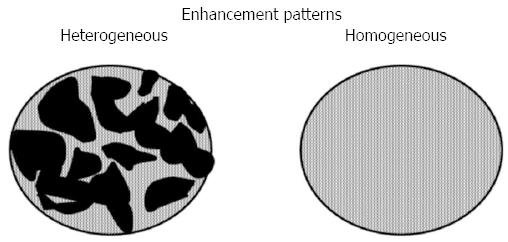
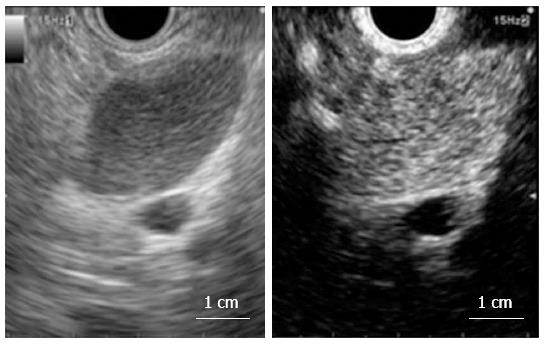
Receiver-operating characteristics (ROC) analysis was used to identify the standard EUS-detected short and long axis cut-off values that would optimize diagnosis of the lymph nodes. Based on a previous report[13], the readers predicted that the lymph nodes were malignant if they had a round shape and/or a sharp edge and/or exhibited hypoechogenicity on standard EUS. The color Doppler EUS images were assessed to determine whether CIV was present[4]. CIV was defined as a tubular structure with a well-defined smooth hyperechoic wall that was ≥ 1 mm in diameter, located toward the center of the lymph node, and demonstrated blood flow on color Doppler EUS (Figure 2A). Based on a previous report[4], the readers predicted that the lymph nodes were malignant if a CIV was absent (Figure 2B). The CH-EUS images were assessed to determine the enhancement patterns, which were classified as being heterogeneous or homogenous[14]. Based on a previous report[14], the readers predicted that the lymph nodes were malignant if heterogeneous enhancement was observed (Figures 3 and 4, Video 1).
Both of the blinded readers initially measured the standard EUS (shape, edge characteristics, and echogenicity), color Doppler EUS (CIV presence/absence), and CH-EUS (heterogeneous/homogenous enhancement pattern) variables separately. Interobserver agreement between the two readers in terms of these measurements was assessed by calculating the κ-coefficient (Supplementary Tables 1-1 to 1-5). Thereafter, if there were discrepant findings between the two readers, they reassessed the relevant image(s) together until an agreement was reached.
| Sex (M:F) | 68:35 | |
| Median age | 65 (35-82) | |
| Median size (long axis × short axis) (mm) | 18 (8-60) × 9 (4-42) | |
| Final diagnosis (n) | Pancreatic carcinoma | 67 |
| Bile duct carcinoma | 21 | |
| Gallbladder carcinoma | 11 | |
| Ampullary carcinoma | 4 | |
The final diagnosis was based on histological and/or cytological analysis of samples obtained by EUS-FNA. After standard EUS, color Doppler EUS, and CH-EUS of each lymph node, EUS-FNA was performed with a 22- or 25-gauge aspiration needle (Echo Tip Ultra, Cook, Winston-Salem, NC, United States). Punctures were repeated until a sample was obtained; the maximum number of passes was five. A cytopathologist was present in the endoscopy room for on-site sample evaluation. After it was confirmed that adequate numbers of cells had been obtained, the samples were processed and evaluated in the pathology department by using Papanicolaou staining for cytology and hematoxylin-eosin staining for histology. If there were multiple apparently visible lymph nodes, EUS-FNA was performed separately on each lymph node: after each aspiration, the needles were changed.
The lymph nodes that were surgically resected after imaging were also assessed by the pathology department for malignancy. For this, 51 patients were included (Figure 1).
The primary objective was to compare the diagnostic accuracy of standard EUS, color Doppler EUS and CH-EUS in terms of the primary end-point, which was the ability to differentiate malignant nodes from benign nodes. The secondary end-point was to compare the accuracy of CH-EUS and EUS-FNA in terms of N-stage diagnosis in patients who underwent surgical resection.
All data were analyzed by using SAS software version 8.2 (SAS Institute, Cary, NC, United States). Differences between the EUS methods in terms of malignant lymph node detection were assessed by using McNemar’s test. A difference with P < 0.01 was regarded as significant. This approach was also used to test differences between benign and malignant lymph nodes in terms of CH-EUS enhancement patterns. McNemar’s test was also used to compare CH-EUS and EUS-FNA in terms of their N-stage diagnostic accuracy in the patients who underwent surgical resection.
Interobserver agreement in terms of the EUS variables described above was also assessed. A κ coefficient of > 0.8 was considered to indicate excellent agreement, > 0.6 was considered to indicate good agreement, and > 0.4 was considered to indicate moderate agreement. The sensitivity, specificity and accuracy with which CH-EUS differentiated malignant from benign lymph nodes were calculated and compared to the values of standard and color Doppler EUS findings (short axis, long axis, shape, edge characteristics, echogenicity and CIV). The numbers of cases of discordance are shown in Supplementary Tables 2-1 to 2-6.
| Sensitivity (95%CI) | Specificity (95%CI) | Accuracy (95%CI) | P value1 | |
| Short axis 13 mm or longer | 72% (34/47) (62-81) | 85% (74/87) (79-90) | 81% (108/134) (73-86) | 0.27 |
| Long axis 20 mm or longer | 72% (34/47) (61-82) | 63% (55/87) (57-68) | 66% (89/134) (59-73) | 0.001 |
| Round shape | 62% (29/47) (51-72) | 75% (65/87) (69-80) | 70% (94/134) (62-77) | 0.008 |
| Sharp edge | 81% (38/47) (71-89) | 30% (26/87) (24-34) | 48% (64/134) (41-53) | < 0.001 |
| Hypoechogenicity | 66% (31/47) (55-76) | 61% (53/87) (55-66) | 63% (84/134) (55-70) | < 0.001 |
| CIV absent | 70% (33/47) (59-80) | 72% (63/87) (66-78) | 72% (96/134) (64-78) | 0.009 |
| Heterogeneous (CH-EUS) | 83% (39/47) (77-89) | 91% (79/87) (86-94) | 88% (118/134) (82-93) |
During the study period, 183 patients suspected of pancreatobiliary disease underwent EUS and were enrolled prospectively. In 109 patients, EUS detected a pancreatobiliary carcinoma and one or more apparently visible intra-abdominal lymph nodes. The total number of detected lymph nodes was 143. The remaining 74 patients were excluded from analysis because a pancreatobiliary carcinoma and/or apparently visible intra-abdominal lymph nodes were not detected. All 109 patients with apparently visible intra-abdominal lymph node(s) in standard EUS immediately underwent color Doppler EUS, CH-EUS, and EUS-FNA. In six patients (nine lymph nodes; 6.3% of the 143 apparently visible lymph nodes detected by standard EUS), the EUS-FNA samples of lymph nodes were inadequate (4 lymph nodes from 2 patients) and failed because the lymph node was in an inaccessible location (5 lymph nodes from 4 patients) (Figure 1). These patients were excluded from the primary analysis cohort. Nevertheless, among these 6 patients, 3 patients underwent surgical resection, and were included in the secondary analysis cohort (Figure 1). The remaining 103 patients (134 lymph nodes) were included in the primary analysis cohort (Figure 1).
Table 1 displays the characteristics of these 103 patients for the primary analysis cohort. The male:female ratio was 68:35 and the median age was 65 (range: 35-82) years. The median long and short axis lengths of the 134 lymph nodes were 18 (range: 8-60) and 9 (range: 4-42) mm, respectively. The final diagnoses were pancreatic carcinoma (n = 67), bile duct carcinoma (n = 21), gallbladder carcinoma (n = 11), and ampullary carcinoma (n = 4). Standard EUS, color Doppler EUS and CH-EUS were successfully performed in all patients and associated adverse effects were not observed. Of the 134 lymph nodes, histological and/or cytological analyses of the samples obtained by EUS-FNA revealed that 47 were malignant lymph nodes and 87 were reactive lymph nodes. Adverse effects of EUS-FNA were also not observed. All 103 patients were followed up for at least 12 mo. None of the patients who were deemed to have benign lymph nodes after EUS-FNA and the other tests, and who did not undergo surgical resection of the nodes, exhibited any signs of lymph node malignancy during follow-up, as indicated by twice yearly standard EUS.
ROC analyses revealed that a short axis of 13 mm or longer and a long axis of 20 mm or longer predicted malignancy with the best sensitivity and specificity (Supplementary Figures 1 and 2). A short axis of 13 mm or longer predicted malignancy with a sensitivity, specificity and accuracy of 72% [95% confidence intervals (CI): 62%-81%)], 85% (95%CI: 79%-90%), and 81% (95%CI: 73%-86%), respectively (Table 2). A long axis of 20 mm or longer predicted malignancy with a sensitivity, specificity and accuracy of 72% (95%CI: 61%-82%), 63% (95%CI: 57%-68%), and 66% (95%CI: 59%-73%), respectively (Table 2). A round shape predicted malignancy with a sensitivity, specificity and accuracy of 62% (95%CI: 51%-72%), 75% (95%CI: 69%-80%), and 70% (95%CI: 62%-77%), respectively (Table 2). A sharp edge predicted malignancy with a sensitivity, specificity and accuracy of 81% (95%CI: 71-89%), 30% (95%CI: 24-34%), and 48% (95%CI: 41-53%), respectively (Table 2). Hypoechogenicity predicted malignancy with a sensitivity, specificity and accuracy of 66% (95%CI: 55%-76%), 61% (95%CI: 55%-66%), and 63% (95%CI: 55%-70%), respectively (Table 2). Interobserver agreement testing revealed good (κ coefficient: 0.63, P < 0.01), moderate (κ coefficient: 0.49, P < 0.01), and moderate (κ coefficient: 0.47, P < 0.01) agreement between the two readers in terms of the shape, edge characteristics, and echogenicity measurements, respectively (Supplementary Tables 2-1 to 2-3).
The absence of a CIV predicted malignancy with a sensitivity, specificity and accuracy of 70% (95%CI: 59%-80%), 72% (95%CI: 66%-78%), and 72% (95%CI: 64%-78%), respectively (Table 2). Interobserver agreement testing revealed good reproducibility between the two readers in terms of this measurement (κ coefficient: 0.69, P < 0.01) (Supplementary Table 2-4).
All 134 lymph nodes yielded high-quality dynamic images on CH-EUS. Interobserver agreement testing revealed excellent reproducibility between the two readers in terms of detecting heterogeneous/homogeneous enhancement patterns (κ coefficient: 0.81, P < 0.01) (Supplementary Table 2-5).
Table 3 lists the number and frequency of lesions in the benign and malignant lymph node groups that had a heterogeneous or homogeneous enhancement pattern after reassessment of discrepant findings by the two blinded readers. Of the 47 malignant lymph nodes, 39 (83%) exhibited heterogeneous enhancement in which the distorted tumor vessels could be clearly visualized (Figures 3 and 4, Video 1). Of the 87 benign lymph nodes, 79 (91%) exhibited homogeneous enhancement (Figures 3 and 5, Video 2). The benign and malignant lymph node groups differed significantly in terms of the frequencies of homogeneous and heterogeneous enhancement (P < 0.01). When heterogeneous enhancement was deemed to indicate malignancy and homogeneous enhancement was deemed to indicate benignity, CH-EUS differentiated malignant from benign lymph nodes with a sensitivity, specificity and accuracy of 83% (95%CI: 77%-89%), 91% (95%CI: 86%-94%), and 88% (95%CI: 82%-93%), respectively (Table 2).
| Number with each enhancement pattern | |||
| Final diagnosis | Heterogeneous | Homogeneous | Total |
| Malignancy | 39 | 8 | 47 |
| Benign | 8 | 79 | 87 |
| Total | 47 | 87 | 134 |
CH-EUS diagnosed malignant lymph nodes with a significantly higher diagnostic accuracy than most of the standard EUS variables (P = 0.001 vs the 20-mm long axis cut-off, P = 0.008 vs the round shape variable, P < 0.001 vs the sharp edge variable, and P < 0.001 vs the hypoechogenicity variable, as determined by McNemar tests) or the color Doppler EUS CIV variable (P = 0.009). However, CH-EUS did not differ significantly from the 13-mm short axis cut-off variable in terms of differentiating malignant from benign lymph nodes (P = 0.27) (Table 2).
Of the 109 patients in whom EUS detected a pancreatobiliary carcinoma and one or more apparently visible intra-abdominal lymph nodes, 48 patients underwent surgical resection and histological examinations of the resected lymph nodes (Figure 1). In addition, three patients whose EUS-FNA samples of lymph nodes were inadequate or failed EUS-FNA because the lymph node was in an inaccessible location underwent surgical resection, and were included in the secondary analysis cohort (Figure 1, Table 4). Thus, the secondary analysis cohort consisted of 51 patients. Comparison of the EUS-FNA and CH-EUS findings relative to surgical specimen histology revealed that six (including failed due to inadequate sampling and inaccessibility) and five of the 51 patients were misdiagnosed by EUS-FNA and CH-EUS, respectively. Thus, EUS-FNA and CH-EUS diagnosed the N-stage in the patients who underwent surgical resection with an accuracy of 88% and 90%, respectively (P = 0.50).
| Sex (M:F) | 32:19 | |
| Median age | 66 (37-79) | |
| Median size (long axis × short axis) (mm) | 20 (8-60) × 10 (4-42) | |
| Final diagnosis (n) | Pancreatic carcinoma | 29 |
| Bile duct carcinoma | 12 | |
| Gallbladder carcinoma | 7 | |
| Ampullary carcinoma | 3 | |
It should be noted that three patients in the secondary analysis cohort were not included in the primary analysis cohort because EUS-FNA failed due to inadequate sampling or inaccessibility of the lymph nodes. All three patients were correctly N-staged by CH-EUS. One of these three patients was shown by standard and color Doppler EUS to have a long axis of 22 mm, a sharp edge, and to lack a CIV: all of these features predicted that the lymph node was malignant. By contrast, CH-EUS revealed that this lymph node had homogeneous enhancement, which was deemed to indicate a benign lymph node. The patient underwent surgery and indeed, histological examination of the lymph node resected during surgery revealed that it was benign. With regard to the remaining two patients, between two and four of the six standard EUS and color Doppler EUS variables predicted that they were benign. By contrast, CH-EUS revealed that it had heterogeneous enhancement, which was deemed to indicate a malignant lymph node. Indeed, histological examination of the lymph nodes resected during surgery revealed that those lymph nodes were malignant.
A study by Gill et al[13] identified several morphological characteristics that can be detected by standard EUS that can help to distinguish between malignant and benign lymph nodes. Multivariable analysis revealed that in particular, a round shape, a sharp edge, and a short axis that exceeded 8.3 mm associated significantly with malignant cytology. However, the predictive accuracy of these features was limited. The present study also assessed the ability of a round shape, a sharp edge, hypoechogenicity, a ≥ 13 mm short axis length, and a ≥ 20 mm long axis length to distinguish between benign and malignant lymph nodes. However, like Gill et al[13], we found that the diagnostic accuracy of these features was limited.
An alternative method is color Doppler EUS. Sawhney et al[4] reported that the absence of CIV on color Doppler EUS is a strong and independent predictor of metastatic lymph node. In our study, however, the absence of CIV on color Doppler EUS did not predict malignancy better than the standard EUS variables. This may reflect differences between our study and theirs in terms of the way the lymph nodes were selected: Sawhney et al[4] only included lymph nodes that were 10 mm or longer, whereas in the present study, smaller lymph nodes were included (64 had a short axis diameter of less than 10 mm). This difference may relate to the fact that we evaluated all apparently visible lymph nodes found during the standard EUS procedure by color Doppler EUS. Therefore, the lymph nodes evaluated in the current study were relatively smaller than those examined by Sawhney et al[4]. Since some small benign lymph nodes may not exhibit CIV, this may have resulted in the relatively lower specificity associated with this variable in our study.
Another alternative method is contrast-enhanced color Doppler EUS with US contrast agent. Kanamori et al[15] reported that defective enhancement on contrast-enhanced color Doppler EUS using the first generation US contrast agent Levovist, (Nihon Schering Co., Ltd., Tokyo, Japan) predicted lymph node malignancy significantly more accurately than standard EUS variables. Hocke et al[8] also reported that that an irregular appearance of the vessels (or the presence of arterial vessels only) on contrast-enhanced Doppler EUS using the second generation US contrast agent SonoVue (BR1, Bracco, Italy) predicted lymph node malignancy significantly better than standard EUS variables. However, as with the study by Sawhney et al[4], the lymph nodes examined in these studies were relatively larger than those in our study.
Recently, the combination of the second generation US contrast agent Sonazoid and low mechanical index imaging techniques has led to CH-EUS being used for perfusion imaging, which facilitates the depiction of tumor vascularity[10,16-18]. Sonazoid resonates with a low acoustic power and thus allows us to perform CH-EUS. We showed previously that this method has an excellent ability to differentiate malignant from benign lesions without Doppler-related artifacts, even when the lesions are small[19]. Heterogeneous enhancement was observed in 39 of 47 (83%) malignant lymph nodes. This is consistent with the observation of a pathology-based study[20] that showed that the vascular architecture of malignant lymph nodes is characterized by caliber fluctuations, an irregular coarse, sinusoid formation, and arteriovenous shunts. In the current study, interobserver agreement regarding CH-EUS results revealed excellent reproducibility between the two readers (κ coefficient: 0.81). Another report also showed that CH-EUS yielded highly reproducible findings with regard to malignant lymph nodes[21]. Indeed, its reproducibility was higher than that of MDCT[22] and all of the standard EUS findings that were measured in the present study. However, it should be noted that in the current study, the readers were experts who had practiced CH-EUS for more than 8 years; each had read the data of more than 500 CH-EUS procedures. It is possible that the reproducibility of CH-EUS findings among beginners may be low, although Gincul et al[21] did not detect significant differences between experts and beginners. Fusaroli et al[23] also reported that among three parameters (uptake, pattern, and washout) of CH-EUS for solid pancreatic lesion, pancreatic cystic lesion, and submucosal lesion, the reproducibility between experienced and non-experienced endosonographers did not differ significantly. This issue must be validated in future series.
Another alternative method is EUS elastography. EUS elastography has been presented as a novel technique to assess tissue elasticity and has been used to differentiate between malignant and benign lymph nodes. Several different variables have been used in EUS elastography as a measure of tissue elasticity, namely, color patterns[24-29], strain ratio[30,31], hue histogram analysis[32,33] and artificial neural networks[34,35]. Wei et al[36] report a meta-analysis that included seven articles and a large number of lymph nodes (368 patients with 431 lymph nodes). The sensitivity and specificity of EUS elastography for the differential diagnosis of benign and malignant lymph nodes were 88%, and 85%, respectively. The area under the summary receiver operating characteristic curve was 0.9456. However, the sensitivity and specificity of this method varied greatly between studies[36]. Thus, CH-EUS should be compared to EUS elastography in terms of its ability to differentiate malignant from benign lymph nodes in further studies. In addition, it may be useful to evaluate whether these imaging methods could complement each other or other methods.
EUS-FNA is also useful for differentiating malignant from benign lymph nodes. Since EUS-FNA is highly specific in terms of identifying malignant lymph nodes, most cases where EUS-FNA reveals the presence of atypical cells in the lymph nodes have a final diagnosis of malignant lymph node[37]. However, false-positive and false-negative EUS-FNA results remain possible. Jason et al[38] report that in their series, the EUS-FNA false-positive and false-negative rates of intra-abdominal lymph node diagnosis were 0.7% and 5.8%, respectively. The present study suffers a limitation in relation to this: we cannot be certain that the EUS-FNA findings of the lymph nodes analyzed in the primary analysis were correct. For this reason, only patients who were followed up for at least 12 mo were included in the primary analysis. None of the patients with apparently benign lymph nodes exhibited signs of lymph node malignancy during this follow-up period.
Another limitation of EUS-FNA is that it cannot be performed in all cases because of intervening vessels and/or the difficult location of the lymph node, which could, for example, lead to an excessively large scope angle or distance from the probe. These problems suggest that CH-EUS technology may complement EUS-FNA-based histological and/or cytological diagnoses. This notion is supported by the four studies that have compared CH-EUS and EUS-FNA previously. All were for pancreatic masses. Napoleon et al[39] report that of five adenocarcinomas that had false-negative EUS-FNA results, CH-EUS revealed hypo-enhancement in four. Gincul et al[21] also report that all five false-negative EUS-FNA cases were correctly classified by CH-EUS. Moreover, Kitano et al[19] report that when CH-EUS was combined with EUS-FNA, the sensitivity of EUS-FNA increased from 92.2% to 100%. Fusaori et al[40] also report that CH-EUS increased the detection of malignant pancreatic lesions in difficult cases (patients with chronic pancreatitis or biliary stents) and helped guide EUS-FNA. The present study showed that at least in the patients who underwent surgical resection, CH-EUS and EUS-FNA did not differ in terms of N-staging diagnostic accuracy. However, CH-EUS correctly N-staged three patients in which EUS-FNA sampling failed because of lymph node location or were inadequate. This is the first report to indicate that CH-EUS complements EUS-FNA in terms of N-staging in patients with pancreatobiliary neoplasms.
The present study had some limitations. Multiple lymph nodes in one patient were included in the primary analysis because it was unclear which of these lymph nodes should be sampled; thus, all apparently visible lymph nodes were sampled. This could have introduced a bias in terms of lymph node selection. In addition, EUS-FNA was the gold standard in the primary analysis, even though the accuracy of EUS-FNA may be limited, as discussed above. Histology of resected specimens yields the most accurate diagnosis. However, it is difficult to identify during surgery which lymph nodes were previously evaluated by standard EUS, color Doppler EUS, or CH-EUS. For this reason, EUS-FNA served as the gold standard in the primary analysis.
In conclusion, CH-EUS depicted the microvasculature of intra-abdominal lymph node very clearly. Thus, it may be a useful modality for differentiating malignant from benign lymph nodes in patients with pancreatobiliary carcinomas and may complement standard EUS, color Doppler EUS and EUS-FNA, all of which have limitations. In addition, it may be helpful for determining the lymph nodes that should be subjected to EUS-FNA. In view of the high accuracy described in this study, in the future, CH-EUS may help to detect the in-operable stage better and thereby helps to avoid unnecessary surgery. Hence, CH-EUS will play an important role in determining the optimal treatment of pancreatobiliary carcinomas. However, given that the sample size of this study was relatively small and all CH-EUS procedures were performed in a single medical unit, an additional study that confirms the value of CH-EUS for differentiating malignant from benign lymph nodes is warranted.
Accurate staging by using the tumor, node and metastasis (TNM) classification system is the most important variable for determining the optimal treatment of pancreatobiliary carcinomas. In particular, since the lymph node stage relates not only to the choice of treatment but also to the prognosis, it is essential that the techniques used for N-staging are reliable.
A cyto-pathological diagnosis via endoscopic ultrasonography (EUS)-fine needle aspiration (FNA) is highly accurate. Noninvasive methods could facilitate EUS-FNA by identifying the target lymph node for EUS-FNA, namely, the lymph node that is most suspicious of malignancy and whose sampling will shape treatment decisions. Standard EUS can help to distinguish between malignant and benign lymph nodes, although the predictive accuracy of these features was limited. US contrast agents that, when combined with contrast harmonic imaging, make it possible to depict the microvasculature in real time. Recently, EUS was equipped with this novel perfusion imaging technique, thus yielding contrast-enhanced harmonic EUS (CH-EUS).
This is the first study to evaluate the diagnostic accuracy of CH-EUS for differentiating malignant from benign lymph node, compared with standard and color Doppler EUS. CH-EUS was more accurate than standard and color Doppler EUS. Notably, three patients with EUS-FNA failure were correctly N-staged by CH-EUS.
The results of this study suggest that it may be a useful modality for differentiating malignant from benign lymph nodes in patients with pancreatobiliary carcinomas and may complement EUS-FNA. In addition, it may be helpful for determining the lymph nodes that should be subjected to EUS-FNA. Application of CH-EUS to staging will help patients avoid unnecessary surgery.
Color Doppler imaging has several limitations, including blooming, overpainting and motion artifacts. It is also difficult to evaluate perfusion by using color Doppler imaging. This problem was recently overcome by a revolution in US technology, namely, contrast harmonic imaging which makes it possible to depict the microvasculature in real time. Recently, EUS was equipped with this novel perfusion imaging technique, thus yielding CH-EUS.
The authors demonstrated the clinical utility of CH-EUS as a diagnostic tool for detecting lymph node metastasis in patients with pancreatobiliary carcinoma. This paper is informative and interesting for the further developments of imaging approaches.
P- Reviewer: Arigami T, Hardt PD, Lee CL, Li YM S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Schomas DA, Quevedo JF, Donahue JM, Nichols FC, Romero Y, Miller RC. The prognostic importance of pathologically involved celiac node metastases in node-positive patients with carcinoma of the distal esophagus or gastroesophageal junction: a surgical series from the Mayo Clinic. Dis Esophagus. 2010;23:232-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Natsugoe S, Yoshinaka H, Shimada M, Sakamoto F, Morinaga T, Nakano S, Kusano C, Baba M, Takao S, Aikou T. Number of lymph node metastases determined by presurgical ultrasound and endoscopic ultrasound is related to prognosis in patients with esophageal carcinoma. Ann Surg. 2001;234:613-618. [PubMed] [Cited in This Article: ] |
| 3. | Sharma A, Fidias P, Hayman LA, Loomis SL, Taber KH, Aquino SL. Patterns of lymphadenopathy in thoracic malignancies. Radiographics. 2004;24:419-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Sawhney MS, Debold SM, Kratzke RA, Lederle FA, Nelson DB, Kelly RF. Central intranodal blood vessel: a new EUS sign described in mediastinal lymph nodes. Gastrointest Endosc. 2007;65:602-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Eloubeidi MA, Wallace MB, Reed CE, Hadzijahic N, Lewin DN, Van Velse A, Leveen MB, Etemad B, Matsuda K, Patel RS. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: a single-center experience. Gastrointest Endosc. 2001;54:714-719. [PubMed] [Cited in This Article: ] |
| 6. | Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479. [PubMed] [Cited in This Article: ] |
| 7. | Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist’s perspective. Am J Clin Pathol. 2003;120:351-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Hocke M, Menges M, Topalidis T, Dietrich CF, Stallmach A. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J Cancer Res Clin Oncol. 2008;134:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video). Gastrointest Endosc. 2008;67:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Romagnuolo J, Hoffman B, Vela S, Hawes R, Vignesh S. Accuracy of contrast-enhanced harmonic EUS with a second-generation perflutren lipid microsphere contrast agent (with video). Gastrointest Endosc. 2011;73:52-63. [PubMed] [Cited in This Article: ] |
| 12. | Sontum PC, Ostensen J, Dyrstad K, Hoff L. Acoustic properties of NC100100 and their relation with the microbubble size distribution. Invest Radiol. 1999;34:268-275. [PubMed] [Cited in This Article: ] |
| 13. | Gill KR, Ghabril MS, Jamil LH, Hasan MK, McNeil RB, Woodward TA, Raimondo M, Hoffman BJ, Hawes RH, Romagnuolo J. Endosonographic features predictive of malignancy in mediastinal lymph nodes in patients with lung cancer. Gastrointest Endosc. 2010;72:265-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Xia Y, Kitano M, Kudo M, Imai H, Kamata K, Sakamoto H, Komaki T. Characterization of intra-abdominal lesions of undetermined origin by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2010;72:637-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Kanamori A, Hirooka Y, Itoh A, Hashimoto S, Kawashima H, Hara K, Uchida H, Goto J, Ohmiya N, Niwa Y. Usefulness of contrast-enhanced endoscopic ultrasonography in the differentiation between malignant and benign lymphadenopathy. Am J Gastroenterol. 2006;101:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Maekawa K. Hepatocellular carcinoma: depiction of tumor parenchymal flow with intermittent harmonic power Doppler US during the early arterial phase in dual-display mode. Radiology. 2001;220:349-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Park BK, Kim B, Kim SH, Ko K, Lee HM, Choi HY. Assessment of cystic renal masses based on Bosniak classification: comparison of CT and contrast-enhanced US. Eur J Radiol. 2007;61:310-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Kitano M, Kamata K, Imai H, Miyata T, Yasukawa S, Yanagisawa A, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for pancreatobiliary diseases. Dig Endosc. 2015;27 Suppl 1:60-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 20. | Shubik P. Vascularization of tumors: a review. J Cancer Res Clin Oncol. 1982;103:211-226. [PubMed] [Cited in This Article: ] |
| 21. | Gincul R, Palazzo M, Pujol B, Tubach F, Palazzo L, Lefort C, Fumex F, Lombard A, Ribeiro D, Fabre M, Hervieu V, Labadie M, Ponchon T, Napoléon B. Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: a prospective multicenter trial. Endoscopy. 2014;46:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Kim JH, Eun HW, Kim KW, Lee JY, Lee JM, Han JK, Choi BI. Intraductal papillary mucinous neoplasms with associated invasive carcinoma of the pancreas: imaging findings and diagnostic performance of MDCT for prediction of prognostic factors. AJR Am J Roentgenol. 2013;201:565-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Fusaroli P, Kypraios D, Mancino MG, Spada A, Benini MC, Bianchi M, Bocus P, De Angelis C, De Luca L, Fabbri C. Interobserver agreement in contrast harmonic endoscopic ultrasound. J Gastroenterol Hepatol. 2012;27:1063-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 26. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 188] [Cited by in F6Publishing: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Itokawa F, Itoi T, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Tanaka R. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Hocke M, Ignee A, Dietrich CF. Advanced endosonographic diagnostic tools for discrimination of focal chronic pancreatitis and pancreatic carcinoma--elastography, contrast enhanced high mechanical index (CEHMI) and low mechanical index (CELMI) endosonography in direct comparison. Z Gastroenterol. 2012;50:199-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Larsen MH, Fristrup CW, Mortensen MB. Intra- and interobserver agreement of endoscopic sonoelastography in the evaluation of lymph nodes. Ultraschall Med. 2011;32 Suppl 2:E45-E50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 32. | Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc. 2012;76:953-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Săftoiu A, Iordache SA, Gheonea DI, Popescu C, Maloş A, Gorunescu F, Ciurea T, Iordache A, Popescu GL, Manea CT. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc. 2010;72:739-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 36. | Xu W, Shi J, Zeng X, Li X, Xie WF, Guo J, Lin Y. EUS elastography for the differentiation of benign and malignant lymph nodes: a meta-analysis. Gastrointest Endosc. 2011;74:1001-109; quiz 1001-109;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Srinivasan R, Bhutani MS, Thosani N, Săftoiu A, Rice DC, Ioncică AM, Eapen GA, Gupta P, Jaganmohan S, Artifon EL. Clinical impact of EUS-FNA of mediastinal lymph nodes in patients with known or suspected lung cancer or mediastinal lymph nodes of unknown etiology. J Gastrointestin Liver Dis. 2012;21:145-152. [PubMed] [Cited in This Article: ] |
| 38. | Korenblit J, Anantharaman A, Loren DE, Kowalski TE, Siddiqui AA. The role of endoscopic ultrasound-guided fine needle aspiration (eus-fna) for the diagnosis of intra-abdominal lymphadenopathy of unknown origin. J Interv Gastroenterol. 2012;2:172-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629-34.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |