Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7805
Peer-review started: December 21, 2014
First decision: January 13, 2015
Revised: March 13, 2015
Accepted: April 3, 2015
Article in press: April 3, 2015
Published online: July 7, 2015
AIM: To investigate whether regional geography influences ethnic and gender trends for the development of gastric cancer (GC).
METHODS: This retrospective analysis of the INVISION patient database at Louisiana State University Health Sciences Center-Shreveport (LSUHSC-S), a southern United States regional hospital, was performed from 2005-2011. Using the international statistical classification of diseases 9 (ICD-9), inpatient, day surgery outpatient, and emergency outpatient diagnosis codes entered into medical records were used to identify GC patients. For each study year, the patients were evaluated for age, ethnicity, and gender, and each patient was counted only once throughout the study. Subsequent patient encounters were counted as visits and separated by inpatient and clinic visits. Complex or severe disease may require more frequent and intensive clinical management; therefore, we evaluated annual clinic visits as “surrogate markers” of disease severity. Finally, we studied the primary diagnosis for Helicobacter pylori (H. pylori) infection (ICD-9 code 41.86) as an additional factor that might increase the risk of GC.
RESULTS: A total of 285 patients were diagnosed with GC at LSUHSC-S between 2005 and 2011. African Americans (181 patients, 89 males and 92 females, 63.5% of total patients) had significantly higher frequencies of GC diagnosis compared with non-Hispanic whites (104 patients, 54 males and 50 females, 36.5% of total patients), at a ratio of 1.74 (P = 0.002). Within each ethnic group, men and women were diagnosed at approximately equal annual rates. Our findings differed significantly from United States national trends, which found that African American females and white females had lower risks for GC than their corresponding male counterparts. The United States national trend between 2005 and 2011 showed that African Americans males had a higher incidence of GC, with an annual mean (per 100000) of 16.31 ± 0.76 compared with white males (9 ± 0.1, P < 0.001), African American females (8.7 ± 0.34, P < 0.001) and white females (4.05 ± 0.07, P < 0.001). Among the GC patients, the number of clinic visits was highest among African American males (195.1 ± 28.1), who had significantly more clinic visits than African Americans females (123 ± 13.02, P < 0.05), white males (41.57 ± 4.74, P < 0.001) and white females (35 ± 8.9, P < 0.001). Similar trends were found for inpatient visits, with an annual mean of 11.43 ± 1.5 for African American males, followed by African American females (7.29 ± 1.36), white males (2.57 ± 0.69) and white females (1.57 ± 0.612). African American males had significantly more inpatient visits than white males (P < 0.001), and African American females had more inpatient visits than white females (P < 0.01). African American patients showed the highest frequency of H. pylori positive status, with approximately 72% vs 28% for the white patients.
CONCLUSION: Increase in GC diagnoses among women at LSUHSC-S is significantly higher than United States national averages, suggesting local geographic and socioeconomic influences may alter GC disease course.
Core tip: Gastric cancer (GC) remains a leading cause of morbidity and mortality. Nationally, AAs reportedly develop GC at twice the rate of Caucasians. Male gender is a significant risk factor for GC development in the United States with a nearly 2:1 male to female dominance. However, at Louisiana State University Health Sciences Center-Shreveport, the annual rates of GC diagnosis among women in either ethnic grouping were statistically indistinguishable from that of their male counterparts. This result indicates that regional geography and socioeconomic factors may contribute to the ethnicity and gender differences observed in patients with GC. Therefore, additional GC surveillance for women, particularly African American females, may improve patient outcomes.
- Citation: Suryawala K, Soliman D, Mutyala M, Nageeb S, Boktor M, Seth A, Aravantagi A, Sheth A, Morris J, Jordan P, Manas K, Cvek U, Trutschl M, Becker F, Alexander J. Gastric cancer in women: A regional health-center seven year retrospective study. World J Gastroenterol 2015; 21(25): 7805-7813
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7805.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7805
Over the past 10 years, there have been significant declines in the incidence and mortality of gastric cancer (GC) in the United States, most likely due to earlier detection and treatment of Helicobacter pylori (H. pylori) infection, as well as decreased consumption of smoked foods[1,2]. However, GC remains a leading cause of morbidity and mortality worldwide; it is ranked as the fourth most common type of cancer and the second most common cause of cancer-related deaths globally[3,4]. The highest incidence rates of GC are now reported in Japan and developing regions of China, the Middle East, Central America and South America[5]. Despite the observed decline in GC incidence in the United States, it is estimated that in 2014, there will be 22220 new cases of gastric malignancy, with 13730 (61.8%) of those cases in males and 8490 (38.2%) in females. The estimated number of total deaths among males and females will be 10990, with 6720 (61.1%) in males and 4270 (38.9%) in females[6].
Numerous risk factors have been implicated in the development of GC, including H. pylori infection, obesity, smoking, diet, atrophic gastritis, ethnicity, gender and age[7-12]. Recent studies have indicated that male gender is the most significant risk factor for the development of gastric malignancy in the United States, with a nearly 2:1 male to female dominance[3,13]. Therefore, male gender appears to represent a major predictor of GC. Additionally, in a study of the survival rates of metastatic GC patients, Yang et al[14] showed that male patients had lower survival rates compared to female patients.
In terms of ethnicity, African Americans (AAs) reportedly develop GC at twice the rate of Caucasians[14]. AAs also had 2 - 6 × greater seropositivity for eight H. pylori infection markers, including the cytotoxin-associated gene A (CagA) and Vacuolating cytotoxin A (VacA) virulence factors, suggesting greater symptomatology during H. pylori infection[15]. These observations show that ethnicity, gender, and H. pylori infection status may cooperate in increasing the risk of GC.
However, studies on ethnicity and gender influences on epidemiology can be confounded by uneven sampling, as well as geographic and socioeconomic factors. For example, in a previous study at LSUHSC-S on inflammatory bowel diseases (IBD), we reported that AAs were diagnosed with IBD at nearly equivalent annual rates as whites; these values exceeded national averages[16]. Because AAs compose 54.7% of the population in the city of Shreveport and 38.9% of the population in the Shreveport-Bossier Metropolitan area, compared to only 13.2% of the national population (as of 2013), studies on racial contributions to disease onset and progression at LSUHSC-S may reveal important trends that are not readily observed in large, homogeneous demographic studies[17,18]. Therefore, to better evaluate racial and gender influences on GC diagnoses in a patient population with an equivalent representation of AAs and whites, from 2005 to 2011, this study examined annual GC diagnoses as a function of gender, ethnicity, age and H. pylori infection status at LSUHSC-S, a tertiary care public health hospital. Lastly, clinic and inpatient were evaluated as possible “surrogate markers” of disease severity[18].
We found that at LSUHSC-S, the annual GC diagnosis rates among women in either racial group (non-Hispanic Caucasians and African-American) were statistically indistinguishable from those of their male counterparts (in each respective ethnic group), unlike United States national averages (i.e., males in each ethnic group had a significantly higher incidence of GC). Therefore, regional geography and socioeconomic factors may contribute to the ethnicity and gender differences observed in GC, and additional GC surveillance for women, particularly African American females, may improve their outcomes.
This retrospective analysis included de-identified patients in the INVISION database at LSUHSC-S from 2005 to 2011. Inpatient, day-surgery outpatient, and emergency outpatient diagnostic codes were extracted from medical records, and all codes represented conclusive diagnoses. Individuals with a primary diagnosis of GC were selected using the international statistical classification of diseases 9 (ICD-9) code 151.0-151.9, encoding for malignant neoplasm of the stomach at an unspecified site. H. pylori infected individuals were identified using the ICD-9 code 41.86 [primary diagnosis code for H. pylori infection, diagnosed by tissue biopsies with subsequent testing for Campylobacter-like organism (CLO), immunohistochemical detection, or urea breath, stool, urine, saliva or serum antigen testing]. For each study year, de-identified patient ages, ethnicity, and gender were evaluated, and each patient was counted once in the study. Subsequent individual de-identified patient encounters were counted as visits and were further separated by hospital visits and clinic visits. This study identified 285 patients (Table 1) and evaluated the following factors: (1) the number of new GC cases diagnosed in each ethnic [AAs and non-Hispanic Whites (Ws)] and gender [female (F)] and male (M)] grouping each year; (2) the number of annual clinic visits per group; (3) the number of annual hospitalizations per group; and (4) the annual numbers of H. pylori diagnoses for each group. Patient gender and ethnicity were self-identified. According to the United State Census Bureau report from 2010, Hispanics account for 4.7% of the total Louisianan population, however, they account for only for 2.5% of the total Shreveport population (vs 41.2% Ws, 54.7% AAs and 1.3% Asians in Shreveport). From the 3 million patients investigated at LSUHSC-S over the seven-year study period, only 2.5% were races other than AAs and Ws, which allowed us to identify only 3 Hispanic patients who matched our previously described inclusion criteria for GC (see above). Therefore, we excluded all 3 Hispanic patients diagnosed with gastric cancer from our study protocol because it was not possible to accurately compare them to Ws and AAs.
| Year/group | WMs | WFs | AAMs | AAFs | Total |
| 2005 | 5 | 10 | 10 | 16 | 41 |
| 2006 | 5 | 11 | 12 | 10 | 37 |
| 2007 | 5 | 3 | 9 | 10 | 27 |
| 2008 | 9 | 7 | 13 | 13 | 42 |
| 2009 | 8 | 8 | 19 | 12 | 47 |
| 2010 | 15 | 4 | 14 | 15 | 48 |
| 2011 | 7 | 7 | 12 | 16 | 42 |
| Total | 54 | 50 | 89 | 92 | 284 |
Statistical group analysis (see biostatistics statement) comparisons were performed using Instat TM software GraphPad 3.06 (GraphPad Software Inc., La Jolla, CA, United States). All comparisons were performed using one-way ANOVAs, with Tukey-Kramer Multiple comparisons test or Student-Newman-Keuls multiple comparisons test (H. pylori diagnosis). Comparisons were considered statistically significant at P < 0.05. All statistical data are expressed as the mean ± SE.
The statistical methods of this study were reviewed by Urska Cvek, Sc.D., MBA, Professor of Computer Science, Director, Laboratory for Advanced Biomed. Inf. and Marjan Trutschl, Sc.D., Abe Sadoff Distinguished Chair in Bioinformatics, Director, Laboratory for Advanced Biomed. Inf. Professor of Computer Science.
Patient data were collected to calculate the annual number of GC cases diagnosed in different population groups. During the study period (2005 to 2011), we identified 285 patients who were diagnosed with GC at LSUHSC-S. We found a total of 181 AA (63.5% of the total number of patients, 89 males and 92 females) and 104 W (36.5% of total patients, 54 males and 50 females) patients who were newly diagnosed with GC. While there was a significant difference between AA and W ethnicities in terms of the annual number of individuals diagnosed with GC, gender did not influence the frequency of GC diagnoses within the racial groups. That is, women in both racial groups were diagnosed at approximately equal proportions as their male counterparts.
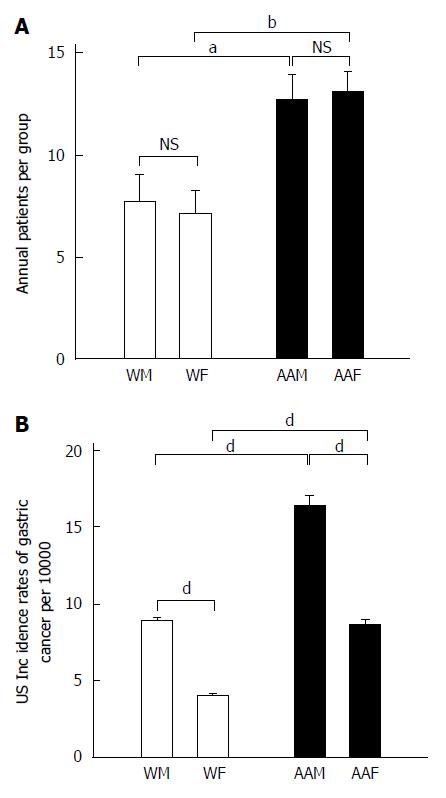
As a group, AAs had significantly more annual diagnoses of GC than whites (P < 0.0002), a ratio of 1:1.74 (Ws:AAs). AAMs and African American females (AAFs) at LSUHSC-S had nearly equal annual proportions of GC (Figure 1A). AAFs had 13.14 ± 0.99 annual GC diagnoses, which was not significantly different from AAMs, who had 12.71 ± 1.23 annual diagnoses. AAFs had significantly higher annual rates of GC diagnoses than either WMs (7.71 ± 1.39, P < 0.05) or WFs (7.14 ± 1.1, P < 0.01), who developed GC at equivalent rates.
We used the United States national trends for GC incidence from 2005 to 2011, as shown in (Figure 1B), as a scale for our results, which reflect diagnoses per 100000 individuals. The national GC incidence (2005 to 2011) was greater among AAMs (mean of 16.3 ± 0.76) than AAFs (8.67 ± 0.34, P < 0.001), WMs (9 ± 0.1, P < 0.001) and WFs (4.05 ± 0.07, P < 0.001). Furthermore, WMs had a higher GC incidence than WFs (P < 0.001). AAFs also showed a statistically significant higher proportion of GC diagnoses than WFs (P < 0.001).
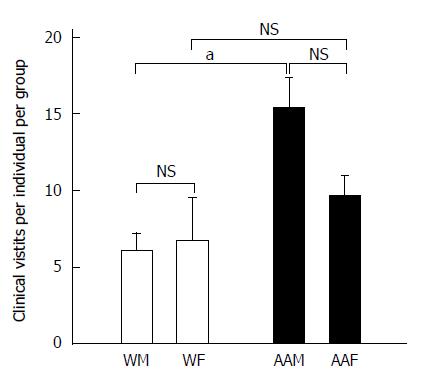
There were 2763 total clinic visits at LSUHSC-S for patients who received a primary GC diagnosis from 2005 to 2011. There were 181 AA patients, repre-senting 63.5% of the total number of patients studied, while 104 patients were Ws, representing the remaining 36.5% (Table 2). AAMs had the greatest number of clinic visits (annual mean of 195.1 ± 28.1), and they had signi-ficantly more clinic visits than AAFs (123 ± 13.02, P < 0.05), WMs (41.57 ± 4.74, P < 0.001) and WFs (35 ± 8.9, P < 0.001), corresponding to an average of 394.7 ± 38.2 visits annually. AAMs had a signifi-cantly greater number of annual clinic visits (15.36 ± 1.94) than WMs (6.12 ± 1.11, P < 0.05) or WFs (6.75 ± 2.8, P < 0.05) (Figure 2). Although not significant, AAMs also had more annual clinic visits per individual than AAFs (9.71 ± 1.27). Because AAMs had the highest number of annual clinic visits as a group (approximately 2.54 × more visits than WMs, 2.27 × more visits than WFs and 1.58 × more visits than AAFs), they may either seek or require more medical attention to manage their disease.
| Year/group | WMs | WFs | AAMs | AAFs | Total |
| 2005 | 53 | 10 | 60 | 108 | 231 |
| 2006 | 48 | 23 | 222 | 148 | 441 |
| 2007 | 22 | 67 | 181 | 106 | 376 |
| 2008 | 29 | 66 | 201 | 78 | 374 |
| 2009 | 51 | 41 | 298 | 148 | 538 |
| 2010 | 52 | 17 | 162 | 98 | 329 |
| 2011 | 36 | 21 | 242 | 175 | 474 |
| Total | 291 | 245 | 1366 | 861 | 2763 |
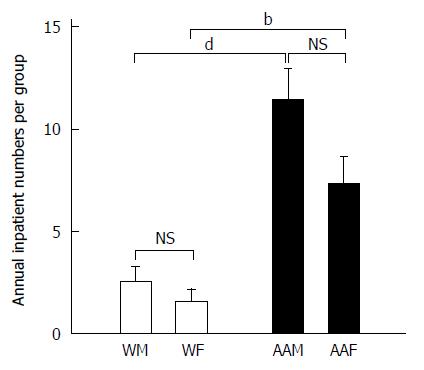
Similar trends were also found when examining inpatient visits for patients with a primary diagnosis of GC at LSUHSC-S between 2005 and 2011 (Figure 3). AAMs again had the highest number of annual inpatient visits (11.43 ± 1.5 visits per year), followed by AAFs, who had 7.29 ± 1.36 visits per year. WMs averaged 2.57 ± 0.69 visits per year, and WFs averaged 1.57± 0.612 visits per year. AAMs were again noted to have statistically more inpatient visits compared to WMs and WFs (both P < 0.001), however, there was no statistical significance between AAMs and AAFs. These findings in AAMs may indicate a more complex course of management that requires more frequent hospitalization.
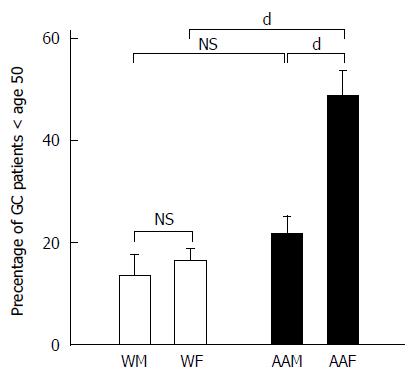
In addition to evaluating the influence of ethnicity and gender on GC, we also studied the influence of age on the frequency with which GC is diagnosed. We divided GC patients into individuals younger than 50 years of age (Figure 4) and individuals 50 years of age or older at the time of diagnosis. Although the average age of diagnosis for gastric cancer is 69, we divided the age groups at 50 years because of variations in trends that were observed. During the 7-year study period, among the 285 patients studied, 71 patients were younger than 50 years of age, and 214 patients were 50 years of age or older. The annual mean percentages of patients younger than 50 years of age diagnosed with GC were 48.46% ± 5.2% for AAFs, 21.6% ± 3.6% for AAMs, 16.44% ± 2.5% for WFs and 13.48% ± 4.2% for WMs. For the patients younger than 50 years of age, AAFs had significantly more GC diagnoses than AAMs (P < 0.001), WFs (P < 0.001) and WMs (P < 0.001). Our data revealed that AAFs showed an apparent earlier onset of GC compared to other groups. In contrast, the annual mean percentages for GC patients (primarily diagnosed at 50 years of age and older) were comparable between genders within each ethnic group, with 35.1% ± 3.1% for AAMs, 27.35% ± 2.2% for AAFs, 17.8% ± 3.34% for WFs and 19.8% ± 3.5% for WMs. AAMs had significantly more GC diagnoses than both WMs (P < 0.01) and WFs (P < 0.01).
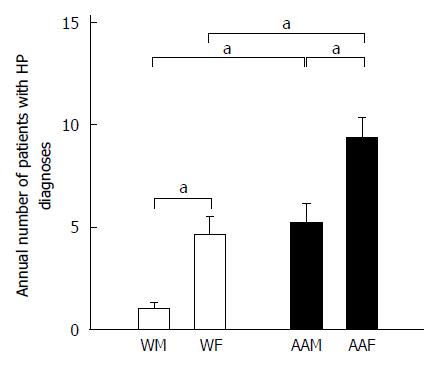
We also used the primary diagnosis of H. pylori infection as a “surrogate marker” of additional risk for GC (Figure 5). Strikingly, AAFs had significantly more H. pylori infection primary diagnoses (65) compared to other groups (36 for AAMs, 32 for WFs, and 7 for WMs). AAFs had more annual H. pylori infection diagnoses (9.29 ± 1) than both AAMs (5.14 ± 1.01, P < 0.05) and WFs (4.6 ± 0.9, P < 0.05). WMs had only 1 ± 0.31 H. pylori infections diagnoses annually, which were significantly fewer, compared to AAMs (P < 0.05). Therefore, because H. pylori infection is known to be a risk factor for GC, it is possible that AAFs have some increased risk because of their higher proportion of H. pylori infection diagnoses compared with other groups. It is unclear whether the relatively higher proportion of H. pylori diagnoses in WFs relative to WMs may help to explain the higher apparent increased risk of GC diagnosis in WFs in our study.
In this retrospective study of GC patients at LSUHSC-S, there was a significant difference in the number of GC diagnoses between African American and white patients. Accordingly Horner et al[19] reported that in the United States, AAs develop GC at approximately twice the rate of white population. Previous studies have identified numerous risk factors, which might be involved in the increased incidence of gastric malignancy in AA populations, including heritable risk, as well as socioeconomic status, dietary and smoking habits, access to health care, local environmental and geographic influences, and regional H. pylori infection epidemiology[20,21]. Our results parallel reported United States national trends in terms of the overall influence of ethnicity, and they appear to be consistent with AA ancestry as a significant risk for the development of GC. However, when ethnicity is considered as a risk factor for the development of GC, it is absolutely important and necessary to state that the observed trends in AAs and Ws are more likely to reflect socioeconomic disproportions (imbalances in work, wealth, income, education, housing and standard of living) rather than biological differences associated with the respective ethnicity. Although recent evidence in the state of Louisiana has linked AA related polymorphisms in the IL-1b gene (particularly the IL1B+3954T allele) with pre-cancerous atrophic gastritis and advanced gastric premalignant lesions, their roles in the epidemiology of GC are only now emerging, and they may still not fully explain the substantial differences in incidences among different ethnic groups within our region compared to United States national trends[22,23]. In comparison, socioeconomic factors are well established in the development of GC and are extensively considered in the following discussion. As described above, additional factors that are believed to be more relevant than the respective ethnic group are barriers to sufficient cancer prevention, early detection, and treatment services, all of which are most likely to significantly impact the reported data in our study.
In the United States, AAs still have significantly lower incomes compared to whites, and socioeconomic disparities remain an important potential factor contributing to their higher proportions of observed GC diagnoses[24]. Low socioeconomic status may also represent an important obstacle towards seeking medical care for AAs. The affordable CARE ACT of 2014 may improve public access to healthcare in the future. However, our study is less likely to be influenced by this bias, as LSUHSC-S provides equal access to healthcare regardless of the ability to pay.
A meta-analysis by Wang et al[2] showed that H. pylori infection is strongly associated with early GC. Although, the prevalence of H. pylori infection in the United States is approximately 30%, AA populations have a higher prevalence that approaches 50%-60%[25]. Interestingly, AAFs accounted for 48% of the total H. pylori infection-diagnosed patients compared to 24% for AAMs, 23% for WFs and 5% for WMs. AAFs had statistically higher proportions of H. pylori infection diagnoses than AAMs (P < 0.05) or WFs (P < 0.05). Similarly, Graham and Malaty found that the prevalence of H. pylori infection was higher in AAs (70%) compared to Ws (34%)[21]. This percentage did not change after adjusting the data for gender, and it remained closely correlated with low socioeconomic standards[20]. Similarly, in our study, AAs represented 69.6% ± 6.1% of the total H. pylori infected individuals compared to 26.3% ± 6.1% for whites. Between 2005 and 2011, AAFs had the highest proportion of H. pylori infections annually. WFs had a higher number of H. pylori infections diagnoses than WMs, although this finding was not statistically significant. This may help to explain the high number of GC diagnoses observed in WFs in our area, which is significantly higher than the United States national average.
In our study, females (of both ethnicities) appear to have almost equal diagnoses as their corresponding male counterparts and, thus, have higher risks for stomach cancer than what was expected based on the United States national average. Importantly, the numbers of AAFs with GC in our study appears to occur at approximately twice the proportion of the national average. Furthermore, WFs in our study also showed a higher proportion of GC diagnoses than what would be anticipated from the reported United States national averages. Several possible reasons might contribute to these findings. At LSUHSC-S, AAFs were found to have the highest proportion of GC in our study, nearly the same as AAMs. AAFs had the highest mean percentage of GC (32.6% ± 2.1%), followed by AAMs (31.2% ± 1.9%), WMs (18.6% ± 2.4%) and WFs (17.6% ± 2.7%). By comparison, AAFs nationally had a lower GC incidence compared to AAMs, and the higher proportion of male cases was also found in whites. In 2009, in a national examination of new GC cases (per 100000 in the United States), AAMs and AAFs had incidence rates of 15.1 and 8.8, respectively, compared to 8.7 and 3.7 for WMs and WFs, respectively[19]. The United States national incidence of GC in AAFs was noted to be approximately half of that in AAMs. In our study, however, AAFs were found to have strikingly higher proportions, nearly double that which was anticipated from national averages and nearly equal to that of AAM. AAFs may be at higher risk of having more advanced GC and having poor prognoses at the time of diagnosis due to poor surveillance. Early onset GC in AAFs in our region could reflect an amalgamation of several risk factors. Combined with other factors, including lack of medical care access and higher incidence of H. pylori infections, AAFs may represent the group with the highest combined risks. The high proportion of AAFs diagnosed with H. pylori infections might be related to the apparent early onset of GC in AAFs observed in our study. WFs in our study also showed a higher risk for GC than that which would be anticipated based on United States national averages, but AAFs had the highest overall proportions, which could reflect lower socioeconomic, educational, occupational and medical care access for women of both ethnicities in this lower income southern United States region.
Because of the high proportions of AAs (58.4%) and women (61.8%) in our study, our data may have more accurate samplings of these groups, and our data support regional differences in the GC risk for women. In addition, because our study involved the population in North Louisiana, we were able to determine different trends in AAs and Ws, thereby eliminating the common problem of an unequal distribution of these ethnic groups within mixed populations. This biasing factor might lead to underestimated risk stratifications within diverse and uneven distributed ethnic societies. Thus, our regional study demonstrates gastric cancer trends within a nearly equally distributed population of African Americans and Whites and could lead to more specific risk stratification for even small ethnic groups in global populations.
It is unclear exactly what accounts for the higher proportions of GC among women of both ethnicities at LSUHSC-S. Relatively lower socioeconomic status, geographic location as well as high rates of H. pylori, and possibly high body mass index may influence the development of GC, but these factors cannot fully explain the observed increased incidence of GC in females of both ethnicities.
In conclusion, Over the past three decades, there has been a significant decline in both the incidence and mortality of GC within the United States, where the incidence rates decreased by 1.7% and 0.8% for men and women, respectively, between 1992 and 2010[26,27]. In this study, however, our data suggest that women in the Northwest Louisiana metropolitan/Shreveport area may be at significantly higher risk, as demonstrated by the increased diagnoses of GC among women of both ethnicities, as well as in the AA populations, compared to United States national averages. The apparent earlier onset of GC seen in AAFs should also be studied more extensively.
Although male gender is a well-established risk factor for GC (and demonstrated in United States national studies), in our region, women have higher overall rates of GC, equal to those of men. While there is a high frequency of H. pylori infections in AAFs at our institution, which might help to explain the equivalent numbers of annual male and female GC patients in our AA population, H. pylori infection is (comparatively) less frequently observed in whites. Therefore, the factors responsible for the increased GC proportion observed in WFs remain unclear.
Our study population evaluated patients in North Louisiana; therefore, we were able to determine different trends in AAs and Ws, thereby eliminating the common problem of an unequal distribution of these ethnic groups within mixed populations. This biasing factor might lead to underestimated risk stratifications within diverse and uneven distributed ethnical societies. Thus, our regional study, demonstrates gastric cancer trends within a nearly equally distributed African American and White population, and it could lead to more specific risk stratification of even small ethnic groups in global populations.
Thus, it is imperative that greater efforts and resources be directed towards improving the healthcare of those individuals at higher risk, including earlier and more advanced screening, smoking education, diet, and weight control, successful eradication of H. pylori infections and availability of medical care. These risk factors are known to confer risk for GC. Guidelines for screening “at risk” groups should be changed to include younger individuals, particularly AAFs. More in depth studies should be performed to investigate interactions between the synergistic risk factors that lead to these findings.
Although no longer the leading cause of worldwide cancer deaths, gastric cancer (GC) remains one of the most frequently diagnosed malignancies, with more than 20000 annual diagnoses in the United States. Because gastric cancer can be influenced by diverse socioeconomic, ethnic background and lifestyle choices, the authors investigated whether regional geographic sampling at a free-care hospital serving primarily economically disadvantaged patients might differ from nationally reported GC statistics.
The current research hotspot indicates that regional demographics can differ substantially from cumulative national data, and note that assumptions about GC incidence and risk may indicate the need to consider how different geographic locations affect risk stratification.
Previous studies of GC risk factors in the United States have suggested increased diagnoses among males and in white (Caucasian) ethnic groupings. Based on cumulative national studies, increased surveillance in these groups might marginalize evaluations in females and in other ethnic groupings, leading to delays in diagnosis and treatment, particularly when regional risks deviate from nationwide findings.
This study showed that data collected over seven years at a southern US regional hospital failed to confirm that male gender and white ethnic groups were at greater risk for GC. Our data suggest that African Americans and females may benefit from at least equal surveillance; the current study found equal risk among genders and ethnic divisions.
In this study, ethnic groupings are self-identified affiliations with either “white” (Caucasian), which is defined as persons of European, North African, or southwest Asian ancestry or “African American”, which is defined as United States residents with total or partial ancestry deriving from the native populations of Sub-Saharan Africa.
In this descriptive/comparative health disparity study, the authors analyzed GC diagnoses (in terms of gender and ethnicity) at a southern regional hospital. The results are interesting and suggest that regional studies may reveal important health care risks that can guide surveillance policies for GC.
P- Reviewer: Borges BD, Fujiwara Y, Li Y, Sun LB S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | La Vecchia C, Negri E, D’Avanzo B, Franceschi S. Electric refrigerator use and gastric cancer risk. Br J Cancer. 1990;62:136-137. [PubMed] [Cited in This Article: ] |
| 2. | Wang C, Yuan Y, Hunt RH. The association between Helicobacter pylori infection and early gastric cancer: a meta-analysis. Am J Gastroenterol. 2007;102:1789-1798. [PubMed] [Cited in This Article: ] |
| 3. | Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 395] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 4. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [PubMed] [Cited in This Article: ] |
| 5. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 6. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2011;64:9-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8789] [Cited by in F6Publishing: 9418] [Article Influence: 941.8] [Reference Citation Analysis (0)] |
| 7. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] [Cited in This Article: ] |
| 8. | Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, Wu XT. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867-2873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol. 2013;24:609-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, Lunet N. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 11. | World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington DC: AICR 2007; . [Cited in This Article: ] |
| 12. | Genta RM. Acid suppression and gastric atrophy: sifting fact from fiction. Gut. 1998;43 Suppl 1:S35-S38. [PubMed] [Cited in This Article: ] |
| 13. | Schlansky B, Sonnenberg A. Epidemiology of noncardia gastric adenocarcinoma in the United States. Am J Gastroenterol. 2011;106:1978-1985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Yang D, Hendifar A, Lenz C, Togawa K, Lenz F, Lurje G, Pohl A, Winder T, Ning Y, Groshen S. Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2:77-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 83] [Reference Citation Analysis (2)] |
| 15. | Epplein M, Signorello LB, Zheng W, Peek RM, Michel A, Williams SM, Pawlita M, Correa P, Cai Q, Blot WJ. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev. 2011;20:826-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Veluswamy H, Suryawala K, Sheth A, Wells S, Salvatierra E, Cromer W, Chaitanya GV, Painter A, Patel M, Manas K. African-American inflammatory bowel disease in a Southern U.S. health center. BMC Gastroenterol. 2010;10:104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Pop-Facts: Demographic Snapshot 2014 Report. Shreveport Bossier MSA. Accessed 2014 October 16. Available from: http://www.lsus.edu/Documents/Offices. [Cited in This Article: ] |
| 18. | U.S. Census Bureau. Shreveport (city) QuickFacts. Accessed 2014 August 15. Available from: http://quickfacts.census.gov/qfd/states/22/2270000.html. [Cited in This Article: ] |
| 19. | Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A. SEER Cancer Statistics Review, 1975-2006. Bethesda, MD: National Cancer Institute 2009; . [Cited in This Article: ] |
| 20. | Bruce MA, Sims M, Miller S, Elliott V, Ladipo M. One size fits all? Race, gender and body mass index among U.S. adults. J Natl Med Assoc. 2007;99:1152-1158. [PubMed] [Cited in This Article: ] |
| 21. | Graham DY, Malaty HM, Evans DG, Evans DJ, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495-1501. [PubMed] [Cited in This Article: ] |
| 22. | Zabaleta J, Camargo MC, Piazuelo MB, Fontham E, Schneider BG, Sicinschi LA, Ferrante W, Balart L, Correa P, Ochoa AC. Association of interleukin-1beta gene polymorphisms with precancerous gastric lesions in African Americans and Caucasians. Am J Gastroenterol. 2006;101:163-171. [PubMed] [Cited in This Article: ] |
| 23. | Zabaleta J, Camargo MC, Ritchie MD, Piazuelo MB, Sierra RA, Turner SD, Delgado A, Fontham ET, Schneider BG, Correa P. Association of haplotypes of inflammation-related genes with gastric preneoplastic lesions in African Americans and Caucasians. Int J Cancer. 2011;128:668-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | U.S. Census Bureau Public Information. Income, Poverty and Health Insurance Coverage in the United States: 2010. Accessed 2014 August 15. Available from: http://www.census.gov/newsroom/releases/archives/income_wealth/cb11-157.html#tablea. [Cited in This Article: ] |
| 25. | Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6-28. [PubMed] [Cited in This Article: ] |
| 26. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 900] [Cited by in F6Publishing: 1163] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 27. | Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 865] [Cited by in F6Publishing: 862] [Article Influence: 86.2] [Reference Citation Analysis (0)] |