Published online Oct 7, 2015. doi: 10.3748/wjg.v21.i37.10688
Peer-review started: March 4, 2015
First decision: April 24, 2015
Revised: May 12, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: October 7, 2015
AIM: To investigate the toxicity and response of intensity-modulated radiotherapy schedule intensified with a simultaneous integrated boost in anal canal cancer.
METHODS: From March 2009 to March 2014, we retrospectively analyzed 41 consecutive patients treated with intensity-modulated radiotherapy (IMRT) and concurrent chemotherapy for anal canal squamous cell carcinoma at our center. Radiotherapy was delivered via simultaneous integrated boost (SIB) technique by helical tomotherapy, and doses were adapted to two clinical target volumes according to the tumor-node-metastasis (TNM) stage: 50.6 Gy and 41.4 Gy in 23 fractions in T1N0, 52.8 Gy and 43.2 Gy in 24 fractions in T2N0, and 55 Gy and 45 Gy in 25 fractions in all patients with N positive and/or ≥ T3, respectively, to planning target volumes 1 and 2. The most common chemotherapy regimen was 5-fluorouracil and mitomycin-based. Human papilloma virus (HPV) p16 expression was performed by immunohistochemistry and evaluated in the majority of patients. Acute and late toxicity was scored according to CTCAe v 3.0 and RTOG scales.
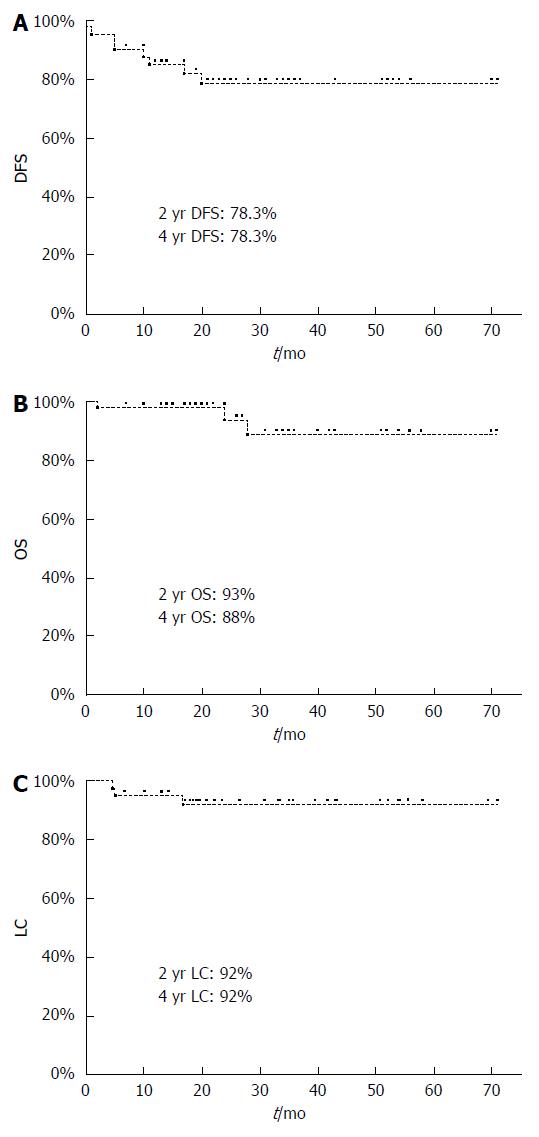
RESULTS: The median follow-up was 30 mo (range: 12-71). Median age was 63 years (range 32-84). The stage of disease was: stage I in 2 patients, stage II in 13 patients, stage IIIA in 12 patients, and stage IIIB in 14 patients, respectively. Two patients were known to be HIV positive (4.9%). HPV p16 expression status was positive in 29/34 (85.3%) patients. The 4-year progression-free survival and overall survival in HPV-positive patients were 78% and 92%, respectively. Acute grade 3 skin and gastrointestinal toxicities were reported in 5% and 7.3% of patients, respectively; patients’ compliance to the treatment was good due to a low occurrence of severe acute toxicity, although treatment interruptions due to toxicity were required in 7.3% of patients. At 6 mo from end of treatment, 36/40 (90%) patients obtained complete response; during follow-up, 5 (13.8%) patients presented with disease progression (local or systemic).
CONCLUSION: In our experience, intensified SIB-IMRT with chemotherapy is very feasible in clinical practice, with excellent results in terms of overall survival and local control.
Core tip: This study evaluated intensity-modulated radiotherapy with simultaneous integrated boost in anal canal cancer of prevalent Human papilloma virus (HPV)-positive patients. The results show excellent outcomes in HPV-positive tumors, and suggest that an intensified radiotherapy schedule associated with chemotherapy is safe and allows the obtaining of oncologic results comparable to the standard schedule without an increase in acute toxicity.
- Citation: Belgioia L, Vagge S, Agnese D, Garelli S, Murialdo R, Fornarini G, Chiara S, Gallo F, Bacigalupo A, Corvò R. Intensified intensity-modulated radiotherapy in anal cancer with prevalent HPV p16 positivity. World J Gastroenterol 2015; 21(37): 10688-10696
- URL: https://www.wjgnet.com/1007-9327/full/v21/i37/10688.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i37.10688
Anal canal cancer is a rare pathology accounting for 2.4% of digestive system cancers[1]. The standard treatment procedure consists of combined radiation-chemotherapy, which allows for the achievement of high rates of local control, overall survival (OS), and disease-free survival (DFS)[2]. However, due to its high toxicity, which can lead to treatment interruptions, different chemotherapy regimens and schedules of RT have been tentatively explored in order to improve treatment compliance[3,4]. Over the last few years, the introduction of intensity-modulated radiotherapy (IMRT) has become increasingly attractive, as it has shown to maintain radiation doses within clinical target volumes, while at the same time reducing the average and threshold doses for Organs-at-Risk (OARs), such as the genitals, perineum, small bowel, and bladder, compared with conventional 3D-Conformal RT[5]. IMRT with a Simultaneous Integrated Boost of Dose (SIB) could be devised as an innovative intensified strategy to escalate radiation doses to sites of macroscopic disease. This approach has its advantages compared to sequential dosage increment, namely: the possibility to deliver different fraction doses to different volumes, shorten overall treatment time (OTT), and guarantee better coverage of Gross Tumor Volume (GTV) with non-target tissue sparing. SIB use has also been investigated in diseases other than anal canal cancer, such as head and neck cancer and cervical cancer, and where it has been demonstrated to be feasible and act to reduce acute toxicity[6-8]. Here we present the results of a study carried out with an intensified radiotherapy regimen associated with chemotherapy that was applied in order to improve oncologic efficacy and reduce overall acute toxicity in patients with squamous cell carcinoma of the anal canal treated at our center.
We analyzed retrospectively 41 consecutive patients with histologically documented anal canal squamous carcinoma (clinical stage T1-4, N0-3) treated between March 2009 and March 2014 at our center, a public university hospital. All tumors originated from the anal canal and could extend to the perianal region in advanced disease.
Pretreatment evaluation included a complete patient history, physical examination, blood chemistry, chest radiogram or chest/abdominal computed tomography (CT), magnetic resonance imaging (MRI) of the pelvis and/or FDG positron emission tomography (PET)-CT, sigmoidoscopy, or transrectal ultrasound in order to assess disease stage. Patients were excluded if they had metastatic disease or prior pelvic radiotherapy.
Evaluation of p16 expression status, as a surrogate of human papilloma virus (HPV) infection, was performed by immunohistochemistry using the automated immunostainer Ventana BenchMark® XT platform (Ventana Medical Systems, Arizona, United States) and anti-p16INK4a antibodies (Ventana Medical Systems, Arizona, United States). p16 is a cyclin-dependent kinase inhibitor with an important role in cellular differentiation and the cell cycle. Overexpression of p16 is found in neoplastic cells, and is strongly associated with the molecular expression of the E7 oncoprotein of HPV.
CT-based planning with a slice thickness ≤ 5 mm from the upper lumbar spine to the mid-femur was performed. Oral contrast was recommended to allow better visualization of the bowel; all patients had a full bladder and a radio-opaque vaginal marker was placed in female patients to assist in target delineation. Patients were scanned in a supine position and immobilized with a thermoplastic mask from the base of the lung to the mid-femur with all-in-one (AIO) solution or COMBIFIXTM for pelvic district. Images were transferred to the Eclipse Treatment Planning System (Varian Medical Systems) for contouring. The gross tumor volume (GTV), clinical tumor volume (CTV), planning target volume (PTV), and avoidance structures (including the bowel bag, bladder, external genitalia, femoral heads, pelvic bones, and intergluteal sulcus) were contoured. Data was sent to the tomotherapy planning station for treatment planning. The GTV, including the primary tumor and macroscopically-involved lymph nodes, was identified using examination, imaging, and endoscopy findings. Two CTVs were delineated: CTV1 and CTV2. CTV1 consisted of the GTV plus 5 mm expansion, while CTV2 included elective nodal stations (perirectal, internal iliac, external iliac, obturator, presacral, and inguinal nodes); an isotropic margin of 5 mm was added to generate Planning Tumor Volumes (PTV1 and PTV2). Treatment consisted of IMRT with SIB delivered by helical tomotherapy (6 MV). Dose prescription for target volumes varied according to the clinical disease stage: 50.6 Gy and 41.4 Gy in 23 fractions in T1N0, 52.8 Gy and 43.2 Gy in 24 fractions in T2N0, and 55 and 45 Gy in 25 fractions in all patients with N+ and/or ≥ T3 prescribed to PTV1 and PTV2, respectively. The plan was accepted if: (1) at least 97% of the volume was covered by 95% of the dose; (2) at least 99% of the volume was covered by 90% of the dose for both PTV1 and PTV2; (3) no more than 1% received more than 5% of the prescription dose for PTV1; and (4) no more than 5% received more than 5% of the prescription dose for PTV2. MVCT scans before each fraction were performed to verify patients’ positioning; image matching was executed using an automatic bone algorithm supervised by experienced medical staff. Normal tissue dose constraints are listed in Table 1.
| Organ | Constraints | |||
| Bladder | Median < 30 Gy | V45 < 35% | ||
| Bowel bag | Median < 20 Gy | V40 < 30% | V45 < 195 mL | |
| Femoral heads | V35 < 15% | V20 < 55% | ||
| Pelvic bones | V10 < 90% | V20 < 75% | V30 < 50% | V40 < 37% |
| External genitalia | Median < 15 Gy | |||
All patients were evaluated for chemotherapy (CHT) by a medical oncologist; most patients received concurrent chemotherapy with a 5-FU continuous venous infusion of 1000 mg/m2 over 96 h and mitomycin-C (MMC) of 10 mg/m2 during the first and last weeks of radiation therapy; in cases of contraindication to polychemotherapy, patients received capecitabine (825 mg/m2 bid/die) concomitant with RT, or underwent exclusive radiotherapy.
Toxicities were graded weekly during chemoradiation and in follow-up (every week in the first month after combined treatment and then at 8 wk post-treatment). Patients underwent rectal examination at 6-8 wk after the completion of radiotherapy course, and then at 3 mo from treatment they underwent pelvic MRI and rectoscopy. Most patients also underwent an endorectal ultrasound and, at 4 mo, a PET/CT was performed to evaluate metabolic response. In patients with complete remission, follow-up investigations were carried out at 3 mo intervals in the first year, and then every 6 mo for 5 years by MRI or endorectal ultrasound alternated with PET/CT. In cases of incomplete response, the clinical and radiological evaluation was repeated every 4 wk until complete remission was recorded[7]. In cases where there was evidence of progression or recurrence, surgery was recommended. Post-treatment biopsies were not routinely performed. Local regional recurrence was defined as recurrence of the disease within the pelvis. All failures were documented by a biopsy. Distant failure was defined as the development of the disease outside the pelvis or inguinal nodes. Acute and late adverse events were measured according to the Common Toxicity Criteria for Adverse Events scale v3.0 and RTOG criteria, respectively[9,10]
The primary endpoints of this study were disease-free survival (DFS) and local control rate (LC), while secondary end points were overall survival (OS), progression-free survival (PFS), and acute and late toxicity. Statistical analysis was performed with JMP v10.0 (SAS institute Inc. Cary, NC) and DFS, LC, and OS rates were calculated with Kaplan Meier non-parametric estimation from treatment start.
From March 2009 to March 2014 we treated 41 patients; their characteristics are summarized in Table 2. The median follow-up was 30 mo (range 12-71 mo); all but one patient had a follow-up of more than 12 mo. The stage of disease was: stage I in 2 patients, stage II in 13 patients, stage IIIA in 12 patients, and stage IIIB in 14 patients, respectively. Evaluation of p16 expression status was positive in 29/34 (85.3%) patients and not available in 6/41 patients; two patients were known to be HIV positive (4.9%).
| Age (yr) | |
| median | 63 |
| range | 32-84 |
| Gender | |
| Male | 3 (7.3) |
| Female | 38 (92.7) |
| Tumor stage | |
| T1 | 3 (7.3) |
| T2 | 19 (46.3) |
| T3 | 10 (24.4) |
| T4 | 9 (22) |
| Nodal stage | |
| N0 | 16 (39) |
| N1 | 18 (44) |
| N2 | 6 (14.6) |
| N3 | 1 (2.4) |
| Staging | |
| I | 2 (4.8) |
| II | 13 (31.8) |
| IIIA | 12 (29.3) |
| IIIB | 14 (34.1) |
| Smoke | |
| Yes | 18 (44) |
| No | 7 (17) |
| Unknown | 16 (39) |
| Histology | |
| SCC | 41 (100) |
| Chemotherapy | |
| MMC + 5-FU | 33 (80.7) |
| MMC + Capecitabine | 4 (9.7) |
| Capecitabine | 2 (4.8) |
| None | 2 (4.8) |
The prescription dose of PTV1 was 55 Gy in 29 patients, 52.8 Gy in 10 patients, and 50.6 Gy in 2 patients; the median dose of PTV1 and PTV2 was 55 Gy (range, 50.6-55 Gy) and 45 Gy (range, 41.4-45 Gy), respectively. All patients completed radiotherapy treatment with the exception of one who interrupted treatment, on his own accord, at 50.6 Gy of the planned 55 Gy and refused the second chemotherapy course. Treatment breaks due to toxicity occurred in 3 patients (7.3%; median 1 d - range 1-3 d) and due to other causes (i.e., holidays or a broken machine) in 27 patients (65.8%; median 1 d- range 1-5 d). The median OTT was 35 d (range 30-40 d). OARs optimization constraints were respected. Concomitant CHT was delivered in 39/41 patients, although 2 patients did not receive CHT for comorbidity. 3/39 patients did not complete chemotherapy courses as planned, mainly because of hematological toxicity (thrombocytopenia and anemia), and 1 patient presented cardiovascular disease (angina pectoris) after first CHT course.
All patients were evaluated for acute toxicity. Grade 2 (11 patients, of whom 10 had diarrhea and 1 had both nausea and diarrhea) and grade 3 (diarrhea) gastrointestinal toxicity was reported in 26.8% and 7.3% of patients, respectively. 29.4% of patients developed grade 2 pain and 24/41 (58.5%) had to be administered major analgesic therapy; only 4 patients were receiving analgesic therapy before treatment start. Grade 3 skin toxicity occurred in 2 patients. 2 T4 stage patients developed a recto-vaginal fistula during RT (at 33 Gy and 19.3 Gy, respectively); one healed on its own after 7 mo from CHT/RT and the other underwent temporary colostomy 2 mo after treatment end. Late toxicity was assessed in 40/41 (97.5%) patients. No patient received a colostomy due to stricture. One patient died due to chemotherapy-related toxicity with pancytopenia and sepsis one week after treatment end. Acute and late toxicities are shown in Tables 3 and 4.
| G0 | G1 | G2 | G3 | G4 | G5 | |
| Gu | 26 (63.4) | 11 (26.8) | 4 (9.8) | / | / | / |
| Skin | 0 (0) | 10 (24.4) | 29 (70.7) | 2 (4.9) | / | / |
| Gi | 10 (24.4) | 17 (41.5) | 11 (26.8) | 3 (7.3) | / | / |
| Pain | 7 (17) | 22 (53.6) | 12 (29.4) | / | / | / |
| Hematological | 31 (75.6) | 4 (9.8) | 3 (7.3) | 2 (4.9) | / | 1 (2.4) |
| G0 | G1 | G2 | G3 | |
| GU (dysuria) | 38 | 0 | 1 (3.8) | 1 (3.8) |
| GI (incontinence) | 29 | 6 (23) | 4 (15.4) | 1 |
| Female genital tract (atrophy of vaginal mucosa) | 37 | 3 (11.5) | 0 | 0 |
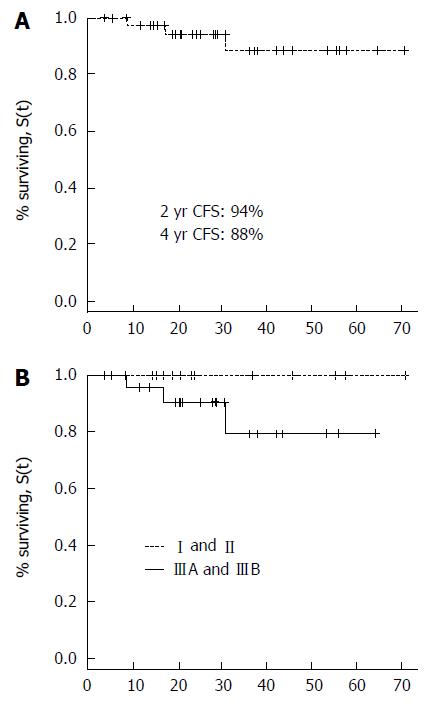
40/41 patients were evaluable, as 1 patient died one week after treatment end. The first imaging evaluation showed complete response (CR) in 25/40 (62%) patients and a partial response (PR) in 15/40 (38%) patients. At 6 mo, 11 of the 15 partial responders had achieved CR. Among the 4 patients with residual tumors shown radiologically at 6 mo, 2 underwent biopsy and were confirmed to have loco-regional persistence, 1 presented systemic and local progression, and 1 had systemic progression; at the time of writing, 2 of said patients are alive and undergoing chemotherapy, while the other 2 patients (both T4 stage) died at 28 and 24 mo from treatment end, respectively. During follow-up, 5/36 patients in complete response presented with disease progression. 1 patient had local recurrence 11 mo after treatment end, but became disease-free after undergoing abdominal-perineal resection. One patient developed lung metastasis after 18 mo, but was treated with SBRT (48 Gy/4 fx - 12 Gy/fraction) and has currently obtained a metabolic complete response. Three patients developed metastatic disease, namely liver metastasis (2 patients) and bone metastasis (1 patient), but after undergoing second-line chemotherapy they presented with stable disease at the last follow-up. Among the 9 patients that presented with an event (persistent or progressive disease) 6 were p16 positive and 3 were p16 negative. The PFS and OS in HPV-positive patients were 78% and 92% at 4 years, respectively. The DFS, CFS, LC, and OS were 78.3%, 94%, 92%, and 93% at 2 years, respectively (Figures 1 and 2).
Over the past 30 years, concurrent FU/MMC-based chemoradiotherapy has been the standard of care in anal carcinoma. This approach enables sphincter preservation in most patients without compromising cure rates. However, CHT/RT is associated with significant acute gastrointestinal, genitourinary, dermatological, and hematological toxicities when conventional radiation therapy techniques are used. As a consequence, prolonged treatment breaks are usually necessary and have been shown to negatively affect local control[11], as highlighted by several studies (v RTOG 9208)[12]. Other trials have demonstrated that IMRT may decrease the incidence of acute toxicity rates, with comparable outcomes to previous clinical trials. The RTOG 0529 trial[13], a phase II study, demonstrated a reduction in grade 3 gastrointestinal, grade 3 dermatologic, and grade 2 hematologic toxicities using IMRT when compared with 3D-CRT. Moreover, RTOG 0529 is the only phase II prospective trial, to our knowledge, in which RT is delivered with a simultaneous integrated boost (SIB)[13].
In our study we used an IMRT-SIB technique, intensifying treatment with a modest accelerated hypofractionated schedule. This schedule allows us to shorten the overall radiotherapy treatment time; our median OTT was 35 d vs the 43 and 49 d reported in RTOG 0529 and RTOG 98-11, respectively; furthermore, we reported very few breaks due to toxicity (7.3% vs 49% when compared with the RTOG 0529 trial rate). This data appears very interesting, and may be explained by the fact that major toxicities are likely to occur when the radiotherapy course is concluded, and thus they do not affect a treatment course that could be administered more easily. We reported a relatively high rate of patients (58.5%) that received major analgesics during treatment, even if they did not encounter grade 3 pain; this is probably due to the fact that patients who presented with basal or breakthrough pain (e.g., during defecation) were well-controlled with minor analgesics or major analgesics, respectively. The acute toxicity rate was comparable with what has been reported in the literature (Table 5).
| Ref. | No. pts | SIB | d/fx | CHT | Gastrointestinal | Genitourinary | Skin | Hematologic | |||||
| G2 | G3 | G2 | G3 | G2 | G3 | G2 | G3 | G4 | |||||
| Pepek et al[26], 2010 | 29 | No | 1.8 | 89% | 76% | 10% | 45% | 3% | 100% | 0 | 45% | 24% | |
| Bazan et al[24], 2010 | 29 | Yes | 1.6-1.8 | 86% | \ | 7% | \ | \ | \ | 21% | \ | 21% | |
| Vieillot et al[28], 2012 | 72 | No | 1.8-2.0 | 85% | 14% | 4% | 4% | 2% | 16% | 16% | 4% | 5% | 4% |
| DeFoe et al[11], 2012 | 78 | No | 1.8 | 98% | 60% | 27.7% | 18.5% | 0 | 91.3% | 29% | 51.4% | 42.9% | 12.9% |
| Kachnic et al[5], 2012 | 43 | Yes | 1.5-1.8 | 100% | 42% | 7% | 5% | 5% | 63% | 5% | 21% | 49% | 12% |
| Kachnic et al[13], 2012 | 52 | Yes | 1.5-1.8 | 100% | 52% | 21% | 13.4% | 1.9% | 52% | 21% | 15.4% | 30% | 27% |
| Chuong et al[29], 2013 | 52 | Yes | 1.8-2.0 | 100% | 39.5% | 9.6% | 26.9% | 0 | 57.7% | 11.5% | 37.8% | 28.8% | |
| Koerber et al[30], 2014 | 68 | Yes | 1.8-2.2 | \ | 47.1% | 19.1% | 63.2% | \ | \ | \ | |||
| Current study | 41 | Yes | 1.8-2.2 | 95% | 26.8% | 7.3% | 9.8% | 0 | 70.7% | 4.9% | 7.3% | 4.9% | \ |
With regards to mid-term late toxicity, no grade 4 late toxicity was observed and no patient underwent surgery for fecal incontinence or stricture. According to these preliminary results, we believe that severe late effects should not affect patients, even if an intensified schedule of radiotherapy with a single SIB dose of 2.2 Gy is adopted in a volume that includes the anal sphincter and muscles involved in anal function. We also have to consider that the analysis of treatment-related toxicity is commonly difficult after radio-chemotherapy for anal cancer. Late effects were not specifically reported in any trial, since the current RTOG late effects instruments are not sufficiently specific, and the fact that quite a few patients appear to adapt even to an impaired function of pelvic organs should be taken into consideration.
An important aspect to analyze is the possibility that the use of a SIB technique allows for the delivery of different doses to different volumes, therefore increasing the dose to GTV. Although the role of dose escalation is still controversial in anal cancer, multivariate analysis of data from the RTOG 98-11 trial showed that positive lymph nodes and a tumor size greater than 5 cm were independent prognostic factors for poor OS; tumor diameter could also be prognostic for colostomy rate and time to colostomy[14]. Similarly, recent multivariate analysis of data from the ACT1 trial also showed that positive lymph nodes are a prognostic indicator for higher local regional failure, anal cancer death, and lower OS[15]. The final analysis of the UNICANCER ACCORD 03 trial did not demonstrate a benefit from high dose radiotherapy, despite a reported statistically insignificant small increase in CFS and LC that lead to further investigation on the role of dosage escalation[16]. It should also be considered that the radiation boost in the ACCORD 03 trial was delivered 3 wk after treatment end, and this gap could have contributed to a reduced possible positive effect of high dose radiotherapy. On the basis of this data and considering that local relapse remains the main site of failure, especially in locally advanced disease, a dose escalation might be reasonable.
In our study, 83% of patients were HPV p16 positive and higher disease control was expected for these patients. Our results for this subgroup of patients are excellent (OS and PFS were 92% and 78% at 4 years, respectively) and comparable with that reported in the literature[17]. A preliminary analysis on HPV-positive vs HPV-negative patients seems to show a trend in favor of the first group, even if the sample number is too small. Other studies have reported that HPV-negative tumors were linked to a poor prognosis[17-19]. Another consideration in our study is that we detected p16 expression by immunohistochemistry, but HPV DNA testing was not performed. In the literature, there are reports that p16 is not a perfect surrogate marker for tumor HPV status, as its specificity might be insufficient and the use of HPV DNA could still be required if patients were to be stratified based on HPV status[19]; this aspect is particularly interesting and has also been demonstrated in diseases other than anal cancer. In oropharyngeal tumors, it is possible to identify a subgroup of patients who are p16 positive but HPV DNA negative, and whose survival is significantly different compared with p16+ and HPV DNA positive patients; the survival curve of this group converged on the survival curve of HPV-negative patients[20]. Based on these observations, it is possible that we registered some HPV false positive tumors, which likely represents another limitation of our study.
In the past few years, there appears to be an increase in the incidence of anal canal cancer, which is probably related to HPV infection. We treated 41 patients at a single center with a relatively short, median follow-up (30 mo), and analyzed them retrospectively. These factors represent the main limitation of this study, but the current literature refer to small groups of patients (18-36 patients) with a short follow up (14-32 mo)[21-27]. Our study therefore aligns with the current knowledge, and our preliminary results in terms of LC and OS are encouraging and in line with those reported in the literature (Table 6).
Our study is distinct from others in terms of radiotherapy dose and fractionation. Further investigations on HPV status are necessary in order to understand if different schedules of radiotherapy (intensified or not) should be delivered according to viral related biology of anal cancer. Our next project is to isolate HPV DNA in patients in order to better investigate this aspect and clarify the crucial aspects of anal function in surviving patients by periodically administering questionnaires on the quality of life for at least 6 mo after treatment end.
Despite different strategies being adopted to minimize local recurrence, it remains a major issue in anal canal cancer. Recently, the importance of human papilloma virus (HPV) has been proven as a predictor of prognosis.
HPV could be used for individualization of treatment. Moreover the introduction of intensity-modulated radiotherapy and integrated boost (SIB-IMRT) technique allows for the reduction of toxicity-related treatments.
SIB allows for the easy modification of the radiotherapy schedule, thereby increasing the effective biological dose without increasing overall treatment time. This aspect could be particularly interesting in HPV-negative patients.
The results of the study show the excellent outcomes in HPV-positive patients and highlight the high tolerance of a moderately-accelerated SIB-IMRT radiotherapy schedule.
IMRT is a radiotherapy technique that allows for the obtaining of better target coverage, better dose distribution, and a reduction of the dose received by normal tissue compared to the previous technique of 3D conformal radiotherapy. SIB also allowed the treatment of different volumes at different doses, as well as allowing an increase of the dose prescribed to the target safely.
This is a good study in which the authors evaluated the prognostic significance of HPV status and the feasibility and outcomes of accelerated intensity-modulated radiotherapy in anal canal cancer patients. The study is well structured and the subject is clear and interesting. The manuscript is correctly written and the conclusions are justified by the results found in the study.
P- Reviewer: Francois E, Ong JJ, Zilli T S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9215] [Cited by in F6Publishing: 9719] [Article Influence: 883.5] [Reference Citation Analysis (3)] |
| 2. | Northover J, Glynne-Jones R, Sebag-Montefiore D, James R, Meadows H, Wan S, Jitlal M, Ledermann J. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer. 2010;102:1123-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 269] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 3. | Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, Thomas CR, Mayer RJ, Haddock MG, Rich TA, Willett C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914-1921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 585] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 4. | Gunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB, Thomas CR, Mayer RJ, Haddock MG, Rich TA. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344-4351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 5. | Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, Willins JD, Ryan DP, Hong TS. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys. 2012;82:153-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Stromberger C, Jann D, Becker ET, Raguse JD, Tinhofer I, Marnitz S, Budach V. Adjuvant simultaneous integrated boost IMRT for patients with intermediate- and high-risk head and neck cancer: outcome, toxicities and patterns of failure. Oral Oncol. 2014;50:1114-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Yi J, Huang X, Gao L, Luo J, Zhang S, Wang K, Qu Y, Xiao J, Xu G. Intensity-modulated radiotherapy with simultaneous integrated boost for locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol. 2014;9:56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Herrera FG, Callaway S, Delikgoz-Soykut E, Coskun M, Porta L, Meuwly JY, Soares-Rodrigues J, Heym L, Moeckli R, Ozsahin M. Retrospective feasibility study of simultaneous integrated boost in cervical cancer using Tomotherapy: the impact of organ motion and tumor regression. Radiat Oncol. 2013;8:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Cancer therapy evaluation program. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Cited in This Article: ] |
| 10. | Radiation therapy oncology group. Available from: http://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityScoringSchema.aspx. [Cited in This Article: ] |
| 11. | DeFoe SG, Beriwal S, Jones H, Rakfal S, Heron DE, Kabolizadeh P, Smith RP, Lalonde R. Concurrent chemotherapy and intensity-modulated radiation therapy for anal carcinoma--clinical outcomes in a large National Cancer Institute-designated integrated cancer centre network. Clin Oncol (R Coll Radiol). 2012;24:424-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Konski A, Garcia M, John M, Krieg R, Pinover W, Myerson R, Willett C. Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92-08. Int J Radiat Oncol Biol Phys. 2008;72:114-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran H. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 424] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 14. | Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, Thomas CR, Mayer RJ, Haddock MG, Rich TA, Willett CG. US intergroup anal carcinoma trial: tumor diameter predicts for colostomy. J Clin Oncol. 2009;27:1116-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Glynne-Jones R, Sebag-Montefiore D, Adams R, Gollins S, Harrison M, Meadows HM, Jitlal M; United Kingdom Coordinating Committee on Cancer Research Anal Cancer Trial Working Party. Prognostic factors for recurrence and survival in anal cancer: generating hypotheses from the mature outcomes of the first United Kingdom Coordinating Committee on Cancer Research Anal Cancer Trial (ACT I). Cancer. 2013;119:748-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Peiffert D, Tournier-Rangeard L, Gérard JP, Lemanski C, François E, Giovannini M, Cvitkovic F, Mirabel X, Bouché O, Luporsi E. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30:1941-1948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 17. | Yhim HY, Lee NR, Song EK, Kwak JY, Lee ST, Kim JH, Kim JS, Park HS, Chung IJ, Shim HJ. The prognostic significance of tumor human papillomavirus status for patients with anal squamous cell carcinoma treated with combined chemoradiotherapy. Int J Cancer. 2011;129:1752-1760. [PubMed] [Cited in This Article: ] |
| 18. | Ravenda PS, Magni E, Botteri E, Manzotti M, Barberis M, Vacirca D, Trovato CM, Dell’Acqua V, Leonardi MC, Sideri M. Prognostic value of human papillomavirus in anal squamous cell carcinoma. Cancer Chemother Pharmacol. 2014;74:1033-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Meulendijks D, Tomasoa NB, Dewit L, Smits PH, Bakker R, van Velthuysen ML, Rosenberg EH, Beijnen JH, Schellens JH, Cats A. HPV-negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br J Cancer. 2015;112:1358-1366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Rietbergen MM, Brakenhoff RH, Bloemena E, Witte BI, Snijders PJ, Heideman DA, Boon D, Koljenovic S, Baatenburg-de Jong RJ, Leemans CR. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Ann Oncol. 2013;24:2740-2745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Deenen MJ, Dewit L, Boot H, Beijnen JH, Schellens JH, Cats A. Simultaneous integrated boost-intensity modulated radiation therapy with concomitant capecitabine and mitomycin C for locally advanced anal carcinoma: a phase 1 study. Int J Radiat Oncol Biol Phys. 2013;85:e201-e207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Tozzi A, Cozzi L, Iftode C, Ascolese A, Campisi MC, Clerici E, Comito T, De Rose F, Fogliata A, Franzese C. Radiation therapy of anal canal cancer: from conformal therapy to volumetric modulated arc therapy. BMC Cancer. 2014;14:833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Janssen S, Glanzmann C, Bauerfeind P, Stieb S, Studer G, Brown M, Riesterer O. Clinical experience of SIB-IMRT in anal cancer and selective literature review. Radiat Oncol. 2014;9:199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Bazan JG, Hara W, Hsu A, Kunz PA, Ford J, Fisher GA, Welton ML, Shelton A, Kapp DS, Koong AC. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer. 2011;117:3342-3351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Kim KH, Chang JS, Keum KC, Ahn JB, Lee CG, Koom WS. Chemoradiotherapy in squamous cell carcinoma of the anal canal: a single institution experience. Radiat Oncol J. 2013;31:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Pepek JM, Willett CG, Wu QJ, Yoo S, Clough RW, Czito BG. Intensity-modulated radiation therapy for anal malignancies: a preliminary toxicity and disease outcomes analysis. Int J Radiat Oncol Biol Phys. 2010;78:1413-1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Bentzen AG, Guren MG, Vonen B, Wanderås EH, Frykholm G, Wilsgaard T, Dahl O, Balteskard L. Faecal incontinence after chemoradiotherapy in anal cancer survivors: long-term results of a national cohort. Radiother Oncol. 2013;108:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Vieillot S, Fenoglietto P, Lemanski C, Moscardo CL, Gourgou S, Dubois JB, Aillères N, Azria D. IMRT for locally advanced anal cancer: clinical experience of the Montpellier Cancer Center. Radiat Oncol. 2012;7:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Chuong MD, Freilich JM, Hoffe SE, Fulp W, Weber JM, Almhanna K, Dinwoodie W, Rao N, Meredith KL, Shridhar R. Intensity-Modulated Radiation Therapy vs. 3D Conformal Radiation Therapy for Squamous Cell Carcinoma of the Anal Canal. Gastrointest Cancer Res. 2013;6:39-45. [PubMed] [Cited in This Article: ] |
| 30. | Koerber SA, Slynko A, Haefner MF, Krug D, Schoneweg C, Kessel K, Kopp-Schneider A, Herfarth K, Debus J, Sterzing F. Efficacy and toxicity of chemoradiation in patients with anal cancer--a retrospective analysis. Radiat Oncol. 2014;9:113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Dasgupta T, Rothenstein D, Chou JF, Zhang Z, Wright JL, Saltz LB, Temple LK, Paty PB, Weiser MR, Guillem JG. Intensity-modulated radiotherapy vs. conventional radiotherapy in the treatment of anal squamous cell carcinoma: a propensity score analysis. Radiother Oncol. 2013;107:189-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Mitchell MP, Abboud M, Eng C, Beddar AS, Krishnan S, Delclos ME, Crane CH, Das P. Intensity-modulated radiation therapy with concurrent chemotherapy for anal cancer: outcomes and toxicity. Am J Clin Oncol. 2014;37:461-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |