Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.475
Peer-review started: April 21, 2014
First decision: May 13, 2014
Revised: June 8, 2014
Accepted: July 11, 2014
Article in press: July 11, 2014
Published online: January 14, 2015
AIM: To identify the mechanisms of chemokine ligand 20 (CCL20)-induced hepatocellular carcinoma (HCC) metastasis and evaluate it as a prognostic marker.
METHODS: Expression of CCL20 was evaluated by immunohistochemistry in HCC tissues from 62 patients who underwent curative resection. The relationship between CCL20 expression and clinicopathologic features was analyzed. Univariate and multivariate analyses were performed to evaluate its predictive value for recurrence and survival of HCC patients. The expression levels of epithelial-mesenchymal transition (EMT)-and signaling pathway-related proteins were evaluated by Western blotting and immunocytochemistry. The effects of CCL20 on HCC cell proliferation and migration were analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenoltetrazolium bromide (MTT) and Transwell assays.
RESULTS: CCL20 immunoreactivity was detected in all 62 patient specimens. CCL20 expression was associated with preoperative alpha-fetoprotein level (P = 0.043), tumor size (P = 0.000), tumor number (P = 0.008), vascular invasion (P = 0.014), and tumor differentiation (P = 0.007). Patients with high CCL20 expression had poorer recurrence-free and overall survivals compared to those with low CCL20 expression (both P < 0.001). CCL20 induced EMT-like changes in HCC cells and increased their proliferation and migration ability (P < 0.05). Western blotting and immunofluorescence staining showed that CCL20 induced an EMT-like phenotype in HCC cells, and increased expression of phosphorylated AKT, β-catenin and vimentin, and decreased E-cadherin expression (P < 0.05). The correlation analysis revealed that high CCL20 expression in HCC tissue specimens was negatively correlated with E-cadherin expression (13.33%, 4/30), and positively correlated with vimentin (90.0%, 27/30), β-catenin (96.67%, 29/30) and p-AKT (76.67%, 23/30) expression.
CONCLUSION: CCL20 expression is associated with HCC recurrence and patient survival and promotes HCC cell proliferation and migration by inducing EMT-like changes via PI3K/AKT and Wnt/β-catenin pathways.
Core tip: This study examined the expression and prognostic value of chemokine ligand 20 in hepatocellular carcinoma. The results indicate that increased expression of this protein regulates the growth and migration of hepatocellular carcinoma cells and epithelial-mesenchymal transition via phosphoinositide kinase-3/AKT, and Wnt/β-catenin signaling pathways and is therefore a potential treatment target.
-
Citation: Hou KZ, Fu ZQ, Gong H. Chemokine ligand 20 enhances progression of hepatocellular carcinoma
via epithelial-mesenchymal transition. World J Gastroenterol 2015; 21(2): 475-483 - URL: https://www.wjgnet.com/1007-9327/full/v21/i2/475.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.475
Hepatocellular carcinoma (HCC) is the sixth most common cancer and third leading cause of cancer death worldwide[1-3]. HCC is associated with a poor outcome and a high rate of mortality due to the high rate of recurrence and metastasis[4-6]. Thus, it is essential to identify novel predictors of recurrence and metastasis. The risk factors for HCC include hepatitis B or C virus infection, alcohol consumption, and non-alcoholic fatty liver disease[7-10]. Moreover, the tumor microenvironment contains various cytokines, chemokines and growth factors that are produced by tumor or stromal cells, which promote tumor initiation, progression and metastasis[11,12].
Chemokine ligand 20 (CCL20), also known as liver activation regulated chemokine or macrophage inflammatory protein-3, is a small cytokine that is strongly chemotactic for lymphocytes and weakly attracts neutrophils[13]. Increasing evidence indicates that CCL20 is related to tumor formation, progression or metastatic processes in many malignancies, including breast and colorectal cancer[14,15]. The proliferation and migration of tumor cells are considered a foundation of cancer survival and development. Tumor progression and metastasis are associated with the induction of epithelial-mesenchymal transition (EMT)[16,17]. In this study, CCL20 expression was examined in HCC patient samples and the relationships with clinicopathologic features and recurrence and patient survival were examined. Moreover, the effects of CCL20 expression on HCC cell EMT, proliferation and migration and involvement of relevant signaling pathways were investigated.
From January 2002 to October 2008, 62 consecutive patients with primary HCC who underwent radical resection at our hospital were enrolled in the study. The diagnosis was confirmed by histologic examination in all cases. All primary tumor tissues were preserved in paraffin for immunohistochemical analyses. None of the patients received preoperative anticancer treatment. Preoperative liver function was evaluated using the Child-Pugh scoring system. Tumor stage was determined according to the tumor-node-metastasis (TNM) classification system of the American Joint Cancer Committee/Union for International Cancer Control (2002). Tumor differentiation was graded using the Edmondson-Steiner classification system. Data was censored at the last follow-up for patients without recurrence or death. Recurrence-free survival (RFS) and overall survival (OS) was defined as the interval between the time of surgery to that of recurrence or death, respectively. This study was approved by the Ethics Committee of our hospital. Written informed consent was obtained from all patients.
Human HCC cell lines Hep3B and Huh7 were maintained in Dulbecco’s Modified Eagle’s Medium (Gibco of Thermo Fisher Scientific Inc., Waltham, MA, United States) supplemented with 10% fetal calf serum and 100 IU/mL penicillin and 100 mg/mL streptomycin in a 5% CO2 incubator at 37 °C. CCL20 (Cat. 360-MP-025; R&D Systems, Minneapolis, MN, United States) was added to the media, which was changed every other day.
Cell proliferation was assessed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenoltetrazolium bromide (MTT) assay. Cells were plated in 96-well culture plates at 5 × 103 cells per well containing 0.2 mL of culture media. After treatment with CCL20 (5 μg/mL) for 24 or 48 h, 0.02 mL of 5 mg/mL MTT was added to each well and incubated for 4 h at 37 °C. The absorbance was measured at 570 nm. Each assay was performed three times independently.
Cell invasion was assessed using Transwell chambers (8 μm pore size; EMD Millipore, Billerica, MA, United States) according to the manufacturer’s instructions. ECM (Sigma-Aldrich, St. Louis, MO, United States) was added to the chamber to form a gel layer, and cells (5 × 104) were added to the upper chamber in the presence of CCL20 at a concentration of 5 μg/mL for 24 or 48 h. The cells migrating to the membrane were enumerated with Giemsa staining. The assay was performed three times independently.
The following primary antibodies used in the experiments were obtained from Santa Cruz Biotechnologies Inc., Dallas, TX, United States: anti-E-cadherin (sc-21791), anti-vimentin (sc-53464), anti-AKT (sc-5298), anti-phospho(p)-AKT (sc-33437), anti-β-catenin (sc-7963) and anti-GAPDH (sc-25778).
Lysates were extracted from cells treated with CCL20 at a concentration of 5 μg/mL for 48 h using lysis buffer with phenylmethylsulfonyl fluoride, and the protein concentration was measured with a BCA protein assay kit (Beyotime, Shanghai, China). Total protein extracts (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat dried milk in Tris-buffered saline (20 mmol/L Tris-HCl, 150 mmol/L NaCl and 0.1% Tween-20, pH 7.5) for 1 h at room temperature and individually incubated overnight at 4 °C with antibodies against E-cadherin (1:1000), vimentin (1:1000), AKT (1:1000), p-AKT (1:1000), β-catenin (1:1000) or GAPDH (1:5000). Signals were detected by using enhanced ECL chemiluminescence (MultiScience Biotech Co., Shanghai, China) and analyzed using Quantity One (Bio-Rad, Hercules, CA, United States).
Immunohistochemical detection of E-cadherin (1:100), vimentin (1:500), p-AKT (1:100) and β-catenin (1:100) was performed on 4 μm-thick sections of specimens which had been fixed in formalin, embedded in paraffin and mounted on slides. Replacement of the primary antibody with mouse- or rabbit-isotype control antibody served as a negative control. Counterstaining of the nucleus was performed using hematoxylin. The staining intensity was scored in four levels (0 = negative, 1 = weak, 2 = moderate, 3 = strong), and the percentages of stained cells at each intensity level were counted. The total immunostaining score was calculated as the sum of each intensity score multiplied by the corresponding percentage. The slides were independently evaluated and scored by three pathologists without knowledge of clinical data.
After reaching confluency, cells plated on coverslips in 6-well dishes were washed twice, fixed with 2% (w/v) formaldehyde and permeabilized with 1% (v/v) Triton X-100. Coverslips were blocked with 10% (w/v) normal goat serum in phosphate-buffered saline at room temperature for 1 h and then incubated in primary antibodies against E-cadherin (1:200), vimentin (1:500), p-AKT (1:200) or β-catenin (1:400) at 4 °C overnight. Cells were washed and incubated with Cy3-labeled secondary antibody (Beyotime) at room temperature for 1 h, and co-stained with DAPI (Sigma-Aldrich) to visualize nuclei. Images were obtained using a fluorescence microscope at magnification × 200.
All analyses in the study were performed using SPSS version 16.0 software (SPSS Inc., Chicago, IL, United States). One-way analyses of variance and Student’s t-tests were used for intergroup comparisons. The correlation between CCL20 expression and clinicopathologic features was examined by the χ2 test. RFS and OS were calculated by the Kaplan-Meier method. Univariate analyses for factors of recurrence and survival were performed using the χ2 test and the log-rank test, respectively. Data are presented as mean ± SD, with P < 0.05 considered as statistically significant.
Of the HCC patient samples, 75.8% (47/62) were from men and 24.2% (15/62) were from women (Table 1). The average age of the patients was 57 years (range: 32-79 years), with 91.9% (57/62) of the patients having Hepatitis B or C viral infections, and 95.2% (59/62) presenting with chronic hepatitis history or liver cirrhosis. The average values of alpha-fetoprotein (AFP) and alanine aminotransferase before surgery were 197 ng/ml and 66 U/L, respectively. TNM stages of all HCC samples were divided into a stage I group (40/62; 64.5%) or a stage II and III group (22/62; 35.5%). The average follow-up period was 42.6 ± 19.8 mo (range: 8-65 mo), and 66.1% (41/62) of patients presented recurrence after surgery. The recurrence sites included the liver (n = 32), lung (n = 4), lymph node (n = 5) and bone (n = 2). OS and RFS were 88.7% (55/62) and 83.9% (52/62) at 1 year, 74.2% (46/62) and 66.1% (41/62) at 3 years, and 53.2% (33/62) and 33.9% (21/62) at 5 years, respectively.
| Clinicopathologic features | Number of patients | CCL20 expression | P value | |
| Low (n = 32) | High (n = 30) | |||
| Age (yr) | ||||
| ≤ 57 | 35 | 19 | 16 | 0.632 |
| > 57 | 27 | 13 | 14 | |
| Gender | ||||
| Male | 47 | 26 | 21 | 0.301 |
| Female | 15 | 6 | 9 | |
| Etiology | 0.167 | |||
| HBV infection | 46 | 26 | 20 | |
| HCV infection | 11 | 2 | 9 | |
| Alcohol | 5 | 4 | 1 | |
| Background liver pathology | 0.122 | |||
| Normal liver | 3 | 2 | 1 | |
| Chronic hepatitis | 20 | 11 | 9 | |
| Liver cirrhosis | 39 | 19 | 20 | |
| AFP (ng/mL) | ||||
| ≤ 197 | 33 | 21 | 12 | 0.043 |
| > 197 | 29 | 11 | 18 | |
| ALT (U/L) | ||||
| ≤ 66 | 28 | 17 | 11 | 0.193 |
| > 66 | 34 | 15 | 19 | |
| Child-Pugh | ||||
| A | 44 | 21 | 23 | 0.338 |
| B | 18 | 11 | 7 | |
| Tumor size (cm) | ||||
| ≤ 5 | 41 | 29 | 12 | 0.000 |
| > 5 | 21 | 3 | 18 | |
| Tumor number | ||||
| Single | 43 | 27 | 16 | 0.008 |
| Multiple | 19 | 5 | 14 | |
| Vascular invasion | ||||
| No | 51 | 30 | 21 | 0.014 |
| Yes | 11 | 2 | 9 | |
| Tumor encapsulation | ||||
| Yes | 24 | 14 | 10 | 0.400 |
| No | 38 | 18 | 20 | |
| Tumor differentiation | ||||
| I + II | 45 | 28 | 17 | 0.007 |
| III + IV | 17 | 4 | 13 | |
| TNM stage | ||||
| I | 40 | 22 | 18 | 0.472 |
| II + III | 22 | 10 | 12 | |
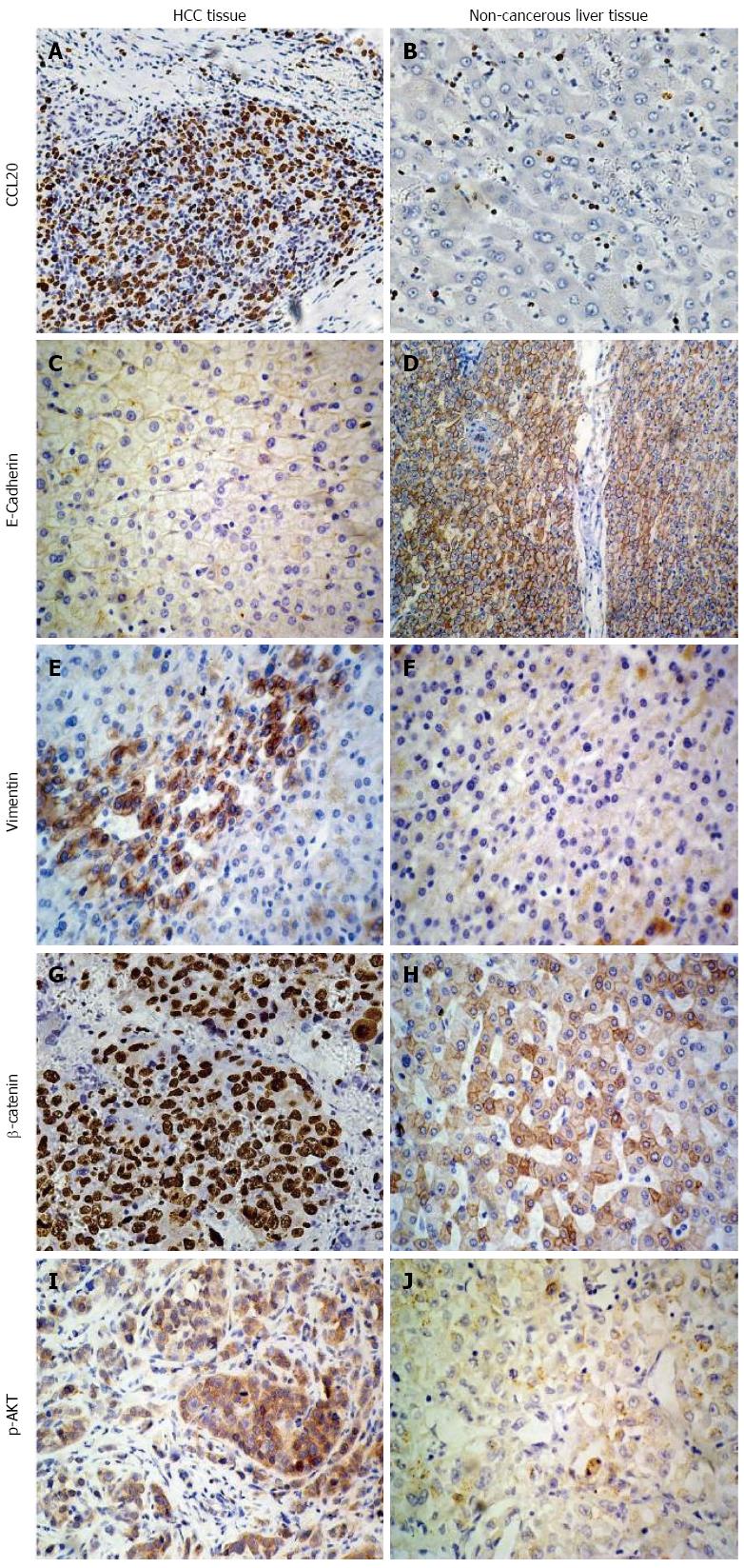
The expression of CCL20 was examined in all 62 HCC samples, and was mainly located in the cytoplasm of HCC cells (Figure 1). The average immunohistochemistry score was 165.0 (range: 85-260). All HCC samples were subsequently divided into low CCL20 group (n = 32) and high CCL20 group (n = 30) by using the average value. The relationships between CCL20 expression and clinicopathologic factors are presented in Table 1. Our findings revealed that CCL20 expression was significantly related to preoperative AFP level (P = 0.043), tumor size (P = 0.000), tumor number (P = 0.008), vascular invasion (P = 0.014), and tumor differentiation (P = 0.007).
Univariate analyses indicated that HCC recurrence was related with vascular invasion (P = 0.042), TNM stage (P < 0.001), and CCL20 expression (P < 0.001) (Table 2). RFS was significantly related with preoperative AFP (P = 0.038), tumor size (P = 0.024), vascular invasion (P = 0.019), TNM stage (P < 0.001), and expression of CCL20 (P < 0.001). OS significantly related with tumor size (P = 0.014), vascular invasion (P = 0.008), tumor encapsulation (P = 0.032), tumor differentiation (P = 0.047), TNM stage (P < 0.001), and CCL20 expression (P < 0.001). There were no significant correlations among the other clinicopathologic factors and recurrence or survivals.
| Factor | Recurrence | P value | RFS (%) | P value | OS (%) | P value | |
| Yes | No | ||||||
| Age (yr) | |||||||
| ≤ 57 | 19 | 16 | 0.184 | 53.2 | 0.211 | 66.7 | 0.135 |
| > 57 | 18 | 9 | 38.1 | 50.6 | |||
| Gender | |||||||
| Male | 26 | 21 | 0.469 | 58.9 | 0.192 | 55.4 | 0.633 |
| Female | 9 | 6 | 42.3 | 64.8 | |||
| Etiology | 0.235 | 0.659 | 0.835 | ||||
| HBV infection | 27 | 19 | 45.7 | 56.3 | |||
| HCV infection | 7 | 4 | 39.2 | 54.5 | |||
| Alcohol | 2 | 3 | 58.4 | 61.7 | |||
| Background liver pathology | 0.953 | 0.784 | 0.947 | ||||
| Normal liver | 2 | 1 | 55.3 | 63.5 | |||
| Chronic hepatitis | 13 | 7 | 45.6 | 57.4 | |||
| Liver cirrhosis | 22 | 17 | 40.9 | 50.2 | |||
| AFP (ng/mL) | |||||||
| ≤ 197 | 15 | 18 | 0.084 | 58.3 | 0.038 | 65.5 | 0.074 |
| > 197 | 19 | 10 | 29.6 | 42.8 | |||
| ALT (U/L) | |||||||
| ≤ 66 | 15 | 13 | 0.371 | 45.6 | 0.881 | 55.3 | 0.942 |
| > 66 | 19 | 15 | 41.5 | 52.9 | |||
| Child-Pugh | |||||||
| A | 24 | 20 | 0.774 | 40.2 | 0.695 | 54.1 | 0.133 |
| B | 11 | 7 | 38.8 | 44.3 | |||
| Tumor size (cm) | |||||||
| ≤ 5 | 21 | 20 | 0.417 | 52.9 | 0.024 | 68.3 | 0.014 |
| > 5 | 14 | 7 | 31.7 | 44.8 | |||
| Tumor number | |||||||
| Single | 25 | 18 | 0.217 | 47.1 | 0.052 | 58.3 | 0.233 |
| Multiple | 13 | 6 | 30.2 | 46.5 | |||
| Vascular invasion | |||||||
| No | 31 | 20 | 0.042 | 54.6 | 0.019 | 62.8 | 0.008 |
| Yes | 8 | 3 | 31.8 | 40.9 | |||
| Tumor encapsulation | |||||||
| Yes | 14 | 10 | 0.649 | 47.6 | 0.443 | 65.3 | 0.032 |
| No | 23 | 15 | 38.2 | 43.1 | |||
| Tumor differentiation | |||||||
| I + II | 21 | 24 | 0.241 | 45.3 | 0.695 | 59.6 | 0.047 |
| III + IV | 11 | 6 | 41.8 | 40.1 | |||
| TNM stage | |||||||
| I | 19 | 21 | < 0.001 | 56.8 | < 0.001 | 66.4 | < 0.001 |
| II + III | 18 | 4 | 20.4 | 35.7 | |||
| CCL20 | |||||||
| Low | 13 | 19 | < 0.001 | 60.9 | < 0.001 | 70.1 | < 0.001 |
| High | 27 | 3 | 21.4 | 30.6 | |||
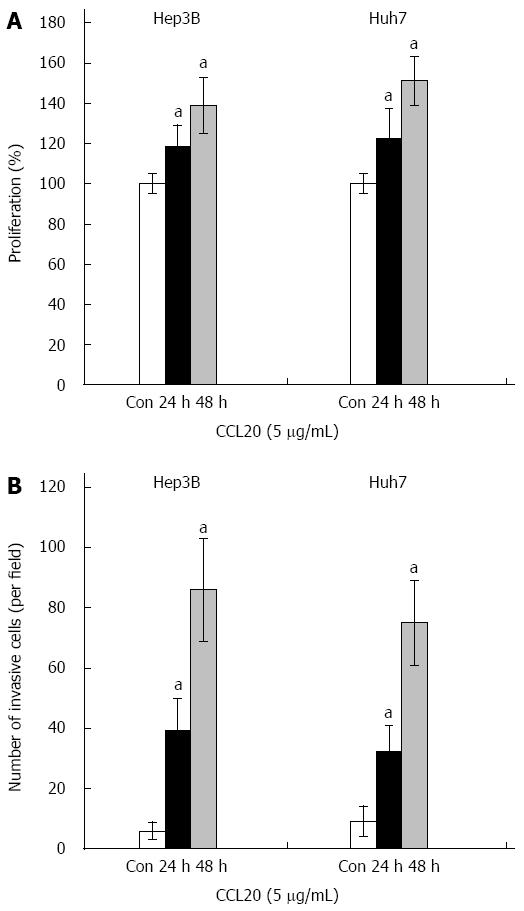
The proliferation of Hep3B and Huh7 HCC cells was significantly enhanced by CCL20 after 24 and 48 h (both P < 0.05) (Figure 2A). Similarly, results of the invasion assay indicated that CCL20 treatment significantly increased invasion of Hep3B and Huh7 HCC cells (both P < 0.05) (Figure 2B).
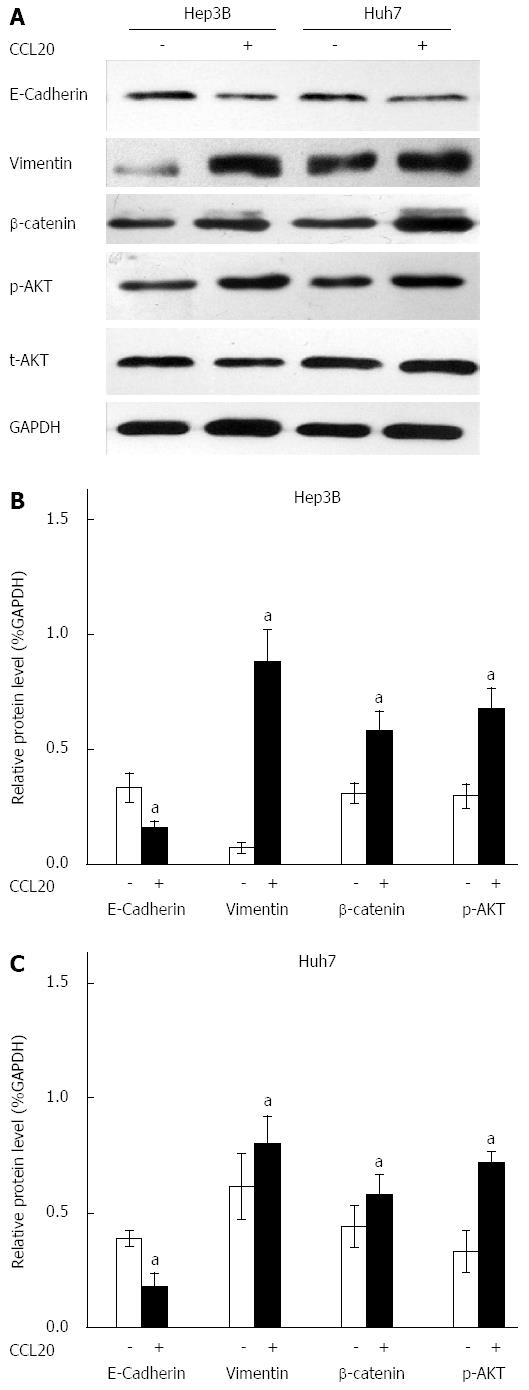
Western blotting results showed that CCL20 at a concentration of 5 μg/mL for 48 h induced an EMT-like phenotype in Hep3B and Huh7 HCC cells. The expression of the epithelial marker E-cadherin was decreased and the mesenchymal marker vimentin was increased significantly by CCL20 (P < 0.05) (Figure 3). Moreover, β-catenin levels and phosphorylation of AKT were also increased compared with controls (P < 0.05).
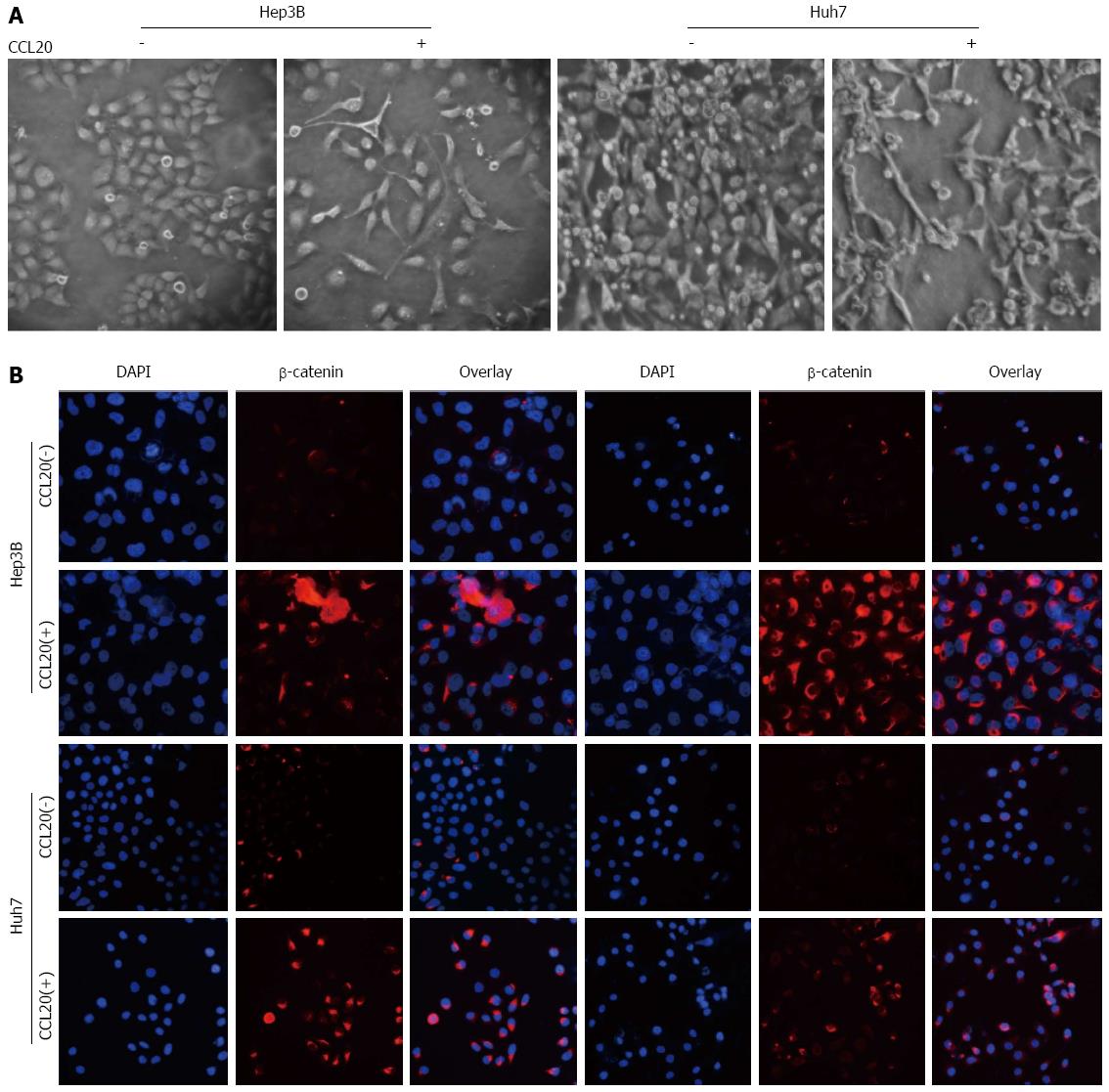
Treatment with CCL20 (5 μg/mL) for 48 h induced an EMT-like phenotype in Hep3B and Huh7 cells (Figure 4A). The detection of β-catenin and p-AKT by immunofluorescence was enhanced by CCL20 treatment (Figure 4B).
EMT markers E-cadherin and vimentin, and cell-signaling pathway-related proteins β-catenin and p-AKT were also measured by immunohistochemistry in patient specimens (Figure 1). Results showed that vimentin, β-catenin and p-AKT levels were much higher and E-cadherin level was lower in samples identified as having high CCL20 expression compared to those with low expression and non-cancerous liver tissues. The correlation analysis revealed that high-CCL20 expression was negatively correlated with E-cadherin expression (4/30; 13.33%), and positively correlated with vimentin (27/30; 90.0%), β-catenin (29/30; 96.67%) and p-AKT (23/30; 76.67%) expression (Table 3).
| E-cadherin | Vimentin | p-AKT | β-catenin | |||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| CCL20 | 4 | 26 | 27 | 3 | 23 | 7 | 29 | 1 |
| χ2 | 13.285 | 14.754 | 9.589 | 17.796 | ||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
Chemokines play a critical role in various biologic events, such as the role of inflammatory responses in regulating the transfer of white blood cells, embryonic development, wound healing, angiogenesis, Th1/Th2 development, leukocyte homeostasis, and lymphatic organ development. Recent studies have suggested that chemokines and their receptors are associated with the pathogenesis, progression and metastasis of many kinds of tumors, including HCC. Li et al[18] found a much higher expression of the CXCL12-CXCR4 axis in HCC specimens than in adjacent, cirrhosis, liver adenocarcinoma, and normal liver tissues. Fujii et al[19] performed in vitro experiments showing that the CCL20-CCR6 axis promotes the growth of Huh7 cells through phosphorylation of mitogen-activated protein kinase. Rubie et al[20] reported that CCL20 was significantly upregulated in HCC specimens. Zheng et al[21] found that CXCR7 expression was increased in HCC tissues and was associated with HCC invasion, adhesion and angiogenesis. Zhou et al[22] found that CXCL5 promotes HCC cell proliferation, invasion and intratumoral neutrophil infiltration and could be a novel prognostic predictor of HCC.
Our results revealed that high expression of CCL20 in HCC tissues was correlated with poor outcome in HCC patients undergoing resection surgery. Our results also indicated that the expression of CCL20 was associated with tumor size, number and tumor differentiation, and with vascular invasion. Results of in vitro experiments indicated that CCL20 can induce EMT-like morphologic changes and promote the proliferation and invasion in HCC cells. Interestingly, CCL20 induced expression of vimentin and downregulated the expression of E-cadherin. These results extend the role of CCL20 in HCC to the context of chemokine-mediated EMT. Several chemokines have been shown to induce EMT in various tumors. Biswas et al[23] found that CXCL13-CXCR5 co-expression regulates EMT of breast cancer cells. Ploenes et al[24] reported that CCL18 induces EMT in lung cancer cells and elevates the invasive potential. Albert et al[25] found that CXCR4 induces EMT in patients with squamous cells carcinoma. Li et al[26] found that SDF-1/CXCR4 signaling induces pancreatic cancer cell invasion and EMT in vitro through non-canonical activation of hedgehog pathway. Matsushita et al[27] showed that CXCL16 plays an important role in liver metastasis of colorectal cancer through the induction of EMT. Our study provides another model of chemokine-mediated EMT, in which CCL20 may play a significant role in HCC progression and metastasis.
A variety of cell signaling pathways have been implicated in the process of EMT. Chang et al[28] found that EMT is associated with activation of the PI3K/AKT/mTOR pathway in prostate cancer radio-resistance. Tsai et al[29] reported that downregulation of PI3K/AKT and Wnt/β-catenin signaling cascades reverses EMT and inhibits breast cancer cell invasiveness. Choi et al[30] demonstrated that PI3K/AKT, ERK1/2 and Smad2/3 pathways are associated with transforming growth factor β1-induced EMT in human lung carcinoma cells. By examining protein expression in patient specimens and in HCC cells in vitro in our study, we showed that CCL20 expression was negatively associated with E-cadherin and positively associated with vimentin, p-AKT, and β-catenin expression. These results suggest that CCL20 expression is involved with the EMT process in HCC and there might be crosstalk between the PI3K/AKT and Wnt/β-catenin signaling pathways.
In conclusion, the present findings indicate that CCL20 expression is correlated with clinicopathologic factors, recurrence and survival in HCC patients, as well as the proliferation, migration and invasion of HCC cells. CCL20 induces EMT-like changes in HCC cells through activation of PI3K/AKT and Wnt/β-catenin signaling pathways. CCL20 may therefore represent a promising molecular marker for predicting outcomes and treatment of HCC patients undergoing resection surgery.
Chemokine ligand 20 (CCL20), also known as liver activation regulated chemokine or macrophage inflammatory protein-3, is a small cytokine that is strongly chemotactic for lymphocytes and weakly attracts neutrophils. Increasing evidence suggests that CCL20 is involved with tumor formation, progression and metastatic processes in many malignancies, including breast and colorectal cancer.
Recent studies on CCL20 have mostly focused on the expression in hepatocellular carcinoma (HCC) tissues. Thus far, no studies have described the underlying mechanism by which CCL20 regulates the growth and metastasis of HCC cells. The results of this study provide new targets and a theoretical basis for the therapy of HCC.
This study demonstrates that CCL20 plays an important role in the proliferation and migration of HCC cells. CCL20 expression could induce an epithelial-mesenchymal transition (EMT)-like change in HCC cells via crosstalk between phosphoinositide kinase-3 (PI3K)/AKT and Wnt/β-catenin signaling pathways. CCL20 may be useful as a molecular monitor of metastasis and recurrence in HCC patients.
CCL20 induces EMT-like changes in HCC cells via crosstalk between PI3K/AKT and Wnt/β-catenin signaling pathways, which may therefore be used as indicators for anti-HCC therapy and prognosis evaluation.
The authors detected expression of CCL20 in HCC tissues and analyzed its prognostic significance for HCC patients. They further identified the PI3K-AKT and Wnt pathways as possible mechanisms for the EMT-like phenotype induced by CCL20.
P- Reviewer: Du Z, Kondo Y, Yang YP, Wang DS S- Editor: Nan J L- Editor: AmEditor E- Editor: Ma S
| 1. | Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237-257. [PubMed] [Cited in This Article: ] |
| 2. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 3. | Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Yeh CT, Huang YH, Liang KH, Chang ML, Hsu CW, Chen YC, Chen TC, Yeh TS, Lee WC. Segregation of signaling proteins as prognostic predictors for local recurrence and distant metastasis in hepatocellular carcinoma. Int J Oncol. 2014;44:491-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Liu L, Dai Y, Chen J, Zeng T, Li Y, Chen L, Zhu YH, Li J, Li Y, Ma S. Maelstrom promotes hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition by way of Akt/GSK-3β/Snail signaling. Hepatology. 2014;59:531-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Okimoto K, Ogasawara S, Chiba T, Kanai F, Yokota H, Motoyama T, Suzuki E, Ooka Y, Tawada A, Iwadate Y. Successful resection of intracranial metastasis of hepatocellular carcinoma. Case Rep Gastroenterol. 2013;7:182-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Lu T, Seto WK, Zhu RX, Lai CL, Yuen MF. Prevention of hepatocellular carcinoma in chronic viral hepatitis B and C infection. World J Gastroenterol. 2013;19:8887-8894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 8. | Kubo S, Takemura S, Sakata C, Urata Y, Uenishi T. Adjuvant therapy after curative resection for hepatocellular carcinoma associated with hepatitis virus. Liver Cancer. 2013;2:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Bhala N, Jouness RI, Bugianesi E. Epidemiology and natural history of patients with NAFLD. Curr Pharm Des. 2013;19:5169-5176. [PubMed] [Cited in This Article: ] |
| 10. | Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, Chen CJ. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Carr BI, Guerra V. HCC and its microenvironment. Hepatogastroenterology. 2013;60:1433-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 12. | Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol. 2012;40:1733-1747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 544] [Cited by in F6Publishing: 577] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 14. | Marsigliante S, Vetrugno C, Muscella A. CCL20 induces migration and proliferation on breast epithelial cells. J Cell Physiol. 2013;228:1873-1883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Vicinus B, Rubie C, Stegmaier N, Frick VO, Kölsch K, Kauffels A, Ghadjar P, Wagner M, Glanemann M. miR-21 and its target gene CCL20 are both highly overexpressed in the microenvironment of colorectal tumors: significance of their regulation. Oncol Rep. 2013;30:1285-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Franco-Chuaire ML, Magda Carolina SC, Chuaire-Noack L. Epithelial-mesenchymal transition (EMT): principles and clinical impact in cancer therapy. Invest Clin. 2013;54:186-205. [PubMed] [Cited in This Article: ] |
| 17. | Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvi-ronmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72:4883-4889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 655] [Reference Citation Analysis (0)] |
| 18. | Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527-533. [PubMed] [Cited in This Article: ] |
| 19. | Fujii H, Itoh Y, Yamaguchi K, Yamauchi N, Harano Y, Nakajima T, Minami M, Okanoue T. Chemokine CCL20 enhances the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. Biochem Biophys Res Commun. 2004;322:1052-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Rubie C, Frick VO, Wagner M, Rau B, Weber C, Kruse B, Kempf K, Tilton B, König J, Schilling M. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scand J Immunol. 2006;63:468-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56:2242-2254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 23. | Biswas S, Sengupta S, Roy Chowdhury S, Jana S, Mandal G, Mandal PK, Saha N, Malhotra V, Gupta A, Kuprash DV. CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res Treat. 2014;143:265-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Ploenes T, Scholtes B, Krohn A, Burger M, Passlick B, Müller-Quernheim J, Zissel G. CC-chemokine ligand 18 induces epithelial to mesenchymal transition in lung cancer A549 cells and elevates the invasive potential. PLoS One. 2013;8:e53068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Albert S, Hourseau M, Halimi C, Serova M, Descatoire V, Barry B, Couvelard A, Riveiro ME, Tijeras-Raballand A, de Gramont A. Prognostic value of the chemokine receptor CXCR4 and epithelial-to-mesenchymal transition in patients with squamous cell carcinoma of the mobile tongue. Oral Oncol. 2012;48:1263-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W, Bhat K, Wang F, Wu E, Wang Z. SDF-1/CXCR4 signaling induces pancreatic cancer cell invasion and epithelial-mesenchymal transition in vitro through non-canonical activation of Hedgehog pathway. Cancer Lett. 2012;322:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 27. | Matsushita K, Toiyama Y, Tanaka K, Saigusa S, Hiro J, Uchida K, Inoue Y, Kusunoki M. Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Ann Surg Oncol. 2012;19 Suppl 3:S518-S527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 29. | Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way TD, Chen WJ. 3,5,4’-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol. 2013;272:746-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Choi JH, Hwang YP, Kim HG, Khanal T, Do MT, Jin SW, Han HJ, Lee HS, Lee YC, Chung YC. Saponins from the roots of Platycodon grandiflorum suppresses TGFβ1-induced epithelial-mesenchymal transition via repression of PI3K/Akt, ERK1/2 and Smad2/3 pathway in human lung carcinoma A549 cells. Nutr Cancer. 2014;66:140-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |