Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11522
Peer-review started: June 29, 2015
First decision: July 13, 2015
Revised: July 31, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: November 7, 2015
This review focuses on new findings about the inflammatory status involved in the development of human liver cirrhosis induced by the two main causes, hepatitis C virus (HCV) infection and chronic alcohol abuse, avoiding results obtained from animal models. When liver is faced to a persistent and/or intense local damage the maintained inflammatory response gives rise to a progressive replacement of normal hepatic tissue by non-functional fibrotic scar. The imbalance between tissue regeneration and fibrosis will determine the outcome toward health recovery or hepatic cirrhosis. In all cases progression toward liver cirrhosis is caused by a dysregulation of mechanisms that govern the balance between activation/homeostasis of the immune system. Detecting differences between the inflammatory status in HCV-induced vs alcohol-induced cirrhosis could be useful to identify specific targets for preventive and therapeutic intervention in each case. Thus, although survival of patients with alcoholic cirrhosis seems to be similar to that of patients with HCV-related cirrhosis (HCV-C), there are important differences in the altered cellular and molecular mechanisms implicated in the progression toward human liver cirrhosis. The predominant features of HCV-C are more related with those that allow viral evasion of the immune defenses, especially although not exclusively, inhibition of interferons secretion, natural killer cells activation and T cell-mediated cytotoxicity. On the contrary, the inflammatory status of alcohol-induced cirrhosis is determined by the combined effect of direct hepatotoxicity of ethanol metabolites and increases of the intestinal permeability, allowing bacteria and bacterial products translocation, into the portal circulation, mesenteric lymph nodes and peritoneal cavity. This phenomenon generates a stronger pro-inflammatory response compared with HCV-related cirrhosis. Hence, therapeutic intervention in HCV-related cirrhosis must be mainly focused to counteract HCV-immune system evasion, while in the case of alcohol-induced cirrhosis it must try to break the inflammatory loop established at the gut-mesenteric lymph nodes-peritoneal-systemic axis.
Core tip: This review focuses on new findings about the inflammatory status involved in the development of human liver cirrhosis, avoiding results obtained from animal models. Liver cirrhosis is induced by the two main causes, infection with hepatitis C virus and chronic alcohol abuse. Detecting differences in the inflammatory status between both cirrhosis etiologies could be useful to identify specific targets for preventive and therapeutic intervention in each case.
- Citation: Martínez-Esparza M, Tristán-Manzano M, Ruiz-Alcaraz AJ, García-Peñarrubia P. Inflammatory status in human hepatic cirrhosis. World J Gastroenterol 2015; 21(41): 11522-11541
- URL: https://www.wjgnet.com/1007-9327/full/v21/i41/11522.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i41.11522
Liver cirrhosis is an end stage hepatic disarrangement characterized by a progressive replacement of the functional hepatic architecture by non-functional fibrotic tissue. Alcoholic liver disease (ALD) and hepatitis C virus (HCV) infection are the two main etiologies of chronic liver diseases leading to cirrhosis and liver-related death in the Western world[1]. Nevertheless, hepatic cirrhosis can be also caused by hepatitis B virus (HBV) infection, hemochromatosis, Wilson´s disease, autoimmune hepatitis, nonalcoholic steatohepatitis associated to diabetes or dislipemy[2]. While the overall death rate caused by cirrhosis has fallen in the last thirty years[3], HCV death rates, closely associated with cirrhosis, have been increasing since the 1990s[4].
Survival of patients with alcoholic cirrhosis (ALC-C) seems to be similar to that of patients with HCV-related cirrhosis (HCV-C). However, it has been reported that the risk to develop ascites is higher in ALC-C, while progression to hepatocellular carcinoma is higher in HCV-C[5]. Most experimental data of ALD are obtained from animal models in stages of mild liver injury (moderate inflammation and steatosis), while severe alcoholic hepatitis (AH) in humans is developed in the phase of cirrhosis associated with severe hepatic failure. Furthermore, extrapolation from murine models to man is not always feasible. Hence, human translational studies are crucial for the development of new therapeutic strategies. It is well established that alcohol withdrawal and brief administration of corticosteroids are critical to ameliorate the survival of patients with AH and ALC-C[6]. However, almost 40% of people with the most severe forms of AH will not improve after that treatment[7].
Clearing HCV infection is difficult owing its high tendency to persist and induce chronic hepatitis C in approximately 75%-80% of infected individuals. For this reason, more research on the key factors required for a successful immune response against HCV is necessary to avoid progression toward hepatic cirrhosis. In this respect, it has been shown that the ability to spontaneously eradicate the virus, as well as the outcome of infection upon treatment with human recombinant interferon (IFN)-α correlate most closely with genetic variations within the region encoding the IFN-λ genes (reviewed by Mihm[8]).
Nevertheless, little is known as for the differences and/or similarities between alcohol- and HCV-induced liver disease at the cellular and molecular levels[9] and, to the best of our knowledge, data on the inflammatory status of ascitic human peritoneal cells are very scarce. Hence, understanding the differences in the immune response mechanisms implicated in the pathogenesis of alcohol- and HCV-induced liver disease will contribute to a better development of individually tailored preventive and therapeutic procedures.
Hepatic damage leading to cirrhosis is the result of a complex mechanism involving, from direct toxic effects to a sustained inflammatory process, driving to the death of hepatocytes and liver fibrosis, mediated by secretion of several cytokines. The inflammatory reaction is the coordinated process by which the liver responds to local insults, trying to restore the original structure and hepatic function. However, if the local damage is persistent and/or intense, the maintained inflammatory response gives rise to a gradual replacement of normal hepatic tissue by non-functional fibrotic scar. The imbalance between tissue regeneration and fibrosis will determine the outcome toward health recovery or hepatic cirrhosis. The involvement of the innate immune response in the pathogenesis of liver cirrhosis has been largely described[10]. Secretion of interleukin (IL)-1β and tumor necrosis factor (TNF)-α by hepatic macrophages, which are accumulated in the liver during chronic hepatic inflammation has been reported[11,12]. Increased hepatic and systemic injury is related with a high production of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-6, IL-17, as well as anti-inflammatory cytokines, IL-10 and transforming growth factor (TGF)-β[13]. Galectin (Gal)-3, a crucial component of the host response in tissue injury and acute inflammation, has also been implicated in the progression toward chronic inflammation after persistent insults. Although normal human hepatocytes do not express Gal-3, it is strongly expressed in the periphery of regenerating nodules in human hepatic cirrhosis produced by a wide range of etiologies[14-16].
Hepatic stellate cells (HSCs) have a fundamental role in liver immunology. These cells are the main storage place for dietary vitamin A, that is required for the suitable function of the immune system, represent a multifaceted source of many soluble immunological active mediators including cytokines and chemokines[17], may function as an antigen presenting cells (APC), and have autophagy capacity. HSCs are crucial sensors of altered liver tissue integrity and able to initiate the activation of cells of the innate immune system[18]. HSC is considered the main fibrogenic cell type in the liver. These cells produce several types of extracellular matrix proteins and, when activated, they lose their Vitamin A and lipids storage compartments and develop a myofibroblastic phenotype[18-20].
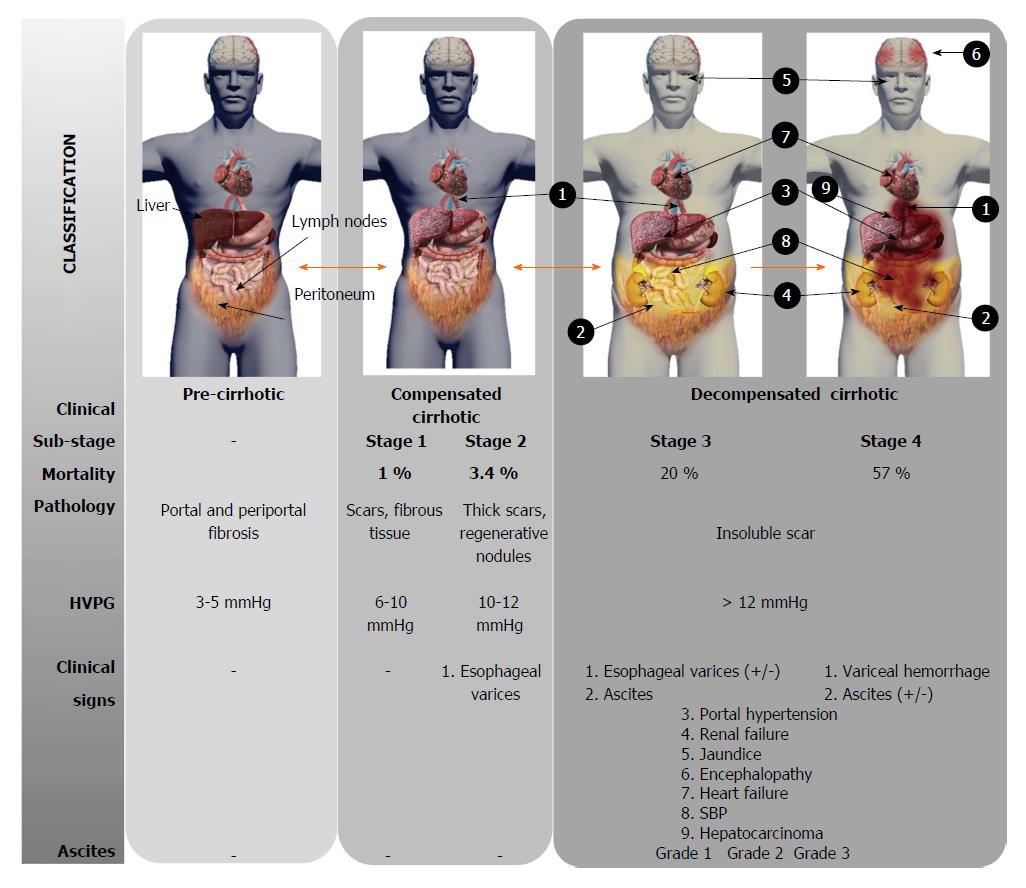
Local accumulation of fibrous tissue isolates normal hepatic lobules, hindering the contact and metabolic exchange between hepatocytes and blood sinusoids, disrupting the normal architecture of the liver, which is steadily replaced by abnormal nodules surrounded by fibrous tissue. This process results in a progressive hepatic loss of function, such as catabolism of toxins and drugs, metabolism regulation of carbohydrates, lipids and proteins, synthesis of multiple proteins and others crucial molecules[21]. Although liver cirrhosis conveys a progressive liver derangement leading to the patient’s death because limited therapeutic strategies are available, there is increasing evidence suggesting that hepatic fibrosis constitutes a potentially bidirectional dynamic process[22]. Several stages are distinguished according to the clinical progression of liver cirrhosis (Figure 1). Frequently, there is an asymptomatic or oligo-symptomatic stage of variable duration referred to as compensated liver cirrhosis followed by the decompensated liver cirrhosis stage, characterized by the presence of ascites, hepatic encephalopathy, esophageal varices and bleeding, jaundice, renal and cardiac failure and frequently, hepatocellular carcinoma development[23]. The Child-Turcotte-Pugh (CTP) and Model for End-Stage Liver Disease (MELD) scores are currently used to estimate the hepatic dysfunction and define prognosis, but they do not provide direct evidence of the stage or dynamic state of cirrhosis[24].
Liver damage in chronic infection by HCV is currently attributed to host’s immune-mediated mechanisms, since HCV itself does not cause a cytopathic effect. Multiple HCV-associated components interact with different elements of the host’s innate and adaptive immune system. In this respect, HCV genome RNA, HCV core and other non-structural proteins activate host innate immune response via different pathogen-associated pattern recognition receptors (PRRs) such as, Retinoic acid inducible gene-I receptor (RIG-I)[25], Toll-like receptors (TLRs)[26,27], Nucleotide-binding oligomerization domain-like receptor family (NLRP3)/caspase-1[28] and protein kinase R[29], expressed on hepatocytes, Kupffer cells, plasmacytoid dendritic cells and natural killer (NK) cells among others. This, triggers specific intracellular signaling pathways leading to secretion of type I and type III IFNs, pro-inflammatory cytokines, chemokines and other antiviral products aimed to interfere and eliminate the viral infection[30]. However, several HCV-components are able to disrupt and interfere with innate and adaptive cellular components impairing the immunoregulatory and cytotoxic activity of several innate and adaptive cell types, such as APCs, NK cells and CD4+ and CD8+ T lymphocytes, among others. HCV regulation of the cross-talk between hepatic resident and recruited cell subtypes seems to dictate the profile of IFNs as well as pro- and anti-inflammatory cytokines production, which will be critical to determine the outcome of natural and treated HCV-infections.
Myeloid mononuclear cells are integrated by circulating blood monocytes, tissue resident macrophages, referred to as Kupffer cells (KCs) in the liver and dendritic cells. These cell types link innate and adaptive immunity and are crucial in the development and maintenance of many inflammatory diseases. The role of KC in the clearance and persistence of HCV has been recently reviewed by Boltjes et al[31]. The number of KC in the liver is increased in chronic HCV infection[32,33], they display an activated phenotype with high mRNA expression levels of CD33 and CD163 receptors[34,35]. Albeit a direct effect of KC on replication of HCV is unknown, it has been described that the TNF-α produced by lipopolysaccharide (LPS)- and HCV-activated KC, enhances HCV infection in hepatoma cells[36]. Furthermore, IL-6, IL-1β, and IFN-β secreted by HCV- or TLR-ligand-stimulated KC pathways[28,37-39] inhibit HCV replication[40,41], suggesting that KC can also exert antiviral effect upon HCV exposure.
Nonetheless, viruses can interfere with the pro-inflammatory activity of macrophages/KC to avoid host defenses. In this respect, several studies have shown that HBV and HCV are able to neutralize the pro-inflammatory activities of immune cells and hepatocytes by interfering with TLR- and RIG-I-signaling[42-46]. However, studies on the effect on human KC are limited, mainly because the difficulty to distinguish between liver infiltrating macrophages and resident KC. Hence, several studies have shown altered TLR responses of blood monocytes obtained from chronic HCV-infected patients, and modulation of cytokine synthesis by HCV proteins[47-49]. Meanwhile, one study described the suppressed effect on the synthesis of type I IFN and on the expression of TRAIL in human isolated KC by HCV core protein through disruption of the toll-like receptor 3 (TLR3)/TIR-domain-containing adapter-inducing interferon-β (TRIF)/TANK binding kinase 1 (TBK1)/Interferon regulatory factor 3 (IRF3) pathway[37]. In turn, chronic exposure of APCs to the core antigen induced hyporesponse to TLR ligands through activation of TLR2, which correlated with liver dysfunction as determined by platelet numbers and prothrombin time levels. Furthermore, stimulation with TLR ligands resulted in decreased synthesis of IL-6 by peripheral blood monocytes from HCV- but not from HBV-infected patients[50].
It has also been described that chronic hepatitis C patients have high levels of serum IL-1β in comparison to healthy controls. Several lines of evidence indicate that secretion of IL-1β by resident hepatic macrophages produces liver inflammation through HCV-induced NLRP3/caspase-1 inflammasome signaling. RNA sequencing analysis from the liver of patients with chronic hepatitis C demonstrated that viral engagement of the NLRP3 inflammasome stimulated IL-1β production leading to pro-inflammatory cytokine, chemokine, and immunoregulatory gene expression networks linked with clinical deterioration[28].
Relating the role of NK cells in the defense against HCV infection, it is assumed that a strong and rapid NK cell response at early stages of the infection induces strong T cell responses leading likely to HCV clearance. In contrast, chronic HCV infection tends to be related with impaired NK cell activity and phenotypes (reviewed by Ahlenstiel[51]) biased towards impaired ability to produce IFN-γ, elevated expression of NKp46-activating receptor, reduced TRAIL and CD107a expression and impaired degranulation compared with controls[52-54]. Hence, persistence of HCV infection is favored by the impaired secretion of IFN-γ, which is required to induce a strong T helper 1 (Th1) mediated cytotoxic response. According with this, Li et al[55] compared the immune state and correlations between cirrhosis produced by HBV and HCV. The levels of IL-6 (Th2 cytokine) in both groups of cirrhotic patients were increased, while the IFN-γ (Th1 cytokine) was only increased in HBV-related cirrhotic patients. Thus, differences were not detected between these cirrhotic groups except in the IFN-γ level.
Furthermore, it has been described that certain chronically activated NKp46high subsets may be especially active against HSC, a crucial player in liver fibrogenesis. The relevance of NK cells in the resolution of HCV infection can be also exemplified by the effect of genetic polymorphisms of killing inhibitory receptors and their Human leukocyte antigen (HLA) ligands on the individual outcome of HCV infection[56]. Many possible mechanisms, by which HCV may impair NK cell cytotoxic and immunoregulatory functions have been described. Thus, Sène et al[57] reported that expression of the Natural killer group 2D receptor (NKG2D) activating receptor on circulating NK cells is down-regulated in chronic HCV infection, although its ligands, MHC class I-related chain (MIC) molecules, are expressed on hepatocytes. This impairs NK cell-mediated cytotoxicity and secretion of IFN-γ. Moreover, stimulation of monocytes with HCV recombinant NS5A protein through TLR4 promoted p38- and phosphatidylinositide 3-kinase (PI3K)-dependent IL-10 secretion, while inhibited the production of IL-12. In turn, IL-10 triggered secretion of TGF-β which down-regulated the expression NKG2D on NK cells, impairing their effector activity. Notably, this effect could be impeded by exogenous IL-15, which could be assayed as adjuvant of current treatments.
Moreover, the up-regulation of KLRG1, an inhibitory transmembrane protein expressed on peripheral blood CD56+ NK cells and antigen-experienced T cells in patients with chronic HCV infection, negatively regulated NK cell numbers and functions through the Akt pathway. Blockage of KLRG1 signaling significantly restored the impaired IFN-γ secretion by NK cells from HCV-infected patients[58].
Besides NK cells, HCV-specific CD8+ cytotoxic T lymphocytes kill HCV-infected cells via the perforin/granzyme lytic pathway, but also by secretion of Fas ligand (CD178 or CD95L) and inflammatory cytokines, mainly IFN-γ. In this respect, impaired T-cell responses in chronic HCV-infected patients have been related with HCV persistent infection. Thus, HCV-specific T-cells become unresponsive and apparently disappear in chronic HCV-infections through several possible mechanisms, such as mutations in critical viral epitopes, insufficient help, expansion of regulatory T-cells (Treg) or clonal anergy.
Tacke et al[59] reported that HCV induces the accumulation of CD33+ myeloid-derived suppressor cells (MDSC) in human peripheral blood mononuclear cells (PBMC), resulting in reactive oxygen species (ROS)-mediated suppression of T-cell responsiveness. Hence, the accumulation of MDSCs during HCV infection may reinforce and maintain HCV persistent infection[59]. Furthermore, patients with microscopic signs of inflammation displayed a significant higher CD8+ T-cell response to five HCV-derived epitopes from core, NS3A, NS3B, NS4B, and NS5B antigens and also a higher CD4+ Th1 response to the HCV core antigen than individuals with signs of fibrosis/cirrhosis. These results indicate that the insufficient T-cell response to HCV is associated with the progression of cirrhosis during chronic HCV infection[60].
Expansion of CD4+ FoxP3+ Treg in chronic HCV-infected liver has been reported[61]. In this respect, the Tim-3 pathway seems to control the effector/regulatory T cell balance by modifying apoptosis and cell proliferation during HCV infection[62]. In turn, Ji et al[63] showed that HCV-infected human hepatocytes express higher levels of TGF-β and Gal-9, and upregulate the expression of both, Tim-3 and regulatory cytokines TGF-β/IL-10 in co-cultured human CD4+ T cells, which evolved into CD25+ FoxP3+ Treg cells. Notably, blockade of Tim-3/Gal-9 ligations abrogated HCV-mediated Treg-cell promotion by HCV-infected hepatocytes, suggesting that interactions of Tim-3/Gal-9 may regulate the development and function of human FoxP3+ Treg cells during HCV infection[63]. More recently, it has been reported that CD4+ T cells expressing HCV-core protein upregulate FoxP3 and IL-10, and suppress CD8+ and CD4+ T cell populations[64].
The role of neutralizing antibodies on the pathogenesis of HCV infections remains unclear. However it is likely that humoral immunity may contribute to the lysis of HCV-infected liver cells via antibody-dependent cellular cytotoxicity.
MicroRNAs (miRNAs) are endogenous small noncoding RNAs that regulate gene expression by binding to mRNAs to interfere with the process of translation[65]. Several miRNAs are related to different liver diseases depending on the etiologies[66], being miR-122 involved in the HCV replication process. Expression of miR- 16, 193b, 199a, 222, 324 in PBMCs[67] and serum levels of miR-122 and miR-155 are elevated in chronic HCV infection and correlate as inflammatory markers[68].
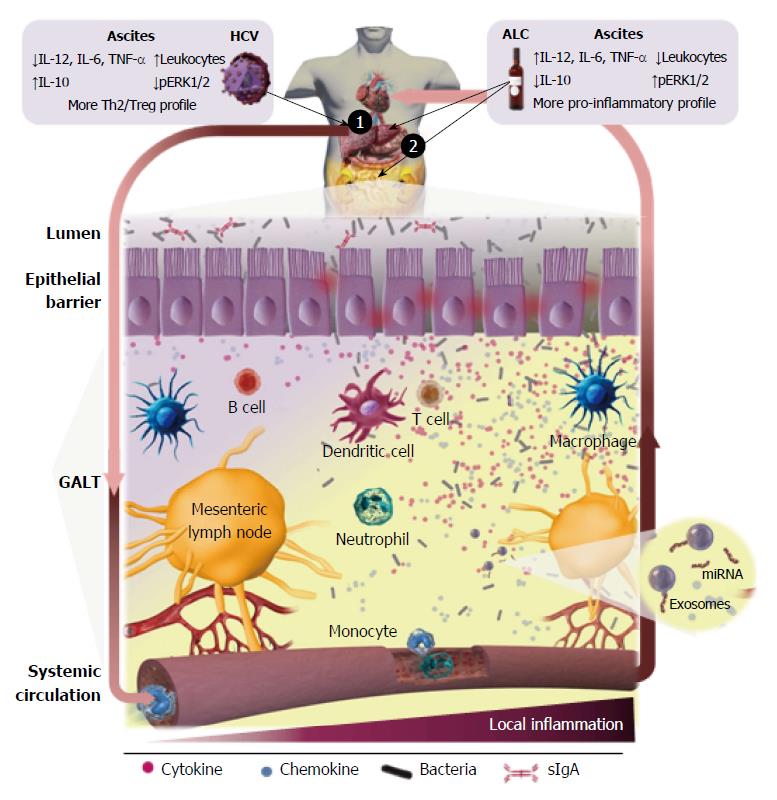
ALD is the result of the combined effect of direct hepatotoxicity of ethanol, which is metabolized into acetaldehyde on hepatocytes[69,70] and increases of the intestinal permeability[71], allowing bacteria and gut-derived bacterial products, mainly endotoxin, translocation in the portal circulation (Figure 2). The microbiota composition is altered and correlates with endotoxemia especially in certain subgroup of alcoholics[72,73]. Endotoxin can be frequently detected in serum and ascites of alcoholic patients. Thus, it is widely accepted that gut-derived endotoxins have a prominent role in the induction of liver damage and aggravation of ALD[74]. Indeed, bacterial decontamination and LPS neutralization improves ALD (reviewed by Szabo[75]). The influence of TLR signaling in experimental and human ALD has been analyzed and recently reviewed by Ceccarelli et al[76]. Although human clinical evidence of the role of TLR in the liver’s inflammatory response in ALD is low, it seems to be in agreement with the experimental data reported in murine models. Stärkel et al[77] reported that liver chronic activation of nuclear factor κ-light-chain-enhancer of activated B cells (NFκB), increased expression of TLR3 and TLR7 and elevated secretion of pro-inflammatory cytokines are associated with human end-stage ALD. Related to this, up-regulation of the MyD88-dependent TLR4/NFκB pathway in AH and non-alcoholic steatohepatitis (NASH) in liver biopsies, where Mallory-Denk bodies (MDBs) formed, has been recently described via the NFκB-CXCR (C-X-C chemokine receptor) 4/7, providing further insights into the mechanism of MDB formation in human liver diseases[78].
Chronic exposure to bacteria and their pathogen associated molecular patterns (PAMP) activates the inflammatory immune response and produces a dysregulation of the homeostatic mechanisms that control the innate and adaptive immune response, leading to AH, cirrhosis and hepatocellular carcinoma. Acute-on-chronic hepatic failure is characterized by acute worsening of cirrhosis, disturbances in systemic and hepatic hemodynamics, marked activation of the sympathetic nervous system and multi-organ failure, all of them associated with dysregulated inflammation. Hence, inflammation drives the progression of ALD from reversible to advanced stages. These alterations predict high death rates in alcohol-related acute-on-chronic liver failure patients[79]. Among the intracellular mechanisms trying to counterbalance a dysregulated inflammatory process have emerged autophagy, an important regulatory mechanism of liver homeostasis under physiological and pathological conditions. Thus, there is increasing evidence that complex pathways of autophagy are involved in liver fibrosis, with profibrogenic activity based on its direct activation of HSC, but also with antifibrogenic effects by its indirect hepatoprotective and anti-inflammatory properties (reviewed by Mallat et al[80] and Ding[81]). Recently, up-regulation of metacaspase1 and chaperones related to protein quality control in liver biopsies from alcoholic patients has been reported, indicating that autophagy is active in human alcoholic steatohepatitis[82].
Nowadays, there is accumulating evidence of an abnormal frequency and/or function of every single component of the human immune system implicated in advanced stages of ALD. In fact the majority of studies on the systemic inflammatory status on human cirrhosis have been performed in ALC-C.
There is increasing evidence of alcohol-induced dysfunction of peripheral blood and resident myeloid mononuclear cells. Thus, it has been described that high circulating endotoxin levels induced by alcohol abuse leads to activation of KC, which causes a hypoxia-reoxygenation injury process[83]. Also, the function of macrophage Fc gamma receptors is altered in patients with ALC-C, and this impairment likely contributes to the high frequency of bacterial infections in such patients[84].
Dysregulation of the M1 (classical)/M2 (alternative) macrophage polarized balance becomes apparent as a central mechanism implicated in the pathogenesis of chronic inflammatory diseases. This suggests that strategies impairing M1 macrophage phenotype and/or enhancing the M2 macrophage polarization could protect against intensified inflammation and in this way they could limit tissue injury. Relating subpopulation of macrophages involved in AH, it has been reported a complex interplay between different types of macrophages M1, M2a, M2b, and M2c in liver biopsies expressing a diverse array of molecules and receptors[85], as well as a new M1/M2 balance regulatory mechanism, that relies on the apoptotic activity of M2 KCs towards their M1 counterparts. These data suggest that inducing M1 KC apoptosis mediated by M2 might be a pertinent strategy to control alcohol-induced inflammation and hepatocyte injury[86]. Notably, it has been recently described the regulatory activity of overexpressed miR-27a on activation and polarization of monocytes induced by alcohol. Alcohol binge drinking in healthy people results in higher frequency of CD16+ and CD68+ and M2-type (CD206+, DC-SIGN+-expressing and IL-10-secreting) CD14+ blood monocytes. Overexpression of miR-27a in monocytes increased the secretion of IL-10 via activation of the ERK signaling pathway by decreasing the expression of ERK inhibitor sprouty2[87].
Furthermore, it has been also described a defect of TLR2 but not TLR4-mediated innate immune response in monocytes from the blood of individuals with compensated chronic ALD[88]. Acute alcohol challenge of monocytes strongly inhibited the NFκB (involved in TLR signaling) activation through mediators of early (LPS) or late (IL-1, TNF-α) stages of inflammation[89].
Recruitment and activation of neutrophils constitutes the first line of cell defense against microbial insults, and is highly mediated by local secretion of IL-8 and IL-17, among others. Circulating neutrophils in both, alcoholic with severe bacterial infections[90] and patients with cirrhosis, are often very low, displaying lower phagocytic activity/capacity, which predicts the development of infection, organ dysfunction and increased mortality[91]. Defective neutrophil function in ALC-C is caused by decreased generation of superoxide anion and defects of degranulation. Neutrophils from patients with cirrhosis and acute AH have decreased phagocytosis in the early stages of bacterial challenge, although their capacity for ingestion and killing of bacteria is greater than neutrophils from individuals with cirrhosis alone[92]. Furthermore, a greater resting burst, revealing neutrophil activation, and a lower phagocytic capacity was associated with significant higher risk of infection, organ failure, and mortality[93]. Neutrophil phagocytic dysfunction in stable cirrhosis was related with increased expression of TLR2 and 4 in inflamed peripheral tissues and excessive production of inflammatory mediators[94]. Related to this, it has been shown that plasma from AH patients induced an increment of the oxidative burst, while it decreased the expression of CXCR1+2, and the phagocytic capacity of neutrophils obtained from healthy donors, concomitantly with a higher expression of TLR2, 4, and 9 and depletion of ATP. Moreover, the presence of the endogenous endotoxin scavenger albumin, prevented the detrimental effect of patients’ plasma on neutrophil TLR expression, phagocytosis and resting burst[95], which attributes an important role to the presence of circulating LPS and others bacterial products.
Although alcohol per se produced an increase in the number and cytotoxic capacity of NK-cells[96], their lytic ability is depressed in the stage of alcoholic cirrhosis, supporting the hypothesis that efficiency of immunosurveillance may be depressed in these patients[97,98]. On the other hand, alcohol promotes collagen accumulation, which has been correlated with inhibition of anti-fibrotic activity of NK cells[99]. Furthermore, NK-derived IFN-γ induces HSC cell cycle arrest and apoptosis[100].
To date little is known about the mechanisms by which adaptive immunity might contribute to hepatic inflammation in ALD. Indeed, activation of the adaptive arm of the immune system may be the consequence of an ongoing alcohol-activated innate immune response. Furthermore, cell damage induced by toxic ethanol metabolites and ROS may produce the breaking of the self-tolerance toward liver components[101]. Regarding the alcohol intake duration, the most relevant findings in peripheral blood were a significant activation of the T-cell population, and specifically of the TCRαβ+ subpopulation, with a higher expression of both HLA-DR and CD11c markers, as well as a significant increment of both, NK cells (CD3-/CD56+) and cytotoxic T cells CD56+. In addition, a decrease in the total number of B cells and their CD5+/CD19+ subset was found[96].
Patients with ALD had decreased number of lymphocytes, but only individuals with advanced fibrosis had a significant increase in the CD4+/CD8+ ratio[102]. Alcoholic patients also display CD57+ T cells expansion, which express significantly higher amounts of cytoplasmic TNF-α and IFN-γ after 6 h of TCR-stimulation than the CD57- counterparts[103]. Defective in vitro proliferative activity of PBMC and impaired or normal production of IL-2, together with an increment of lymphoid cells bearing T activation markers and rIL-2 responsiveness was early reported from ALC-C patients[104,105].
It has been recently described that ALD patients lymphocytes express high levels of the immune inhibitory receptors, PD1 and Tim-3 and their respective ligands, CD274 and Gal-9, which modulate the balance between protective immunity and host immune-mediated damage. These lymphoid cells produce lower levels of IFN-γ and higher IL-10 by chronic endotoxin exposure. Furthermore, these effects can be reversed by blocking PD1 and Tim-3, increasing the antimicrobial activity of T cells and neutrophils[106].
It has been described a sharp reduction in the frequency and absolute number of alcoholics’ CD5+ B lymphocytes as well as a decrease in the percentage of CD5- CD45RAhigh B cells, leaving many patients with a B cell population that was mostly CD19+ CD5- CD45RAlow. This subpopulation is phenotypically analogous to the described IgM-producing CD5- CD45RAlow subset, reported by others, and may be enriched for autoantibody-producing cells[107].
Patients with ALD have antibodies against alcohol dehydrogenase (ADH), as well as ADH-specific T-cell responses, which have been associated with alcohol intake in ALC-C patients. Anti-ADH titers are also associated with disease severity and active alcohol consumption. ADH peptides promoted the secretion of IFN-γ, IL-4, and IL-17 from PBMCs of patients with ALC-C. IL-4 secretion was lower in active drinking vs abstinence, while IL-17 production was higher. The intensity of the predominant Th1 responses correlated with disease severity[108].
A prospective study reported an association between the presence of antibodies against alcohol-modified hepatocytes and an augmented risk of developing ALC-C[109]. Furthermore, alcohol abusers with lipid-peroxidation derived antibodies have a five-fold higher presence of increased levels of TNF-α in plasma than heavy drinkers with these antibodies within the normal range[110]. Furthermore, the association of high TNF-α and lipid-peroxidation-induced antibodies raises by 11-fold the risk of progressing toward advanced ALD. Of note, the combination of steatosis and elevated titers of antibodies against lipid peroxidation-derived products is an independent predictor of severe fibrosis/cirrhosis in alcohol-drinking people with chronic hepatitis C[111]. B cells from ALC-C patients produced spontaneously more IgA than healthy controls. Moreover, the production of IgA by oligodeoxynucleotides with CpG motifs-activated B lymphocytes was significantly increased compared to controls. These results strongly suggest that TLR priming of B cells could account in part, for the hyperimmunoglobulinemia detected in ALC-C patients[112], although it is also established that hyperimmunoglobulinemia in chronic hepatic diseases is mostly caused by the collateral circulation secondary to portal hypertension, antigens and endotoxins from gut that bypass the liver and contact with the antibody-producing cells[113].
Alcohol intake activates the innate immune system and induces several pro-inflammatory cytokines such as IL-1β and TNF-α, inducing hepatocellular damage. Although it is well established that mediators of the inflammatory process participate in ALD pathogenesis, the exact contribution of inflammasomes in ALD is still unclear[100]. In this respect, blocking IL-1β activity with recombinant IL-1RA (anakinra) ameliorated alcohol-induced hepatic inflammation and damage. IL-1β gene polymorphisms have been correlated with AH[114]. Furthermore, high expression of IL-18 has been associated with advanced ALD[115]. Besides PAMP, inflammation is also elicited by damage associated molecular patterns (DAMP). It has been recently described that endogenous signals of metabolic danger, such as uric acid and ATP, are implicated in inflammatory cross-talk between hepatocytes and immune cells and have a prominent participation in alcohol-induced liver inflammation[116]. Activation of inflammasomes in liver biopsies from AH patients has been associated with MDBs formation, suggesting that MDB could be indicative of the extent of inflammasome activation[117]. More studies are needed to elucidate the exact alcohol-responsive cells in vivo and to clarify the role of inflammasome components associated with ALD.
Human ALD is also associated with the activation of the IL-17 pathway. In AH, liver infiltration with IL-17-secreting cells is a crucial feature that might contribute to liver neutrophil recruitment and it correlates to MELD score in ALD. PBMC of ALD patients produce higher levels of IL-17, and their CD4+ T cells are highly Th17. The IL-17 receptor is expressed by HSCs, which recruited neutrophils after IL-17 stimulation in a dose-dependent manner through IL-8 and Gro-α secretion in vitro[118]. Activation of the innate immune system results in elevation of hepatoprotective mediators such as IL-6[119], and anti-inflammatory cytokines, such as IL-10, which play a relevant role in meliorating alcoholic liver damage and inflammation[120]. However, chronic alcohol intake moderates the signaling pathways triggered by those cytokines, thereby decreasing their anti-inflammatory and hepatoprotective activities, and contributing to progression of ALD[121,122]. Thus, while compensated ALC-C is characterized by serum increases of IL-6 and decreases of IL-10, patients with decompensated ALC-C, besides increased IL-6, have also increased concentrations of TNF-α and IL-8. In turn, TGF-β1 and IL-10 levels were alike to those found in healthy controls. Hence, significant alteration of the balance between pro-inflammatory and anti-inflammatory mediators is characteristic of compensated and especially of decompensated ALC-C[123]. Recently, fibroblast growth factor-inducible 14, a molecule included in the TNF receptor superfamily, was described to be over-expressed in the liver, especially by hepatic progenitors, in patients with AH and it correlated with aggravated stages[124].
Serum IL-12 levels, an important cytokine driving Th1 cell-mediated responses, were higher in AH, ALC-C and alcoholic steatosis patients than in a control group. IL-12 presented good sensitivity and specificity in the diagnosis of ALD[125].
Increased serum levels of activin-A (a component of the TGF superfamily, involved in hepatic fibrosis, regeneration and stem cell differentiation) only in patients with ALC-C or hepatocellular carcinoma suggest its potential role in the pathophysiology of ALD[126].
Up-regulation of the pro- and anti-inflammatory cytokine system and simultaneous desensitization of effector cells in patients with ALC-C, could explain the attenuated systemic inflammatory response to chronic endotoxemia. This dysregulation of the immune state may produce the failure of the host defenses against infections, which are frequent complications of ALC-C[127].
A recent work has suggested that alcohol drinking increases the activity of the complement system in the liver, contributing to the inflammation-associated pathogenesis of AH[128].
The main neutrophil’s chemoattractant, IL-8 is activated in ALD, especially in AH, and it correlated with liver damage. The levels of IL-8 can reflect the stage and severity of ALD, and may predict the survival of patients with AH[129,130]. Liver samples from AH show up-regulated expression of the CXC subfamily members IL-8, Gro-α, CXCL5, CXCL6, CXCL10, and platelet factor 4 and correlate with neutrophils infiltration. The expression of CC chemokine CCL2 (C-C ligand-2), but not CCL5, is also increased. Higher expression of IL-8, CXCL5, Gro-γ, and CXCL6 levels are related with worse prognosis[131]. On the other hand, CXCL5 levels are lower in the plasma of people with chronic liver disease, which suggests that CXCL5 might be implicated in its pathogenesis[132,133]. The CXCL9 (ligand of CXCR3) has also effects on the liver, in fact it is released by the liver and might contribute to hepatic and extrahepatic organ malfunction. High levels of CXCL9 are associated with higher mortality in cirrhotic patients with severe portal hypertension receiving transjugular intrahepatic portosystemic shunt[134]. A recent study has described that elevated serum levels of CXCL1, mostly expressed by mononuclear cells activated by LPS, together with a polymorphism in the gene encoding for this molecule are risk factors for ALC-C[135].
CCL20 is a strong chemotactic factor for lymphocytes that binds to the chemokine receptor CCR6. Hepatic and serum levels of CCL20 are elevated in people with AH and correlated with the grade of fibrosis, endotoxemia, portal hypertension, disease severity scores and short term mortality[136].
Several members of the miRNAs family are affected by the presence of alcohol, producing an abnormal miRNA profile in the liver and peripheral blood in ALD. The functions and advances on the effect of circulating miRNAs in human alcoholic diseases, mainly focusing on inflammation and cell survival after ethanol/LPS treatment, have been reviewed by McDaniel et al[137], Szabo[75] and Gao and Bataller[138].
Systemic blood, portal and hepatic levels of Gal-3 are increased in patients with ALC-C and it has been described to be negatively associated with liver function[139].
A role for osteopontin in AH has been described. Hepatic expression and serum levels of osteopontin are highly elevated in AH compared to normal livers and other classes of chronic liver diseases, and correlated with short-term survival[140].
A comprehensive transcriptional study using oligonucleotide microarrays on hepatic biopsies from cirrhotic patients evidenced that there are genes differentially expressed between ALC-C and HCV-C. Various of the gene expression changes detected in the HCV-induced cirrhotic livers were associated with the activation of the innate antiviral immune response, while differences between chronic liver injury due to HCV or ethanol seem to be more associated with the regulation of lipid metabolism and deposition of extracellular matrix components concomitantly with others related with macrophage activation[1,3,4,9,141]. When severity, measured by CTP classification, of cirrhosis from each etiology was compared, a different gene expression pattern was identified in clinical stages of ALC-C[9], but not in HCV-induced disease. Thus, CTP class A ALC-C livers displayed unique expression patterns for genes involved in the inflammatory response, including those acting on macrophage activation and migration, as well as oxidative stress and lipid metabolism. This is in agreement with the above mentioned studies demonstrating that the exposition to alcohol activates the innate immune response and induces several pro-inflammatory, hepatoprotective and anti-inflammatory cytokines[142]. However, chronic alcohol intake diminishes the intracellular signaling cascades induced by several cytokines, decreasing their anti-inflammatory and hepatoprotective actions, and then, contributing to the development of ALD[122].
Transcriptome studies of human liver samples has revealed that the expression of several TNF superfamily receptors is up-regulated in the liver of patients with AH compared with healthy individuals or patients with other hepatic diseases. Among them, Fn14, was the only TNF superfamily molecule exclusively up-regulated in AH compared with other hepatic diseases and correlated with both short-term mortality and portal hypertension severity[124].
Advanced oxidation protein products (AOPP) are oxidative stress markers with a pro-inflammatory activity that accumulate in hepatic cirrhosis. AOPP level positively correlated with the Child-Pugh score in ALC-C but not in HCV-C, and the correlation with the markers of chronic inflammation, more specifically TNF-α, was stronger in ALC-C. This suggested that oxidative stress may be a mediator of chronic inflammatory status at the initial stage of ALC-C. In turn, AOPP in HCV-C was less related with the inflammation, although a significant correlation with antioxidant defenses could be detected[143].
The size of the spleen, recorded by sonography and computed tomography, also varies by the etiology of the cirrhosis, being in the alcohol group significantly smaller than in the hepatitis C and NASH groups[144].
Ascites is the most common complication of liver cirrhosis. Thus, 60% of patients with compensated cirrhosis develop ascites within 10 years during the course of their disease[145]. As liver fibrosis progresses the presence of regenerative nodules and bioactive molecules increases, driving to portal hypertension. Ascites only appears when portal hypertension and, specifically sinusoidal hypertension, has developed producing arterial splanchnic vasodilatation. This causes an increment of capillary pressure and permeability, and a decreased effective volume of arterial blood. The increase of plasma volume and cardiac output are adaptable mechanisms for this reduction. Activation of both the sympathetic nervous system and renin-angiotensin-aldosterone system induce a compensatory retention of sodium and water, thereby leading to the accumulation of ascites[146]. The ascites is classified on the basis of quantitative criterion and determines the clinical treatment: grade 1 corresponds to mild, only detectable by ultrasound and is not treated, grade 2 corresponds to moderate ascites, evident by slight symmetrical abdominal distension, which is treated by reduction of sodium intake and diuretics, and grade 3 is a large ascites volume with manifest abdominal distension, which is treated by large-volume paracentesis, followed by limitation of sodium intake and diuretics (Figure 1). The presence of ascites in cirrhotic patients usually goes parallel with worsening of the clinical state and predicts a poor prognosis, thus many patients are referred for liver transplantation after development of ascites[147]. Bacterial infections are frequent complications appearing in patients with cirrhosis and ascites, being spontaneous bacterial peritonitis (SBP) the most habitual and clinically relevant[148].
Commensal microbiota has defined lines of communication in peaceful coexistence with the host, without induction of pro-inflammatory responses in healthy conditions. Bacterial translocation (BT) defined as displacement of bacteria and/or bacterial components from the gut to the mesenteric lymph nodes (MLNs) (Figure 2) is a crucial physiological process for the host´s immunity training. In contrast, throughout the course of liver cirrhosis there is a ‘‘pathological increase of BT’’[149] due to changes and overgrowth of intestinal microbiota[150], an increase of intestinal permeability and the dysregulation of the gut associated lymphoid tissue (GALT) immune response (revised by Wiest et al[151]). Thus, bacterial products and/or alive bacteria of intestinal origin come across the intestinal wall[152], reaching the MLN and ascitic fluid from where they can be carried by the lymph toward the blood circulatory system, and finally disseminated toward other organs. The dysregulation and deterioration of the immune system mechanisms paralleled the disease progression[10,151].
Significant increases of LPS and LBP have been detected in cirrhotic patients[153]. Bacterial DNA may be also present by BT in ascites of as many as one-third of cirrhotic patients with non-neutrocytic and culture-negative ascitic fluid[154] and it could be also detected in peripheral blood[155]. On the other hand, and in contrast to that reported in hospitalized patients (30%-40%), bacterial DNA is rarely detected in serum and ascitic fluid of cirrhotic outpatients with non-neutrocytic ascites[156]. Bacterial peptides (glyceraldehyde-3-phosphate dehydrogenase A, Porin OmpC and HSP60) have been also detected in ascitic fluid from a group of patients with advanced cirrhosis and culture-negative ascites[157].
In stages of cirrhosis without increased permeation of viable bacteria, the systemic inflammation developed in response to the HCV infection or alcohol abuse described above, induces small but continuous increases in translocation of bacterial products, which trigger an increased pro-inflammatory cytokine response and release of ROS within the GALT in order to improve the anti-bacterial defenses.
The intestinal inflammation in cirrhotic patients could be suggested by the elevated fecal polymorphonuclear leukocytes elastase concentrations[158]. Patients with advanced hepatic cirrhosis, and particularly those with ascites, had augmented local production of TNF-α in MLNs, whereas the levels of IL-6 were not different between cirrhotic and controls. This could be attributed to BT and might contribute to systemic alterations of cirrhosis[159].
The translocation of bacterial DNA triggers an increased pro-inflammatory cytokine response with high levels of TNF-α, IL-2, IL-6, IL-12, increased expression of the complement system proteins C3b, membrane attack complex and C5a[160-162], and release of ROS and nitric oxide (NO) through the inducible form of NO synthase[155]. The translocation of LPS induces the production of TGF-β, IL-6, IL-1β and hyaluronan by peritoneal cells in ascites[163]. The presence of bacterial peptides in ascitic fluid of patients with advanced cirrhosis and culture-negative ascites, is associated with elevated levels of C3b and membrane attack complex proteins and a marked inflammatory response with higher levels of TNF-α and IFN-γ[157].
The peritoneal macrophages from individuals with decompensated cirrhosis and culture-negative ascites, present a predominant activated state[158,164,165]. These cells show a pre-activated immune status at baseline, with high CD54, CD86 and HLA-DR surface marker expression levels, increased phosphorylated levels of ERK1/2, PKB and c-Jun intracellular signaling molecules, and enhanced secretion of IL-6. This fact probably reveals that consecutive episodes of BT boost a sustained immune response in these patients, even in the momentary absence of bacterial products. This primed status would favor an IL-6-controlled rapid response against repeated BT episodes[164]. In vitro studies performed with peritoneal macrophages obtained from ascites of cirrhotic patients, have demonstrated that secretion of pro-inflammatory cytokines IL-1β, TNF-α and IL-6 from this clinical situation strongly depends on the MAPK signaling pathways, while the PI3K-Akt (also known as Protein kinase B) route plays a relevant role in the regulation of the anti-inflammatory function mediated by IL-10[166,167]. The inhibitors of mitogen-activated protein kinase kinase (MEK1) and c-Jun N-terminal kinases (JNK) reduced the secretion of IL-1β, IL-6 and TNF-α and could serve as therapeutic agents for pharmaceutical intervention to decrease hepatic damage, reducing the inflammatory response associated to liver failure. On the contrary, PI3K-Akt inhibitors suppressed the production of IL-10, and increased the release of IL-1β, mostly by enhancing the release of intracellular IL-1β and caspase-1 toward the extracellular medium. Therefore, the inhibitors of PI3K-Akt are discarded as potential therapeutic agents in hepatic fibrosis, as these drugs would promote the inflammatory response. Macrophages from non-infected ascitic fluids showed constitutive activation of caspase-1 and a notable increase in the expression of IL-18, IL-1β, and AIM2 compared to blood macrophages. The activation of the inflammasome in vitro did not require a priming signal, supporting the pre-activated state of these cells[167,168].
As described above, hepatic cirrhosis patients present an altered B lymphocyte function and increased systemic immunoglobulin levels. However, in spite that the serum[113] and salivary[150] levels of secretory IgA (sIgA) are elevated in these patients, the concentrations of fecal sIgA as well as the secretion of mucosal sIgA into the jejunum are decreased[150,158].
Exosomes are small membrane vesicles released from many cell types which contain functional proteins, mRNAs and miRNAs. Exosomes can be engulfed by other cells modulating their functions and playing an important role in the control of immune responses[169]. Exosomes purified from ascites of hepatic cirrhosis patients, have proved to induce the production of IL-1β, IL-6 and TNF-α through NFκB/STAT3 activation in a TLR-dependent way, after internalization by monocytic cells in vitro[170]. Furthermore, increased levels of free miR-212 have been detected in the gut after alcohol consumption, resulting in the inhibition of the expression of the tight junction (TJ) protein ZO-1, increasing the gut permeability[171].
The fibrinolytic activity of plasma from patients with decompensated cirrhosis and ascites is higher than that of patients without ascites. In turn, the ascites from such patients has even higher fibrinolytic activity than their plasma, as suggested by: the lower levels of plasminogen and fibrinogen, the elevated fragment D-dimer and fibrin split levels, and the short euglobulin lysis time (fibrinolytic activity) found in the ascitic fluid. Since ascites fluid re-enters the systemic circulation via the thoracic duct or/and via a natural peritoneovenous shunt, ascites can be considered as a pathological fluid that contributes to the systemic fibrinolytic state found in most patients with ascites[172].
The peritoneal immune response differs from the systemic one in non-infected cirrhotic patients with ascites, and could potentially determine the likelihood for future adverse events. Hence, subjects with advanced liver cirrhosis and ascites showed significantly greater levels of IL-2, IL-4, IL-6, IL-8, IL-10, monocyte chemotactic protein (MCP)-1, TNF-α, vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) in the ascitic fluid compared to serum, while the levels of IL-1α, IL-β and IFN-γ were not statistically different[173].
The local inflammation caused by BT in this cirrhotic stage further loosens intestinal TJ-function augmenting intestinal permeability[174] and perpetuating BT. Alterations in TJ-proteins have been demonstrated in duodenal biopsies with diminished expression of occludin and claudin-1 that progressively increase from crypt to tip of the villi[175,176]. Therefore, under cirrhotic situations, loosening of TJs may allow an increased accessibility of viable bacteria and bacterial components to areas of free passage. As cirrhosis advances, the gut evolved into a major source of activated immune cells and pro-inflammatory cytokines, inducing and maintaining a systemic inflammatory status[10].
Progressively, in advanced decompensated cirrhosis a combination of severe impaired functional capacity of the liver, a sustained failure of both the mononuclear phagocytic system and the protein synthesis involved in the immune response is established. Furthermore, the increased intestinal permeability that allows the epithelial crossing of viable bacteria from gut, and the consequent deterioration of the systemic inflammatory status, determine the difficulty of the immune system to solve this situation. Then, it switches into a immunodeficient state, which characterizes severe decompensated cirrhosis with extra-hepatic organ failure stages[10].
Prostaglandin E2 (PGE2), likely produced by circulating monocytes, KC and resident macrophages, has been recently identified as a major contributor to the immunosuppressive status in acutely decompensated cirrhosis. In addition, the decrease of albumin (that reduces the bioavailability of PGE2) levels present in these patients seems to be associated with the increased free PGE2 levels[177].
Ascites from cirrhotic patients presents a reduced opsonic activity as a consequence of reduced concentrations of C3 and C4[178,179]. The concentration of C3 is the main factor to confer local defense against infection of ascitic fluid. Hepatic production of C3 and its concentration in ascites are significantly decreased in patients with advanced cirrhosis, and also in those who develop SBP respect to cirrhotic patients without infection[180]. On the basis of these findings, defects of opsonophagocytic activity seem to contribute in the increased susceptibility to infection in cirrhotic patients[181].
Neutrophil chemotaxis and phagocytosis were decreased in cirrhotic patients with previous episodes of bacterial infection compared with non-infected subjects. The expression of complement receptor 3 (CR3) in neutrophils from peripheral blood was significantly increased in cirrhotic patients, whereas it was significantly reduced in elicited neutrophils of cirrhotic patients with previous bacterial infection[182]. Lower respiratory burst in response to Escherichia coli was detected during infection in hepatic cirrhosis[183]. These data suggest that impaired neutrophils recruitment to the infection site caused by BT and defective phagocytic activity may contribute to bacterial infections in cirrhotic patients with advanced liver disease.
The ascitic NO concentrations registered at diagnosis of an infectious episode are associated with the frequency of clinical complications and death[184,185]. Increased systemic TNF-α[186] and ascitic Macrophage inflammatory protein (MIP)-1β[187] levels at admission have been found in cirrhotic patients who subsequently developed SBP compared with those of uninfected patients, and also a more severe immune response is detected in cirrhotic patients with SBP compared with non-cirrhotic patients with sepsis[188].
Infected ascitic fluid of decompensated cirrhotic patients displays a decrease of both the CD4/CD8 ratio (due to reduction in CD4 and maintenance of CD8) and T cell receptor (TCR) γδ expression, while the levels of TNF-α were increased compared with non-infected ascites. In turn, ascitic levels of IL-8, IL-10, IL-12 and TNF-α were higher in infected patients defined by ascitic positive bacterial culture[189]. Intracytoplasmic levels of IL-4, the major defining Th2 cytokine, were no different between infected and non-infected groups. These data suggested the predominant cytotoxic response, especially Th1, in cases of decompensated liver cirrhosis with ascitic infections, and the possible role of TNF-α in the pathogenesis of ascites infections[189]. Furthermore, levels of soluble receptors of TNF (sTNFR) in the ascitic fluid were found to be elevated in patients with SBP. The levels of TNF-α, IL-6, IL-1β, C-reactive protein, and the anti-inflammatory molecules, soluble IL-1 receptor antagonist (sIL-1Ra), sTNFR55 were elevated in patients with SBP compared to cirrhotic controls[190,191]. SBP was also associated with significantly higher chemokines (IL-8, Gro-α and MCP-1) soluble adhesion molecule ICAM-1, which could be implicated in the peritoneal infiltrate in patients with SBP[192].
As prognosis markers of bacterial infections and more concretely of the Syndrome of systemic inflammatory response in cirrhosis, besides the clinical indexes MELD and Child-Pugh, the presence of bacterial DNA[193], LPS binding protein and C reactive protein[194], IL-22[195], HLA-DR’s expression and IL-10[164,196], among others have been proposed.
Kiyici et al[189], also segregated cirrhotic patients by the viral or non-viral cirrhotic etiology, showing that the levels of TNF-α were higher in the infected group than in non-infected only in viral etiology; IL-8 was higher in the infected group than in non-infected only in non-viral cirrhosis; and no differences were detected on IL-12 and IL-10 cytokine levels between infected or non-infected group respect to the etiology. Related to this, our group has reported that cirrhosis promoted by HCV is associated with a prevalent immune inhibitory status in both ascites and isolated peritoneal M-DM, contrasting with the cirrhosis induced by alcohol[197]. Ascites associated with HCV-C contains a significant higher number of leukocytes (T lymphocyte, polymorphonuclear and monocyte cell subpopulations) than ALC-C. These findings did not agree with the cellular distributions recorded in the peripheral blood. This indicates that the absolute cell number and population distribution of peritoneal leukocytes in ascites from cirrhotic patients vary with the underlying cause and do not match the situation in peripheral blood. Moreover, the findings also reveal that leukocyte migration towards the peritoneal cavity is not a passive process induced by hemodynamic alterations associated to cirrhosis, like portal hypertension, but is more the consequence of an active chemoattractant-induced process, recruiting leukocytes not only from the blood, but also probably, from impaired lymphatic drainage. Hence, a specific differential profile of chemoattractant stimuli must be implicated in the recruitment of each particular cell population, depending on the cirrhotic etiology. These results[197] also revealed that ascites from HCV-C patients displays a significant lower concentration of IL-12 and a higher level of IL-10 than the corresponding from the ALC-C group, indicating a predominant Th2/Treg pattern in the pathogenesis of advanced HCV-C. However, when cytokine levels of ascitic fluid were compared with the number of macrophages contained there, or secreted in vitro by isolated peritoneal macrophages, besides discrepancies in IL-12, the concentration of IL-6 and TNF-α were also different between both groups of cirrhotic patients. Moreover, this fact was related with a lower baseline ERK1/2 phosphorylation in HCV-C than that detected in the ALC-C group. Peritoneal macrophages are “differently primed” by the in vivo pathophysiological environment and maintain their inflammatory differentiation pattern for at least 24 h, being more pro-inflammatory under ALC-C condition. This “alert state” could be useful for preventing the development of SBP in recurrent events of intestinal BT in ALC-C patients. Peritoneal macrophages showed a predominant immune inhibited status in the last-stages of HCV-induced liver damage compared with ALC-C[60], which may be produced to avoid immune-mediated decompensation. The drop of significant differences in the IL-10 levels with respect to the number of peritoneal macrophages, clearly suggests that other cell populations are contributing to the total amount of this anti-inflammatory cytokine in ascites of the HCV-C group, essentially Treg cells. However, this does not imply that these cells in HCV-C are functionally exhausted or endotoxin-tolerant as described[198], since they are able to be further activated by several PAMP agonists. In fact, the relative increase of IL-6 secretion and ERK1/2 phosphorylation after stimulation with LPS was significantly higher in HCV-C than in ALC-C patients. Similar results were observed for IL-6 secretion induced by oligodeoxynucleotides (ODN) and Candida albicans, and IL-10 induced by ODN from HCV-C patients. These findings also indicate the differential expression and/or susceptibility to agonists of PRRs in the macrophages contained in ascites from both groups of cirrhotic patients.
The smaller inflammatory profile of ascites from HCV-C patients compared with those with alcoholic etiology could also be the consequence of the hypothetically lower frequency of intestinal BT in HCV-C patients.
This review focuses on new findings about the inflammatory status involved in the development of human hepatic cirrhosis induced by the two major causes, infection with hepatitis C virus or chronic alcohol abuse, avoiding results obtained from animal models. Described differences between the inflammatory status of cirrhosis caused by these etiologies highlight the interest to perform new studies that allow to obtain further insights into the cellular and molecular mechanisms implicated in this disease. Advances in this field will contribute to a better development of individually tailored preventive and therapeutic procedures.
We thanks the collaboration of Ángel J. Guirao Bermejo with the computer modelling (3ds Max, Autodesk, California, United States) and drawing (Adobe Photoshop CC, Adobe System Incorporated, California, United States) of the figures.
P- Reviewer: El-Karaksy HM, Gong ZJ, Tovo CV S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Mas VR, Fassnacht R, Archer KJ, Maluf D. Molecular mechanisms involved in the interaction effects of alcohol and hepatitis C virus in liver cirrhosis. Mol Med. 2010;16:287-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003;327:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 3. | Chen CM, Yoon YH, Yi HY, Lucas DL. Alcohol and hepatitis C mortality among males and females in the United States: a life table analysis. Alcohol Clin Exp Res. 2007;31:285-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in hepatitis C-related mortality in the United States, 1995-2004. Hepatology. 2008;47:1128-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Wiegand J, Kühne M, Pradat P, Mössner J, Trepo C, Tillmann HL. Different patterns of decompensation in patients with alcoholic vs. non-alcoholic liver cirrhosis. Aliment Pharmacol Ther. 2012;35:1443-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Toshikuni N, Izumi A, Nishino K, Inada N, Sakanoue R, Yamato R, Suehiro M, Kawanaka M, Yamada G. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol. 2009;24:1276-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 555] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 8. | Mihm S. Activation of Type I and Type III Interferons in Chronic Hepatitis C. J Innate Immun. 2015;7:251-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Lederer SL, Walters KA, Proll S, Paeper B, Robinzon S, Boix L, Fausto N, Bruix J, Katze MG. Distinct cellular responses differentiating alcohol- and hepatitis C virus-induced liver cirrhosis. Virol J. 2006;3:98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 726] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 11. | Ramachandran P, Iredale JP. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol. 2012;56:1417-1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Dasarathy S. Inflammation and liver. JPEN J Parenter Enteral Nutr. 2008;32:660-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519-526. [PubMed] [Cited in This Article: ] |
| 15. | Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA. 2006;103:5060-5065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 16. | Butscheid M, Hauptvogel P, Fritz P, Klotz U, Alscher DM. Hepatic expression of galectin-3 and receptor for advanced glycation end products in patients with liver disease. J Clin Pathol. 2007;60:415-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899-d1914. [PubMed] [Cited in This Article: ] |
| 18. | Weiskirchen R, Tacke F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg Nutr. 2014;3:344-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 104] [Reference Citation Analysis (0)] |
| 19. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3381] [Cited by in F6Publishing: 3795] [Article Influence: 199.7] [Reference Citation Analysis (3)] |
| 20. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2084] [Cited by in F6Publishing: 2032] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 21. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Pellicoro A, Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Fibrogenesis Tissue Repair. 2012;5:S26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | de Franchis R, Dell’Era A. Non-invasive diagnosis of cirrhosis and the natural history of its complications. Best Pract Res Clin Gastroenterol. 2007;21:3-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 25. | Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 557] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 26. | Wang N, Liang Y, Devaraj S, Wang J, Lemon SM, Li K. Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J Virol. 2009;83:9824-9834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55:666-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 328] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 29. | Arnaud N, Dabo S, Maillard P, Budkowska A, Kalliampakou KI, Mavromara P, Garcin D, Hugon J, Gatignol A, Akazawa D. Hepatitis C virus controls interferon production through PKR activation. PLoS One. 2010;5:e10575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Horner SM, Gale M Jr. Regulation of hepatic innate immunity by hepatitis C virus. Nat Med. 2013;19:879-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 31. | Boltjes A, Movita D, Boonstra A, Woltman AM. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol. 2014;61:660-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Khakoo SI, Soni PN, Savage K, Brown D, Dhillon AP, Poulter LW, Dusheiko GM. Lymphocyte and macrophage phenotypes in chronic hepatitis C infection. Correlation with disease activity. Am J Pathol. 1997;150:963-970. [PubMed] [Cited in This Article: ] |
| 33. | McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260-269. [PubMed] [Cited in This Article: ] |
| 34. | Burgio VL, Ballardini G, Artini M, Caratozzolo M, Bianchi FB, Levrero M. Expression of co-stimulatory molecules by Kupffer cells in chronic hepatitis of hepatitis C virus etiology. Hepatology. 1998;27:1600-1606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Fletcher NF, Sutaria R, Jo J, Barnes A, Blahova M, Meredith LW, Cosset FL, Curbishley SM, Adams DH, Bertoletti A. Activated macrophages promote hepatitis C virus entry in a tumor necrosis factor-dependent manner. Hepatology. 2014;59:1320-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology. 2010;138:305-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 39. | Broering R, Wu J, Meng Z, Hilgard P, Lu M, Trippler M, Szczeponek A, Gerken G, Schlaak JF. Toll-like receptor-stimulated non-parenchymal liver cells can regulate hepatitis C virus replication. J Hepatol. 2008;48:914-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Zhu H, Liu C. Interleukin-1 inhibits hepatitis C virus subgenomic RNA replication by activation of extracellular regulated kinase pathway. J Virol. 2003;77:5493-5498. [PubMed] [Cited in This Article: ] |
| 41. | Zhu H, Shang X, Terada N, Liu C. STAT3 induces anti-hepatitis C viral activity in liver cells. Biochem Biophys Res Commun. 2004;324:518-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Lin W, Kim SS, Yeung E, Kamegaya Y, Blackard JT, Kim KA, Holtzman MJ, Chung RT. Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. J Virol. 2006;80:9226-9235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1933] [Cited by in F6Publishing: 1854] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 44. | Miyazaki M, Kanto T, Inoue M, Itose I, Miyatake H, Sakakibara M, Yakushijin T, Kakita N, Hiramatsu N, Takehara T. Impaired cytokine response in myeloid dendritic cells in chronic hepatitis C virus infection regardless of enhanced expression of Toll-like receptors and retinoic acid inducible gene-I. J Med Virol. 2008;80:980-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Woltman AM, Boonstra A, Janssen HL. Dendritic cells in chronic viral hepatitis B and C: victims or guardian angels? Gut. 2010;59:115-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. 2011;6:e15324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (2)] |
| 47. | Abe T, Kaname Y, Hamamoto I, Tsuda Y, Wen X, Taguwa S, Moriishi K, Takeuchi O, Kawai T, Kanto T. Hepatitis C virus nonstructural protein 5A modulates the toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J Virol. 2007;81:8953-8966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 48. | Chung H, Watanabe T, Kudo M, Chiba T. Hepatitis C virus core protein induces homotolerance and cross-tolerance to Toll-like receptor ligands by activation of Toll-like receptor 2. J Infect Dis. 2010;202:853-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Szabo G, Dolganiuc A. Hepatitis C and innate immunity: recent advances. Clin Liver Dis. 2008;12:675-92, x. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Chung H, Watanabe T, Kudo M, Chiba T. Correlation between hyporesponsiveness to Toll-like receptor ligands and liver dysfunction in patients with chronic hepatitis C virus infection. J Viral Hepat. 2011;18:e561-e567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Ahlenstiel G. The natural killer cell response to HCV infection. Immune Netw. 2013;13:168-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151-160, 1151-1160, 1160.e1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 320] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 53. | Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325-335.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 54. | Varchetta S, Mele D, Mantovani S, Oliviero B, Cremonesi E, Ludovisi S, Michelone G, Alessiani M, Rosati R, Montorsi M. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology. 2012;56:841-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Li WY, Jiang YF, Jin QL, Zhang H, Feng XW, Niu JQ. Immunologic characterization of posthepatitis cirrhosis caused by HBV and HCV infection. J Biomed Biotechnol. 2010;2010:138237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 916] [Cited by in F6Publishing: 895] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 57. | Sène D, Levasseur F, Abel M, Lambert M, Camous X, Hernandez C, Pène V, Rosenberg AR, Jouvin-Marche E, Marche PN. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 2010;6:e1001184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 58. | Wang JM, Cheng YQ, Shi L, Ying RS, Wu XY, Li GY, Moorman JP, Yao ZQ. KLRG1 negatively regulates natural killer cell functions through the Akt pathway in individuals with chronic hepatitis C virus infection. J Virol. 2013;87:11626-11636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55:343-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 60. | Sreenarasimhaiah J, Jaramillo A, Crippin J, Lisker-Melman M, Chapman WC, Mohanakumar T. Lack of optimal T-cell reactivity against the hepatitis C virus is associated with the development of fibrosis/cirrhosis during chronic hepatitis. Hum Immunol. 2003;64:224-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Claassen MA, de Knegt RJ, Tilanus HW, Janssen HL, Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. 2010;52:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 62. | Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, Jia ZS, Wang KS, Yao ZQ. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol. 2012;189:755-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Ji XJ, Ma CJ, Wang JM, Wu XY, Niki T, Hirashima M, Moorman JP, Yao ZQ. HCV-infected hepatocytes drive CD4+ CD25+ Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway. Eur J Immunol. 2013;43:458-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Fernandez-Ponce C, Dominguez-Villar M, Aguado E, Garcia-Cozar F. CD4+ primary T cells expressing HCV-core protein upregulate Foxp3 and IL-10, suppressing CD4 and CD8 T cells. PLoS One. 2014;9:e85191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Ambros V. The functions of animal microRNAs. Nature. 2004;431:350-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7919] [Cited by in F6Publishing: 8280] [Article Influence: 414.0] [Reference Citation Analysis (0)] |
| 66. | Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 455] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 67. | Chang CC, Lin CC, Hsieh WL, Lai HW, Tsai CH, Cheng YW. MicroRNA expression profiling in PBMCs: a potential diagnostic biomarker of chronic hepatitis C. Dis Markers. 2014;2014:367157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Bala S, Szabo G. MicroRNA Signature in Alcoholic Liver Disease. Int J Hepatol. 2012;2012:498232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 69. | Aravinthan A, Pietrosi G, Hoare M, Jupp J, Marshall A, Verrill C, Davies S, Bateman A, Sheron N, Allison M. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS One. 2013;8:e72904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Tang C, Liang X, Liu H, Guo L, Pi R, Yang J. Changes in mitochondrial DNA and its encoded products in alcoholic cirrhosis. Int J Clin Exp Med. 2012;5:245-250. [PubMed] [Cited in This Article: ] |
| 71. | Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 461] [Cited by in F6Publishing: 445] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 72. | Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966-G978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 493] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 73. | Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 74. | Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 419] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 75. | Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 469] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 76. | Ceccarelli S, Nobili V, Alisi A. Toll-like receptor-mediated signaling cascade as a regulator of the inflammation network during alcoholic liver disease. World J Gastroenterol. 2014;20:16443-16451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Stärkel P, De Saeger C, Strain AJ, Leclercq I, Horsmans Y. NFkappaB, cytokines, TLR 3 and 7 expression in human end-stage HCV and alcoholic liver disease. Eur J Clin Invest. 2010;40:575-584. [PubMed] [Cited in This Article: ] |
| 78. | Liu H, Li J, Tillman B, Morgan TR, French BA, French SW. TLR3/4 signaling is mediated via the NFκB-CXCR4/7 pathway in human alcoholic hepatitis and non-alcoholic steatohepatitis which formed Mallory-Denk bodies. Exp Mol Pathol. 2014;97:234-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015;35:724-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 80. | Mallat A, Lodder J, Teixeira-Clerc F, Moreau R, Codogno P, Lotersztajn S. Autophagy: a multifaceted partner in liver fibrosis. Biomed Res Int. 2014;2014:869390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Ding WX. Induction of autophagy, a promising approach for treating liver injury. Hepatology. 2014;59:340-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Mendoza AS, Dorce J, Peng Y, French BA, Tillman B, Li J, French SW. Levels of metacaspase1 and chaperones related to protein quality control in alcoholic and nonalcoholic steatohepatitis. Exp Mol Pathol. 2015;98:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605-G611. [PubMed] [Cited in This Article: ] |
| 84. | Gomez F, Ruiz P, Schreiber AD. Impaired function of macrophage Fc gamma receptors and bacterial infection in alcoholic cirrhosis. N Engl J Med. 1994;331:1122-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Lee J, French B, Morgan T, French SW. The liver is populated by a broad spectrum of markers for macrophages. In alcoholic hepatitis the macrophages are M1 and M2. Exp Mol Pathol. 2014;96:118-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 387] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 87. | Saha B, Bruneau JC, Kodys K, Szabo G. Alcohol-induced miR-27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol. 2015;194:3079-3087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 88. | Pimentel-Nunes P, Roncon-Albuquerque R, Gonçalves N, Fernandes-Cerqueira C, Cardoso H, Bastos RP, Marques M, Marques C, Alexandre Sarmento J, Costa-Santos C. Attenuation of toll-like receptor 2-mediated innate immune response in patients with alcoholic chronic liver disease. Liver Int. 2010;30:1003-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Mandrekar P, Dolganiuc A, Bellerose G, Kodys K, Romics L, Nizamani R, Szabo G. Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation? Alcohol Clin Exp Res. 2002;26:1609-1614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 90. | Perlino CA, Rimland D. Alcoholism, leukopenia, and pneumococcal sepsis. Am Rev Respir Dis. 1985;132:757-760. [PubMed] [Cited in This Article: ] |
| 91. | Taylor NJ, Nishtala A, Manakkat Vijay GK, Abeles RD, Auzinger G, Bernal W, Ma Y, Wendon JA, Shawcross DL. Circulating neutrophil dysfunction in acute liver failure. Hepatology. 2013;57:1142-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 196] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 93. | Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 94. | Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 95. | Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jürgens G, Hallström S, Jalan R. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G15-G22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Laso FJ, Madruga JI, López A, Ciudad J, Alvarez-Mon M, San Miguel J, Orfao A. Distribution of peripheral blood lymphoid subsets in alcoholic liver cirrhosis: influence of ethanol intake. Alcohol Clin Exp Res. 1996;20:1564-1568. [PubMed] [Cited in This Article: ] |
| 97. | Charpentier B, Franco D, Paci L, Charra M, Martin B, Vuitton D, Fries D. Deficient natural killer cell activity in alcoholic cirrhosis. Clin Exp Immunol. 1984;58:107-115. [PubMed] [Cited in This Article: ] |
| 98. | Laso FJ, Madruga JI, Girón JA, López A, Ciudad J, San Miguel JF, Alvarez-Mon M, Orfao A. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology. 1997;25:1096-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 100. | Negash AA, Gale M Jr. Hepatitis regulation by the inflammasome signaling pathway. Immunol Rev. 2015;265:143-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Albano E. Role of adaptive immunity in alcoholic liver disease. Int J Hepatol. 2012;2012:893026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 102. | Matos LC, Batista P, Monteiro N, Ribeiro J, Cipriano MA, Henriques P, Girão F, Carvalho A. Lymphocyte subsets in alcoholic liver disease. World J Hepatol. 2013;5:46-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Song K, Coleman RA, Alber C, Ballas ZK, Waldschmidt TJ, Mortari F, LaBrecque DR, Cook RT. TH1 cytokine response of CD57+ T-cell subsets in healthy controls and patients with alcoholic liver disease. Alcohol. 2001;24:155-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Devière J, Denys C, Schandene L, Romasco F, Adler M, Wybran J, Dupont E. Decreased proliferative activity associated with activation markers in patients with alcoholic liver cirrhosis. Clin Exp Immunol. 1988;72:377-382. [PubMed] [Cited in This Article: ] |
| 105. | Girón-González JA, Alvarez-Mon M, Menéndez-Caro JL, Manzano L, Abreu L, Yebra M, Durántez-Martínez A. T lymphocytes from alcoholic cirrhotic patients show normal interleukin-2 production but a defective proliferative response to polyclonal mitogens. Am J Gastroenterol. 1994;89:767-773. [PubMed] [Cited in This Article: ] |
| 106. | Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2015;148:590-602.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 107. | Cook RT, Waldschmidt TJ, Cook BL, Labrecque DR, McLatchie K. Loss of the CD5+ and CD45RAhi B cell subsets in alcoholics. Clin Exp Immunol. 1996;103:304-310. [PubMed] [Cited in This Article: ] |
| 108. | Lin F, Taylor NJ, Su H, Huang X, Hussain MJ, Abeles RD, Blackmore L, Zhou Y, Ikbal MM, Heaton N. Alcohol dehydrogenase-specific T-cell responses are associated with alcohol consumption in patients with alcohol-related cirrhosis. Hepatology. 2013;58:314-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Takase S, Tsutsumi M, Kawahara H, Takada N, Takada A. The alcohol-altered liver membrane antibody and hepatitis C virus infection in the progression of alcoholic liver disease. Hepatology. 1993;17:9-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Vidali M, Hietala J, Occhino G, Ivaldi A, Sutti S, Niemelä O, Albano E. Immune responses against oxidative stress-derived antigens are associated with increased circulating tumor necrosis factor-alpha in heavy drinkers. Free Radic Biol Med. 2008;45:306-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Vidali M, Occhino G, Ivaldi A, Rigamonti C, Sartori M, Albano E. Combination of oxidative stress and steatosis is a risk factor for fibrosis in alcohol-drinking patients with chronic hepatitis C. Am J Gastroenterol. 2008;103:147-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Massonnet B, Delwail A, Ayrault JM, Chagneau-Derrode C, Lecron JC, Silvain C. Increased immunoglobulin A in alcoholic liver cirrhosis: exploring the response of B cells to Toll-like receptor 9 activation. Clin Exp Immunol. 2009;158:115-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 113. | Liu WT, Jing YY, Han ZP, Li XN, Liu Y, Lai FB, Li R, Zhao QD, Wu MC, Wei LX. The injured liver induces hyperimmunoglobulinemia by failing to dispose of antigens and endotoxins in the portal system. PLoS One. 2015;10:e0122739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Takamatsu M, Yamauchi M, Maezawa Y, Saito S, Maeyama S, Uchikoshi T. Genetic polymorphisms of interleukin-1beta in association with the development of alcoholic liver disease in Japanese patients. Am J Gastroenterol. 2000;95:1305-1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Hanck C, Manigold T, Böcker U, Kurimoto M, Kölbel CB, Singer MV, Rossol S. Gene expression of interleukin 18 in unstimulated peripheral blood mononuclear cells of patients with alcoholic cirrhosis. Gut. 2001;49:106-111. [PubMed] [Cited in This Article: ] |
| 116. | Petrasek J, Iracheta-Vellve A, Saha B, Satishchandran A, Kodys K, Fitzgerald KA, Kurt-Jones EA, Szabo G. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol. 2015;98:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 117. | Peng Y, French BA, Tillman B, Morgan TR, French SW. The inflammasome in alcoholic hepatitis: Its relationship with Mallory-Denk body formation. Exp Mol Pathol. 2014;97:305-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 119. | Deviere J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77:221-225. [PubMed] [Cited in This Article: ] |
| 120. | Diez Ruiz A, Santos Perez JL, Lopez Martinez G, Gonzalez Calvín J, Gil Extremera B, Gutierrez Gea F. Tumour necrosis factor, interleukin-1 and interleukin-6 in alcoholic cirrhosis. Alcohol Alcohol. 1993;28:319-323. [PubMed] [Cited in This Article: ] |
| 121. | Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 317] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 122. | McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G497-G502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 123. | Daniluk J, Szuster-Ciesielska A, Drabko J, Kandefer-Szerszeń M. Serum cytokine levels in alcohol-related liver cirrhosis. Alcohol. 2001;23:29-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 124. | Affò S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millán C, Loaeza-del-Castillo A, Altamirano J. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2013;62:452-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 125. | Tung KH, Huang YS, Yang KC, Perng CL, Lin HC, Lee SD. Serum interleukin-12 levels in alcoholic liver disease. J Chin Med Assoc. 2010;73:67-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Voumvouraki A, Notas G, Koulentaki M, Georgiadou M, Klironomos S, Kouroumalis E. Increased serum activin-A differentiates alcoholic from cirrhosis of other aetiologies. Eur J Clin Invest. 2012;42:815-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 127. | von Baehr V, Döcke WD, Plauth M, Liebenthal C, Küpferling S, Lochs H, Baumgarten R, Volk HD. Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumour necrosis factor receptors as well as effector cell desensitisation. Gut. 2000;47:281-287. [PubMed] [Cited in This Article: ] |
| 128. | Shen H, French BA, Liu H, Tillman BC, French SW. Increased activity of the complement system in the liver of patients with alcoholic hepatitis. Exp Mol Pathol. 2014;97:338-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 129. | Huang YS, Chan CY, Wu JC, Pai CH, Chao Y, Lee SD. Serum levels of interleukin-8 in alcoholic liver disease: relationship with disease stage, biochemical parameters and survival. J Hepatol. 1996;24:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 130. | Zimmermann HW, Seidler S, Gassler N, Nattermann J, Luedde T, Trautwein C, Tacke F. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One. 2011;6:e21381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 131. | Dominguez M, Miquel R, Colmenero J, Moreno M, García-Pagán JC, Bosch J, Arroyo V, Ginès P, Caballería J, Bataller R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 132. | Tacke F, Zimmermann HW, Trautwein C, Schnabl B. CXCL5 plasma levels decrease in patients with chronic liver disease. J Gastroenterol Hepatol. 2011;26:523-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 133. | Karlmark KR, Wasmuth HE, Trautwein C, Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol. 2008;2:233-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 134. | Berres ML, Asmacher S, Lehmann J, Jansen C, Görtzen J, Klein S, Meyer C, Strunk HM, Fimmers R, Tacke F. CXCL9 is a prognostic marker in patients with liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. J Hepatol. 2015;62:332-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 135. | Nischalke HD, Berger C, Lutz P, Langhans B, Wolter F, Eisenhardt M, Krämer B, Kokordelis P, Glässner A, Müller T. Influence of the CXCL1 rs4074 A allele on alcohol induced cirrhosis and HCC in patients of European descent. PLoS One. 2013;8:e80848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 136. | Affò S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, Millán C, Coll M, Caviglia JM, Arroyo V. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782-1792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 137. | McDaniel K, Herrera L, Zhou T, Francis H, Han Y, Levine P, Lin E, Glaser S, Alpini G, Meng F. The functional role of microRNAs in alcoholic liver injury. J Cell Mol Med. 2014;18:197-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 138. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1244] [Cited by in F6Publishing: 1342] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 139. | Wanninger J, Weigert J, Wiest R, Bauer S, Karrasch T, Farkas S, Scherer MN, Walter R, Weiss TS, Hellerbrand C. Systemic and hepatic vein galectin-3 are increased in patients with alcoholic liver cirrhosis and negatively correlate with liver function. Cytokine. 2011;55:435-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 140. | Morales-Ibanez O, Domínguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E, Houchi H, Affò S, Sancho-Bru P, Altamirano J. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology. 2013;58:1742-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 141. | Uchimura Y, Sata M, Kage M, Abe H, Tanikawa K. A histopathological study of alcoholics with chronic HCV infection: comparison with chronic hepatitis C and alcoholic liver disease. Liver. 1995;15:300-306. [PubMed] [Cited in This Article: ] |
| 142. | Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (2)] |
| 143. | Zuwała-Jagiełło J, Pazgan-Simon M, Simon K, Warwas M. Advanced oxidation protein products and inflammatory markers in liver cirrhosis: a comparison between alcohol-related and HCV-related cirrhosis. Acta Biochim Pol. 2011;58:59-65. [PubMed] [Cited in This Article: ] |
| 144. | Kashani A, Salehi B, Anghesom D, Kawayeh AM, Rouse GA, Runyon BA. Spleen size in cirrhosis of different etiologies. J Ultrasound Med. 2015;34:233-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 145. | Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122-128. [PubMed] [Cited in This Article: ] |
| 146. | Kuiper JJ, van Buuren HR, de Man RA. Ascites in cirrhosis: a review of management and complications. Neth J Med. 2007;65:283-288. [PubMed] [Cited in This Article: ] |
| 147. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1070] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 148. | Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669-74; quiz 675-6. [PubMed] [Cited in This Article: ] |
| 149. | Such J, Guarner C, Enriquez J, Rodriguez JL, Seres I, Vilardell F. Low C3 in cirrhotic ascites predisposes to spontaneous bacterial peritonitis. J Hepatol. 1988;6:80-84. [PubMed] [Cited in This Article: ] |
| 150. | Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 151. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 506] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 152. | Berg RD. Bacterial translocation from the gastrointestinal tract. J Med. 1992;23:217-244. [PubMed] [Cited in This Article: ] |
| 153. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 154. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 155. | Francés R, Muñoz C, Zapater P, Uceda F, Gascón I, Pascual S, Pérez-Mateo M, Such J. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860-864. [PubMed] [Cited in This Article: ] |
| 156. | Sersté T, Bert F, Leflon-Guibout V, Chauvet C, Marcon E, Asselah T, Francoz C, Durand F, Lebrec D, Valla D. Detection of bacterial DNA in serum and ascitic fluid of asymptomatic outpatients with cirrhosis and non-neutrocytic ascites. Liver Int. 2011;31:494-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Caño R, Llanos L, Zapater P, Pascual S, Bellot P, Barquero C, Pérez-Mateo M, Such J, Francés R. Proteomic evidence of bacterial peptide translocation in afebrile patients with cirrhosis and ascites. J Mol Med (Berl). 2010;88:487-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 158. | Saitoh O, Sugi K, Lojima K, Matsumoto H, Nakagawa K, Kayazawa M, Tanaka S, Teranishi T, Hirata I. Increased prevalence of intestinal inflammation in patients with liver cirrhosis. World J Gastroenterol. 1999;5:391-396. [PubMed] [Cited in This Article: ] |
| 159. | Genescà J, Martí R, Rojo F, Campos F, Peribáñez V, Gónzalez A, Castells L, Ruiz-Marcellán C, Margarit C, Esteban R. Increased tumour necrosis factor alpha production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut. 2003;52:1054-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 160. | Francés R, Zapater P, González-Navajas JM, Muñoz C, Caño R, Moreu R, Pascual S, Bellot P, Pérez-Mateo M, Such J. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology. 2008;47:978-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 161. | Francés R, Rodríguez E, Muñoz C, Zapater P, De la ML, Ndongo M, Pérez-Mateo M, Such J. Intracellular cytokine expression in peritoneal monocyte/macrophages obtained from patients with cirrhosis and presence of bacterial DNA. Eur J Gastroenterol Hepatol. 2005;17:45-51. [PubMed] [Cited in This Article: ] |
| 162. | Francés R, González-Navajas JM, Zapater P, Muñoz C, Caño R, Pascual S, Márquez D, Santana F, Pérez-Mateo M, Such J. Bacterial DNA induces the complement system activation in serum and ascitic fluid from patients with advanced cirrhosis. J Clin Immunol. 2007;27:438-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 163. | Sánchez-Rodríguez A, Criado M, Flores O, Olveira-Martín A, Martín-Oterino JA, Esteller A. Correlation of high levels of hyaluronan and cytokines (IL-1beta, IL-6, and TGF-beta) in ascitic fluid of cirrhotic patients. Dig Dis Sci. 2000;45:2229-2232. [PubMed] [Cited in This Article: ] |
| 164. | Ruiz-Alcaraz AJ, Martínez-Esparza M, Caño R, Hernández-Caselles T, Recarti C, Llanos L, Zapater P, Tapia-Abellán A, Martín-Orozco E, Pérez-Mateo M. Peritoneal macrophage priming in cirrhosis is related to ERK phosphorylation and IL-6 secretion. Eur J Clin Invest. 2011;41:8-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 165. | Ahmed AM, Bomford A, Nouri-Aria KT, Davies T, Smith R, Williams R. Peritoneal macrophages from patients with cirrhotic ascites show impaired phagocytosis and vigorous respiratory burst. Results Immunol. 2011;1:53-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 166. | Tapia-Abellán A, Ruiz-Alcaraz AJ, Hernández-Caselles T, Such J, Francés R, García-Peñarrubia P, Martínez-Esparza M. Role of MAP kinases and PI3K-Akt on the cytokine inflammatory profile of peritoneal macrophages from the ascites of cirrhotic patients. Liver Int. 2013;33:552-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 167. | Tapia-Abellán A, Ruiz-Alcaraz AJ, Antón G, Miras-López M, Francés R, Such J, Martínez-Esparza M, García-Peñarrubia P. Regulatory role of PI3K-protein kinase B on the release of interleukin-1β in peritoneal macrophages from the ascites of cirrhotic patients. Clin Exp Immunol. 2014;178:525-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Lozano-Ruiz B, Bachiller V, García-Martínez I, Zapater P, Gómez-Hurtado I, Moratalla A, Giménez P, Bellot P, Francés R, Such J. Absent in melanoma 2 triggers a heightened inflammasome response in ascitic fluid macrophages of patients with cirrhosis. J Hepatol. 2015;62:64-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 169. | Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581-593. [PubMed] [Cited in This Article: ] |
| 170. | Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, Marmé F, Umansky L, Umansky V, Eigenbrod T. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691-36702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 171. | Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 172. | Agarwal S, Joyner KA, Swaim MW. Ascites fluid as a possible origin for hyperfibrinolysis in advanced liver disease. Am J Gastroenterol. 2000;95:3218-3224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 173. | Attar BM, Moore CM, George M, Ion-Nedelcu N, Turbay R, Zachariah A, Ramadori G, Fareed J, Van Thiel DH. Procalcitonin, and cytokines document a dynamic inflammatory state in non-infected cirrhotic patients with ascites. World J Gastroenterol. 2014;20:2374-2382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 174. | Kalaitzakis E, Johansson JE, Bjarnason I, Björnsson E. Intestinal permeability in cirrhotic patients with and without ascites. Scand J Gastroenterol. 2006;41:326-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 175. | Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 123] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 176. | Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI, Nieuwoudt M, van Wyk SG, Vieira W, Pretorius E. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58:1125-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 177. | O’Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, Newson J, Karra E, Winstanley A, Alazawi W. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20:518-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 178. | Runyon BA, Morrissey RL, Hoefs JC, Wyle FA. Opsonic activity of human ascitic fluid: a potentially important protective mechanism against spontaneous bacterial peritonitis. Hepatology. 1985;5:634-637. [PubMed] [Cited in This Article: ] |
| 179. | Runyon BA. Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis. Hepatology. 2007;8:632-635. [PubMed] [Cited in This Article: ] |
| 180. | Mustafa G, Khan M, Alam K, Rahman S, Ahmad N, Alam S, Ahmed S. Study on ascitic fluid complement 3 level in cirrhotic patients with spontaneous bacterial peritonitis and without spontaneous bacterial peritonitis. Hepatogastroenterology. 2007;54:1905-1907. [PubMed] [Cited in This Article: ] |
| 181. | Ono Y, Watanabe T, Matsumoto K, Ito T, Kunii O, Goldstein E. Opsonophagocytic dysfunction in patients with liver cirrhosis and low responses to tumor necrosis factor-alpha and lipopolysaccharide in patients’ blood. J Infect Chemother. 2004;10:200-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 182. | Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182:526-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 183. | Bruns T, Peter J, Hagel S, Herrmann A, Stallmach A. The augmented neutrophil respiratory burst in response to Escherichia coli is reduced in liver cirrhosis during infection. Clin Exp Immunol. 2011;164:346-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 184. | Such J, Hillebrand DJ, Guarner C, Berk L, Zapater P, Westengard J, Peralta C, Soriano G, Pappas J, Francés R. Nitric oxide in ascitic fluid is an independent predictor of the development of renal impairment in patients with cirrhosis and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2004;16:571-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 185. | Garcia-Tsao G, Angulo P, Garcia JC, Groszmann RJ, Cadelina GW. The diagnostic and predictive value of ascites nitric oxide levels in patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:17-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 186. | Such J, Hillebrand DJ, Guarner C, Berk L, Zapater P, Westengard J, Peralta C, Soriano G, Pappas J, Runyon BA. Tumor necrosis factor-alpha, interleukin-6, and nitric oxide in sterile ascitic fluid and serum from patients with cirrhosis who subsequently develop ascitic fluid infection. Dig Dis Sci. 2001;46:2360-2366. [PubMed] [Cited in This Article: ] |
| 187. | Lesińska M, Hartleb M, Gutkowski K, Nowakowska-Duława E. Procalcitonin and macrophage inflammatory protein-1 beta (MIP-1β) in serum and peritoneal fluid of patients with decompensated cirrhosis and spontaneous bacterial peritonitis. Adv Med Sci. 2014;59:52-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 188. | Byl B, Roucloux I, Crusiaux A, Dupont E, Devière J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;104:1492-1497. [PubMed] [Cited in This Article: ] |
| 189. | Kiyici M, Nak SG, Budak F, Gurel S, Oral B, Dolar E, Gulten M. Lymphocyte subsets and cytokines in ascitic fluid of decompensated cirrhotic patients with and without spontaneous ascites infection. J Gastroenterol Hepatol. 2006;21:963-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 190. | Rodríguez-Ramos C, Galan F, Díaz F, Elvira J, Martín-Herrera L, Girón-González JA. Expression of proinflammatory cytokines and their inhibitors during the course of spontaneous bacterial peritonitis. Dig Dis Sci. 2001;46:1668-1676. [PubMed] [Cited in This Article: ] |
| 191. | Yildirim B, Sari R, Isci N. Patients with spontaneous bacterial peritonitis, and malignant and cirrhotic ascites. J Natl Med Assoc. 2005;97:276-280. [PubMed] [Cited in This Article: ] |
| 192. | Girón-González JA, Rodríguez-Ramos C, Elvira J, Galán F, Del Alamo CF, Díaz F, Martín-Herrera L. Serial analysis of serum and ascitic fluid levels of soluble adhesion molecules and chemokines in patients with spontaneous bacterial peritonitis. Clin Exp Immunol. 2001;123:56-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 193. | Zapater P, Francés R, González-Navajas JM, de la Hoz MA, Moreu R, Pascual S, Monfort D, Montoliu S, Vila C, Escudero A. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology. 2008;48:1924-1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 194. | Papp M, Vitalis Z, Altorjay I, Tornai I, Udvardy M, Harsfalvi J, Vida A, Kappelmayer J, Lakatos PL, Antal-Szalmas P. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 2012;32:603-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 195. | Kronenberger B, Rudloff I, Bachmann M, Brunner F, Kapper L, Filmann N, Waidmann O, Herrmann E, Pfeilschifter J, Zeuzem S. Interleukin-22 predicts severity and death in advanced liver cirrhosis: a prospective cohort study. BMC Med. 2012;10:102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 196. | Berry PA, Antoniades CG, Carey I, McPhail MJ, Hussain MJ, Davies ET, Wendon JA, Vergani D. Severity of the compensatory anti-inflammatory response determined by monocyte HLA-DR expression may assist outcome prediction in cirrhosis. Intensive Care Med. 2011;37:453-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 197. | Tapia-Abellán A, Martínez-Esparza M, Ruiz-Alcaraz AJ, Hernández-Caselles T, Martínez-Pascual C, Miras-López M, Such J, Francés R, García-Peñarrubia P. The peritoneal macrophage inflammatory profile in cirrhosis depends on the alcoholic or hepatitis C viral etiology and is related to ERK phosphorylation. BMC Immunol. 2012;13:42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 198. | Lin CY, Tsai IF, Ho YP, Huang CT, Lin YC, Lin CJ, Tseng SC, Lin WP, Chen WT, Sheen IS. Endotoxemia contributes to the immune paralysis in patients with cirrhosis. J Hepatol. 2007;46:816-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |