Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4875
Peer-review started: October 28, 2014
First decision: December 11, 2014
Revised: January 4, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: April 28, 2015
AIM: To compare two different laparotomy methods for modeling rabbit VX2 hepatocarcinoma.
METHODS: Thirty New Zealand rabbits were randomly divided into two groups: A and B. Group A was assigned a traditional laparotomy method (embedding tumor fragments directly into the liver with tweezers). Group B was subjected to an improved laparotomy method (injection of tumor fragments into the liver through a 15 G syringe needle). The operation time, incision length, incision infection rate, and mortality rate were compared between the two groups after laparotomy. Magnetic resonance imaging (MRI) was performed to evaluate tumor formation rates and the characteristics of the tumors 2 wk after laparotomy.
RESULTS: The mean operation times for the two groups (Group A vs Group B) were 23.2 ± 3.4 min vs 17.5 ± 2.9 min (P < 0.05); the incision length was 3.3 ± 0.5 cm vs 2.4 ± 0.6 cm (P < 0.05); and the mortality rate after 2 wk was 26.7% vs 0% (P < 0.05); all of these outcomes were significantly different between the two groups. The incision infection rates in the two groups were 6.7% vs 0% (P > 0.05), which were not significantly different. MRI performed after 2 weeks showed that the tumor formation rates in the two groups were 90.9% vs 93.3% (P > 0.05). These rates were not significantly different between the two groups. The celiac implantation rate and abdominal wall metastasis rate in the two groups were 36.4% vs 13.3% (P < 0.05) and 27.2% vs 6.7% (P < 0.05), respectively, which were significantly different between the two groups.
CONCLUSION: The tumor formation rates were not significantly different between the two methods for modeling rabbit VX2 hepatocarcinoma. However, the improved method is recommended because it has certain advantages.
Core tip: A crucial issue in studying liver cancer is the establishment of an animal model to simulate human liver cancer. There are various ways to establish rabbit VX2 hepatocarcinoma, and using an open laparotomy implant is a widely adopted classical method. We injected tumor fragments into the left lobe of the rabbit liver, deviating from the abdominal wall using a 15 G syringe needle instead of building a sinus using tweezers. This improved method is recommended because of advantages such as decreased injury to the liver, shorter operation time, lower death rates, reduced abdominal cavity implantation and fewer abdominal wall invasions.
- Citation: Chen Z, Kang Z, Xiao EH, Tong M, Xiao YD, Li HB. Comparison of two different laparotomy methods for modeling rabbit VX2 hepatocarcinoma. World J Gastroenterol 2015; 21(16): 4875-4882
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4875
Liver cancer, the third leading cause of cancer death worldwide, is a primary malignancy originating in the liver. There are approximately 60-70 million liver cancer deaths each year, 50% of which occur in China. Most patients are diagnosed in intermediate or advanced stages of the disease[1,2]. Early detection and diagnosis of liver cancer are an important issue that must be addressed. It is crucial to establish an animal model simulating human liver cancer to study this disease. VX2 carcinoma is a type of animal tumor that was derived from a rabbit papilloma by Shope and Hurst[3]. Rabbit VX2 hepatocarcinoma is an ideal model of liver cancer and is widely used for imaging and other experimental studies due to the rapid growth of these tumors and their similarity to human hepatocellular carcinoma[4-6]. There are various ways to establish rabbit VX2 hepatocarcinoma, including the widely adopted classical method of open laparotomy implantation. In open laparotomy implantation, the implant substance can be a cell suspension or tumor fragments, with the latter being reported to show a higher success rate[7]. However, traditional laparotomy implantation methods may cause spill-out into the celiac. We developed a modified method that uses a syringe to inject tumor fragments to improve the success rate of implantation. Here we compared the two different methods for implanting VX2 tumors, and the results are presented below.
Thirty New Zealand white rabbits of both sexes weighing 2.0-3.0 kg were provided by the experimental animal center of Second Xiangya Hospital of Central South University. The animal experiments were performed in accordance with a protocol approved by the animal care committee and were in compliance with institutional guidelines (Permit Number: 2012-0087).
Basic surgical instruments and supplies were used, including a 15 G needle connected to a 5 mL syringe.
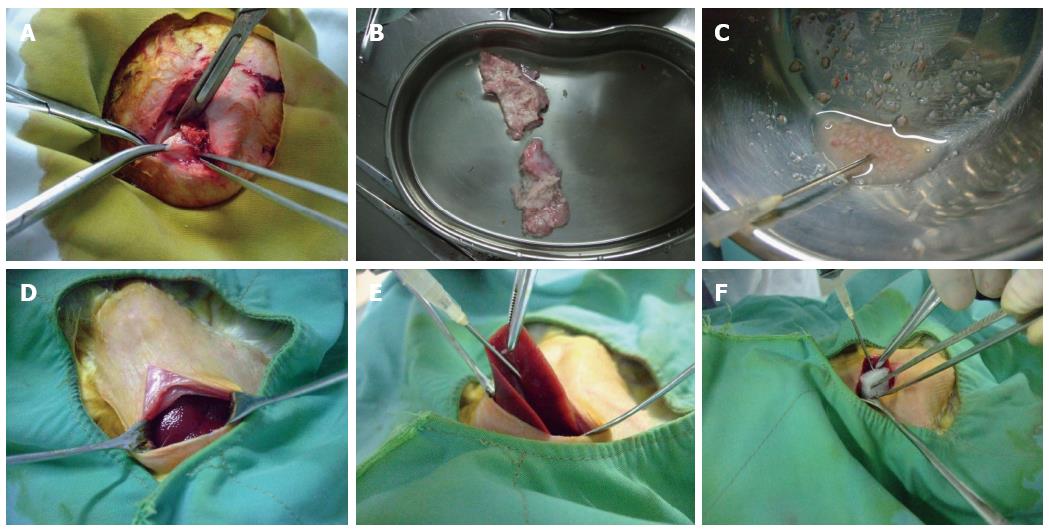
Establishment of the rabbit VX2 liver carcinoma model: The 30 New Zealand white rabbits were divided into two groups using a random number table, with 15 rabbits being placed in each group. Direct embedding of tumor fragments was performed on the rabbits in Group A (traditional open method group). Group B was subjected to the modified method, in which tumor fragments were injected with a 15 G needle connected to a 5 mL syringe. Four hours before the operation, the rabbits were fasted and only given water. The hair around the operation field was shaved using a razor. Intramuscular injections of gentamicin (80000 units) were administered before the operation and once a day after the operation for a total of two days. All rabbits were anesthetized with 2 mL/kg chloral hydrate via ear vein injection.
Tumor-bearing rabbits were anesthetized with chloral hydrate. Tissue at the edge of the tumor was removed under sterile conditions. Then, the tumor was placed in physiological saline solution for washing, and necrotic tissue, fascia and other connective tissues were removed. This procedure was followed by soaking the tumor in physiological saline solution and cutting it into small pieces (0.5-1 mm diameter) using scissors. Then, the incision in the tumor-bearing rabbits was sutured, and gentamicin (80000 units) was injected intramuscularly.
Healthy rabbits were anesthetized and placed on a rabbit operating table with their limbs fixed at the four corners. Routine sterilization of the surgical field was performed, and a vertical incision was made below the xiphoid. When the liver was exposed, the 15 rabbits in Group A were subjected to the following conventional open laparotomy implantation method: stretching of the left lobe using smooth forceps, puncturing of the liver tissue to build a sinus with an ostium diameter of approximately 2 mm using ophthalmic scissors, insertion of tumor fragments into the sinus, covering the sinus with gelfoam, and suturing the incision after confirming that there was no obvious bleeding. Group B was subjected to the following modified implantation method: exposure of the middle lobe of the liver using smooth forceps, insertion of a 15 G needle sideways and upward into the visceral surface of the liver lobe and then injection of 0.5 mL of tumor fragments with a 5 mL syringe. During this process, we made sure that the tumor fragments were injected into the liver parenchyma. We could see that the surface of the puncture site was intumescent and white. Finally, the puncture site was covered with gelfoam after removal of the needle, and the incision was sutured after confirming that there was no obvious bleeding. The modified implantation method is shown in Figure 1.
Postoperative indicators: Observed indicators included the operation time, incision size, infection rate, rabbit survival after 2 wk, and tumor formation as assessed by magnetic resonance imaging (MRI).
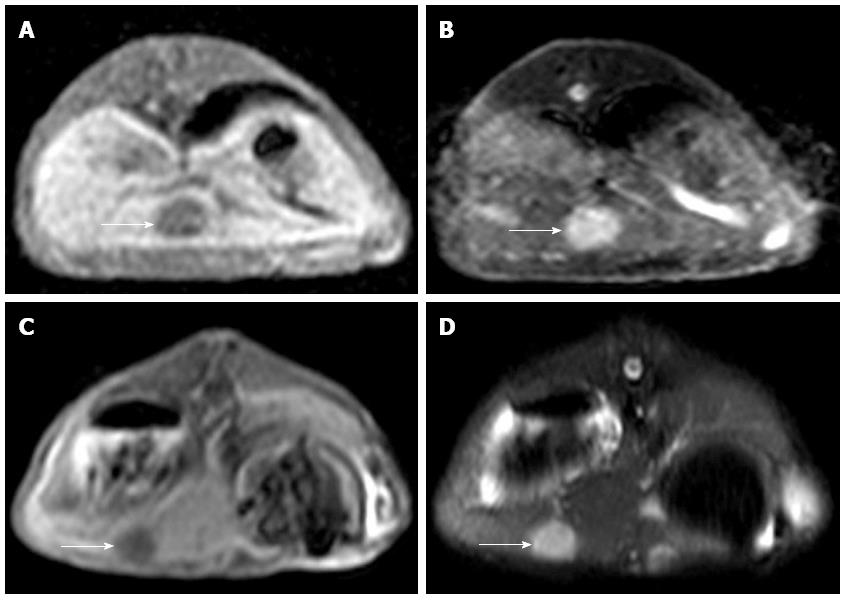
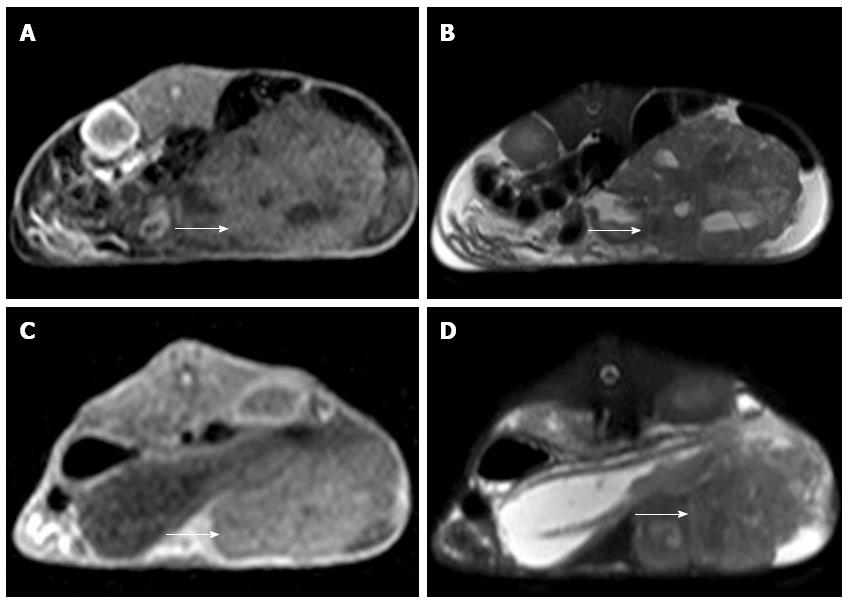
MRI detection: All of the rabbits in Groups A and B were subjected to 3 T MRI to assess the implant. Successful implantation criteria were as follows: intrahepatic nodules or space-occupying masses identified via MRI with a slightly low signal on T1WI and slightly high signal on T2WI. Celiac implantation and abdominal wall violation were also assessed via MRI. All scans were performed on a 3-T MR scanner (Philips Achieva 3.0-T X-series, Phillips Healthcare, The Netherlands) using the 16-element phased array of a SENSEXL Torso coil. The imaging protocol included the following parameters: (1) coronal sense turbo spin-echo T2-weighted fat-suppressed MRI [repetition time (TR)/echo time (TE), 1565/70 ms; average, 3; flip angle, 90 degrees; matrix size, 252 × 193; field of view (FOV), 24 cm × 24 cm; section thickness, 3 mm; and gap, 0.3 mm]; (2) axial plane sense turbo spin-echo T2-weighted fat-suppressed MRI (TR/TE, 1565/70 ms; average, 3; flip angle, 90 degrees; matrix size, 252 × 192; FOV, 24 cm × 24 cm; section thickness, 4 mm and gap, 0.4 mm); and (3) axial plane sense turbo spin-echo T1-weighted MRI (T1WI; TR/TE, 10/2.3 ms; average, 3, flip angle, 15 degrees; matrix, 252 × 193; FOV, 24 cm × 24 cm; section thickness, 4 mm; and gap, 0.4).
Following MRI examination, the experimental rabbits were sacrificed via air embolism, and the liver was anatomized to observe the growth of the tumors. The tumors were removed and fixed in formalin. Pathological testing was performed to confirm the histopathology of the intrahepatic nodules or space-occupying masses found through MRI.
The data were analyzed using SPSS 17.0 software. All of the data for continuous variables are expressed as the median and range. The t-test was employed to compare the differences between the medians of continuous variables for operation time and incision size. The Fisher exact test or χ2 test was used to compare proportions between groups regarding incision infections, death rates, inoculation success rates, celiac implantation and abdominal wall violation. Statistical significance was defined as P < 0.05.
Five rabbits in Group A (traditional laparotomy method, 15) died within two weeks. Four of these rabbits died on the day of operation, and coagulated blood that may have resulted from liver rupture was found in their abdominal cavities during autopsy. The other rabbit died four days after the operation. Festering was observed on the abdominal wall, which may have been due to infection.
No death was observed in Group B (modified laparotomy method, 15) within two weeks. Two of the remaining 11 rabbits in Group A and 1 of the 15 rabbits in Group B developed an incision infection. After disinfection of the incision and injection of antibiotic treatment, the infections of 1 of the rabbits in Group A and the 1 rabbit in Group B were relieved. The statistical data showed that the operation time for Group B was shorter than that for Group A. Additionally, the incision length was shorter, and postoperative mortality at 2 wk was lower in Group B than in Group A. There was no obvious difference in the postoperative incision infection rate between the two groups. The data for comparison of the two groups are shown in Tables 1 and 2.
| Group | Mean operation time (min) | Mean incision size (cm) |
| A (traditional laparotomy method) | 21.2 ± 2.6 | 3.3 ± 0.5 |
| B (modified laparotomy method) | 17.6 ± 2.4 | 2.6 ± 0.4 |
| t test | 3.903 | 2.928 |
| P value | 0.001 | 0.007 |
| Group | Number of infected rabbits | Number of non-infected rabbits | Postoperative incision infection rate (%) | Mortality in each group | Survival in each group | Mortality rate (%) |
| A (traditional laparotomy method) | 2 | 9 | 18.2 | 5 | 10 | 33.3 |
| B (modified laparotomy method) | 1 | 14 | 6.7 | 0 | 15 | 0 |
| P value | 0.556 | 0.042 |
MRI examination was performed on the remaining 10 experimental rabbits in Group A and 15 in Group B to evaluate the inoculation success rate and tumor growth. Nine out of 10 of the remaining rabbits in Group A and 14 out of 15 of the rabbits in Group B were implanted successfully (Figure 2). Six of the 10 rabbits in Group A and 2 of the 15 in Group B appeared to exhibit celiac implantation (Figure 3). Five of the 10 rabbits in Group A and 1 of the 15 in Group B appeared to show abdominal wall invasion (Figure 4). There was no significant difference between the two groups in the implantation success rate. The celiac implantation ratio and the frequency of invasion of the abdominal wall in Group A were higher than in Group B. The MRI findings for the two groups are shown in Table 3.
| Group | Successfully implanted | Unsuccessfully implanted | Successful implantation rate | With celiac implantation | No celiac implantation | Celiac implantation rate | With abdominal wall invasion | No abdominal wall invasion | Abdominal wall invasion rate |
| A (traditional laparotomy method) | 9 | 1 | 90% | 6 | 4 | 60% | 5 | 5 | 50% |
| B (modified laparotomy method) | 14 | 1 | 93.3% | 2 | 13 | 13.3% | 1 | 14 | 6.7% |
| P value | 1 | 0.028 | 0.023 |
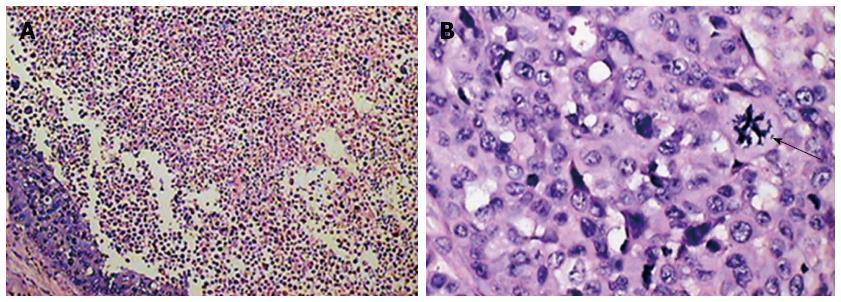
It was confirmed that the intrahepatic nodules or masses observed by MRI were VX2 hepatocarcinomas and that they shared common characteristics with human hepatic carcinoma. The histological findings are shown in Figure 5.
There are many methods for establishing a rabbit VX2 hepatocarcinoma model, such as puncture under computed tomography (CT)[8] or ultrasound (US)[9] guidance, direct percutaneous puncture inoculation[10,11], open implantation[12-15], and perfusion via the hepatic artery or portal vein[16]. Because special equipment and laboratory space are needed for implantation under CT or US guidance, the application of this procedure is limited. In contrast, direct percutaneous puncture inoculation presents the disadvantage of a lower success rate. Therefore, open implantation, which shows an acceptable success rate[17], is now widely used. However, it is extremely easy for invasion of nearby tissues and liver damage to occur after conventional open implantation. To improve the success rate of modeling, researchers have proposed several modifications[18].
The difference between the improved laparotomy method and the conventional method is that implantation of tumor fragments is accomplished via injection instead of by building a sinus using tweezers. This subtle change has many advantages. First, because the rabbit liver is thin, brittle and fragile, it is likely to bleed after conventional piercing. Once bleeding occurs, it is difficult to stop under experimental conditions. The modified laparotomy method involving injection with a 15 G syringe needle can markedly decrease both bleeding and the death rate caused by liver rupture. Second, the conventional method can lead to abdominal cavity transplantation[12], as the tumor fragments inserted with tweezers can easily enter the abdominal cavity. Additionally, the sinus becomes wider after tumor insertion, which also facilitates the spilling of tumor tissue out of the sinus. Third, Lee et al[19] found that the left lobe of the rabbit liver is preferable for implanting VX2 carcinoma. Yoon et al[17] showed that implanting VX2 carcinoma in the left lobe of the rabbit liver, which deviates from the abdominal wall, was effective in preventing tumor invasion in the muscular tissue of the rabbit abdominal wall. We were able to perform implantation effectively using the improved puncture method developed in this study. Fourth, compared with building a sinus in liver tissue using tweezers, injection requires a smaller operating field and surgical incision, decreases injury and shortens the incision and suturing time. Therefore, this method can improve efficiency, decrease surgical risk, and be easily performed.
Both Groups A and B showed a high success rate, as the process of laparotomy occurred under direct vision, which can ensure that the tumor tissue becomes embedded into the liver tissue. The infection rate did not differ between the two groups, due to the aseptic procedure, correct incision and suturing performed by the same skilled operator.
Considering the above-mentioned analysis, we concluded that there was no difference in the tumor formation rate between the improved and conventional laparotomy methods for modeling rabbit VX2 hepatocarcinoma. Nevertheless, the improved method is recommended because it has several advantages, such as decreasing injury to the liver, shorter operation time, a lower death rate, less abdominal cavity implantation and fewer abdominal wall invasions.
In conclusion, we found that the tumor formation rates were not significantly different between the two methods for modeling rabbit VX2 hepatocarcinoma. However, the improved method is recommended because it shows several advantages.
Early detection and diagnosis of liver cancer are an important issue that must be addressed. It is crucial to establish an animal model simulating human liver cancer to study the disease. Rabbit VX2 hepatocarcinoma is an ideal model of liver cancer that is widely used. There are various ways to establish rabbit VX2 hepatocarcinoma, including the widely adopted classical method of open laparotomy implantation. However, the traditional laparotomy implantation method presents some disadvantages. Therefore, the authors developed a modified method to improve the modeling of this disease.
To investigate hepatocarcinoma more completely, we needed to establish an ideal liver cancer model that is stable and controllable and in which the implanted lesion is limited.
The authors injected tumor fragments into the left lobe of the rabbit liver, deviating from the abdominal wall, using a 15 G syringe needle, instead of implanting tumor fragments by building a sinus using tweezers.
The improved method is recommended because of showing advantages such as decreased injury to the liver, shorter operation time, a lower death rate, less abdominal cavity implantation and fewer abdominal wall invasions.
Open laparotomy implantation is a widely adopted classical method for establishing rabbit VX2 carcinoma. In this study, the authors improved this method, which could be useful in the study of liver cancer.
This is an important field of study and the authors examine an interesting approach. Overall the writing and phrasing should be improved. In addition, the weaknesses of the various models could be discussed more. Indeed, the most interesting aspect to the study may rather be the description of the method as the results are so dramatically related to sample size. Therefore the text should more closely reflect the procedural aspect of the study.
P- Reviewer: Lawless MW, Ouyang YY S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Kudo M. Hepatocellular carcinoma 2009 and beyond: from the surveillance to molecular targeted therapy. Oncology. 2008;75 Suppl 1:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 3. | Shope RE, Hurst EW. Infectious papillomatosis of rabbits: with a note on the histopathology. J Exp Med. 1933;58:607-624. [PubMed] [Cited in This Article: ] |
| 4. | Nitta N, Sonoda A, Seko A, Ohta S, Nagatani Y, Tsuchiya K, Otani H, Tanaka T, Kanasaki S, Takahashi M. A combination of cisplatin-eluting gelatin microspheres and flavopiridol enhances anti-tumour effects in a rabbit VX2 liver tumour model. Br J Radiol. 2010;83:428-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Deng J, Virmani S, Yang GY, Tang R, Woloschak G, Omary RA, Larson AC. Intraprocedural diffusion-weighted PROPELLER MRI to guide percutaneous biopsy needle placement within rabbit VX2 liver tumors. J Magn Reson Imaging. 2009;30:366-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Merkle EM, Boll DT, Boaz T, Duerk JL, Chung YC, Jacobs GH, Varnes ME, Lewin JS. MRI-guided radiofrequency thermal ablation of implanted VX2 liver tumors in a rabbit model: demonstration of feasibility at 0.2 T. Magn Reson Med. 1999;42:141-149. [PubMed] [Cited in This Article: ] |
| 7. | Sun JH, Zhang YL, Nie CH, Yu XB, Xie HY, Zhou L, Zheng SS. Considerations for two inoculation methods of rabbit hepatic tumors: Pathology and image features. Exp Ther Med. 2012;3:386-390. [PubMed] [Cited in This Article: ] |
| 8. | Wang Z, Yang G, Nie P, Fu J, Wang X, Liu D. Dynamical observation on biological progression of VX2 liver tumors to identify the optimal time for intervention in animal models. PLoS One. 2013;8:e74327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 454] [Reference Citation Analysis (0)] |
| 9. | Zou X, Liu Q, Zhou X, He G, Yu M, Han Z, Meng X, Su H. Ultrasound-guided percutaneous laser and ethanol ablation of rabbit VX2 liver tumors. Acta Radiol. 2013;54:181-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Lin WY, Chen J, Lin Y, Han K. Implantation of VX2 carcinoma into the liver of rabbits: a comparison of three direct-injection methods. J Vet Med Sci. 2002;64:649-652. [PubMed] [Cited in This Article: ] |
| 11. | Thorstensen O, Isberg B, Svahn U, Jorulf H, Venizelos N, Jaremko G. Experimental tissue transplantation using a biopsy instrument and radiologic methods. Invest Radiol. 1994;29:469-471. [PubMed] [Cited in This Article: ] |
| 12. | Virmani S, Harris KR, Szolc-Kowalska B, Paunesku T, Woloschak GE, Lee FT, Lewandowski RJ, Sato KT, Ryu RK, Salem R. Comparison of two different methods for inoculating VX2 tumors in rabbit livers and hind limbs. J Vasc Interv Radiol. 2008;19:931-936. [PubMed] [Cited in This Article: ] |
| 13. | Zhou CW, Li FQ, Qin Y, Liu CM, Zheng XL, Wang ZB. Non-thermal ablation of rabbit liver VX2 tumor by pulsed high intensity focused ultrasound with ultrasound contrast agent: Pathological characteristics. World J Gastroenterol. 2008;14:6743-6747. [PubMed] [Cited in This Article: ] |
| 14. | Saad-Hossne R, Teixeira FV, Denadai R. In vivo assessment of intratumoral aspirin injection to treat hepatic tumors. World J Hepatol. 2013;5:372-378. [PubMed] [Cited in This Article: ] |
| 15. | Virmani S, Rhee TK, Ryu RK, Sato KT, Lewandowski RJ, Mulcahy MF, Kulik LM, Szolc-Kowalska B, Woloschak GE, Yang GY. Comparison of hypoxia-inducible factor-1alpha expression before and after transcatheter arterial embolization in rabbit VX2 liver tumors. J Vasc Interv Radiol. 2008;19:1483-1489. [PubMed] [Cited in This Article: ] |
| 16. | Burgener FA, Violante MR. Comparison of hepatic VX2-carcinomas after intra-arterial, intraportal and intraparenchymal tumor cell injection. An angiographic and computed tomographic study in the rabbit. Invest Radiol. 1979;14:410-414. [PubMed] [Cited in This Article: ] |
| 17. | Yoon CJ, Chung JW, Park JH, Yoon YH, Lee JW, Jeong SY, Chung H. Transcatheter arterial chemoembolization with paclitaxel-lipiodol solution in rabbit VX2 liver tumor. Radiology. 2003;229:126-131. [PubMed] [Cited in This Article: ] |
| 18. | Cao W, Wan Y, Liang ZH, Duan YY, Liu X, Wang ZM, Liu YY, Zhu J, Liu XT, Zhang HX. Heated lipiodol as an embolization agent for transhepatic arterial embolization in VX2 rabbit liver cancer model. Eur J Radiol. 2010;73:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Lee KH, Liapi E, Buijs M, Vossen J, Hong K, Georgiades C, Geschwind JF. Considerations for implantation site of VX2 carcinoma into rabbit liver. J Vasc Interv Radiol. 2009;20:113-117. [PubMed] [Cited in This Article: ] |