Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.10927
Revised: March 19, 2014
Accepted: May 29, 2014
Published online: August 21, 2014
AIM: To determine the miss rate for colorectal flat adenomas during colonoscopy and the risk factors.
METHODS: Flat adenomas are frequently missed during colonoscopy. However, the risk factors that influence their miss rates are unclear. This was a multicenter, retrospective study in which patients diagnosed with colorectal adenomas at a diagnostic colonoscopy and followed within 3 mo by a second therapeutic colonoscopy were pooled out from the established database. The “per-patient” and “per-adenoma” adenoma miss rates (AMR) for overall adenomas and flat adenomas, and patient-, adenoma-, and procedure-related risk factors potentially associated with the “per-adenoma” AMR for flat adenomas were determined.
RESULTS: Chromoscopy and high-definition colonoscopy were not taken under consideration in the study. Among 2093 patients with colorectal adenomas, 691 (33.0%) were diagnosed with flat adenomas, 514 with concomitant protruding adenomas and 177 without. The “per-patient” AMR for flat adenomas was 43.3% (299/691); the rates were 54.3% and 11.3%, respectively, for those with protruding adenomas and those without (OR = 9.320, 95%CI: 5.672-15.314, χ2 = 99.084, P < 0.001). The “per-adenoma” AMR for flat adenomas was 44.3% (406/916). In multivariate analysis, older age, presence of concomitant protruding adenomas, poor bowel preparation, smaller adenoma size, location at the right colon, insufficient experience of the colonoscopist, and withdrawal time < 6 min were associated with an increased “per-adenoma” AMR for flat adenomas. The AMR for flat adenomas was moderately correlated with that for overall adenomas (r = 0.516, P < 0.0001). The AMR for flat adenomas during colonoscopy was high.
CONCLUSION: Patient’s age, concomitant protruding adenomas, bowel preparation, size and location of adenomas, proficiency of the colonoscopist, and withdrawal time are factors affecting the “per-adenoma” AMR for flat adenomas.
Core tip: The miss rate for flat adenomas during colonoscopy is high. Patient’s age, concomitant protruding adenomas, bowel preparation, size and location of adenomas, proficiency of the colonoscopist, and withdrawal time are factors affecting the “per-adenoma” adenoma miss rate for flat adenomas.
- Citation: Xiang L, Zhan Q, Zhao XH, Wang YD, An SL, Xu YZ, Li AM, Gong W, Bai Y, Zhi FC, Liu SD. Risk factors associated with missed colorectal flat adenoma: A multicenter retrospective tandem colonoscopy study. World J Gastroenterol 2014; 20(31): 10927-10937
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/10927.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.10927
Colorectal cancer (CRC) is the most common malignant tumor and the second leading cause of cancer-related deaths in the world[1]. CRC mainly originates from colorectal adenomas[2]. According to the morphology, Kudo et al[3] classified colorectal adenomas into protruding and flat ones. It has been reported that colorectal flat adenomas have a greater tendency to develop into severe dysplasia and carcinoma than protruding adenomas[4,5]. However, because of their flat morphology and low awareness among colonoscopists, many flat adenomas are missed during colonoscopy although they are often visible. Therefore, flat adenomas are not only difficult to detect, but also easy to miss[6-8]. Specifically, the miss rates for flat adenomas during colonoscopy range from 35% to 60%, which are much higher than those (4%-19%) seen in the protruding type of adenomas[6,9,10]. It is believed that undetected or missed adenomas may play an important role in the incidence of interval cancers[11].
Colonoscopy has been considered as the “golden standard” in detection of colorectal adenomas and plays an important role in CRC prevention. In addition, colonoscopic polypectomy with follow-up monitoring has been proven to decrease the incidence of colorectal cancer, mainly in the left colon[12]. However, colorectal adenomas, especially flat adenomas, are frequently missed during colonoscopy as mentioned above. Flat adenomas are frequently localized at the right part of the colon, and it has been suggested that the high miss rate for flat adenomas at the right colon contributes to the high incidence of cancer at the right colon after colonoscopy[13]. Therefore, it is extremely important and essential to recognize and identify these flat neoplastic lesions at an early stage. The use of new techniques such as chromoendoscopy or magnifying narrow-band imaging during colonoscopy in recent years appears to significantly improve the detection of colorectal flat adenomas during colonoscopy[4,10,13,14]; however, controversy exists[9,15].
It is critical to determine the miss rate of colonoscopy for colorectal flat adenomas, and more importantly, to identify the risk factors that are associated with the increased flat adenoma miss rates. However, there are only few studies evaluating the miss rates of flat adenomas[9,15]. Moreover, the risk factors influencing the miss rate for flat adenomas have not been explored and thus are not understood. Therefore, this multicenter study aimed to determine the miss rates in detection of colorectal flat adenomas during colonoscopy and the risk factors that influence the miss rates.
This was a multicenter, retrospective study in which patients diagnosed with colorectal adenomas at a diagnostic colonoscopy and followed within 3 mo by a second therapeutic colonoscopy[16] between September 2009 and September 2011 from four Chinese hospitals were pooled out from the database established in the computerized system for colonoscopy. The study proposal was approved by the ethics committees of these four institutions (Medical Ethics Committee of Nanfang Hospital, Southern Medical University; Medical Ethics Committee of Wuxi City People’s Hospital Affiliated with Nanjing Medical University; Ethics Committee of Mianyang Central Hospital; Medical Ethics Committee of Shenzhen Longgang Central Hospital). All patients gave written informed consent at the first and second colonoscopy to allow their colonoscopy data to be used for this research purpose.
The patients enrolled in this study had to meet the following criteria: (1) they were 18 years old or over; (2) the colonoscope reached the cecum (i.e., completion of colonoscopy); (3) the interval duration between the first and second colonoscopy procedures was less than 90 d; the first colonoscopy was only for diagnosis, and the second colonoscopy was for therapeutic purpose and with good bowel preparation; (4) colonic images were properly taken at various parts of the colon; it was essential that the cecum, appendiceal orifice or ileocecal valve was clearly pictured after insertion of the colonoscope (to indicate the completion of the colonoscope insertion) and the images of the rectum were properly taken during the withdrawal of the colonoscope (to ensure calculation of the withdrawal time); and (5) the colonoscopists at the first colonoscopy had performed normal total colonoscopy on more than 100 cases; the colonoscopists at the second colonoscopy had performed colonoscopy on more than 1000 cases, with more than 150 cases annually. Cases with colorectal cancer, polyposis syndrome (defined as conditions where a patient had more than 100 polyps in the intestine, including familial polyposis syndrome, serrated polyposis syndrome, Peutz-Jeghers syndrome, juvenile polyposis syndrome, and Cronkhite-Canada syndrome), inflammatory bowel disease, partial large bowel resection or insufficient data required for analysis were excluded from this study.
Large bowel preparation was performed by using polyethylene glycol electrolyte solution, sodium phosphate or mannitol based on the hospital practice and guidelines. Patients were examined first in the left lateral decubitus position. Then, the patient was placed in the supine position or on the right side as needed to facilitate intubation of the cecum. Colonoscopy procedures were carried out by using electronic colonoscopies CF-240 I and CF-260 I (Olympus, Tokyo, Japan) in our study. In cases with areas suspicious of adenoma, dye solution (3-6 mL of 0.2% indigo carmine, Nanjing Weichuang Medical Technology Co., Ltd., Nanjing, China) was sprayed directly onto the areas with a 20 mL syringe to allow a cushion of air to push the dye through the biopsy channel. Images of any lesions were taken. Endoscopic mucosal resection, endoscopic piecemeal mucosal resection or endoscopic submucosal dissection was used for endoscopic resection of flat adenomas.
The primary endpoints were the miss rate and the associated risk factors of flat adenoma. The second colonoscopy was used as the reference standard, and any adenomas that were identified at the first colonoscopy were not counted at the second colonoscopy. Thus, all adenomas that were identified at the second colonoscopy but not detected at the first colonoscopy were defined as missed adenomas. The adenoma miss rate (AMR) was calculated using both patient and adenoma based analyses. The “per-patient” AMR was calculated by the number of patients with missed adenoma(s) divided by the total number of patients examined. The “per-adenoma” AMR was calculated by the number of missed adenoma(s) divided by the total number of adenomas detected at both examinations. Accordingly, the AMR for flat adenomas was also calculated.
The risk factors potentially associated with the miss of adenomas, flat adenomas in particular, included in the study were patient-related, such as demographic and clinical characteristics, and quality of bowel preparation; adenoma-related, such as the size and location, and pathologic classification; and procedure-related, such as proficiency and specialty of the colonoscopist, colonoscopy operative mode, and withdrawal time. A total of 29 colonoscopists were involved in this study. The proficiency of colonoscopists was defined by the cumulative cases of colonoscopy as (1) more than 1000; (2) between 500 to 1000; and (3) less than 500 cases. The specialty of colonoscopists was defined as gastroenterology and non-gastroenterology. Quality of bowel preparation was assessed by the colonoscopist at colonoscopy, which was graded as being excellent (no or minimal solid stool and only clear fluid requiring suction), adequate (collections of semi-solid debris that are cleared with washing/suction) or poor (solid or semi-solid debris that cannot be cleared) as previously described[17]. Cases with excellent and adequate bowel preparation were grouped together as good bowel preparation.
The mode of colonoscopy operation was defined as one-person (i.e., only the endoscopist personally advanced the endoscope during insertion.) or two-person (i.e., colonoscopy was performed with a nurse or assistant actively advancing the colonoscope during insertion) technique. The withdrawal time was defined as the time taken for colonoscopy withdrawal to the rectum minus the time taken for colonoscopy insertion to the cecum based on the times recorded on the images. The withdrawal time of the colonoscopy for a particular colonoscopist was represented by the average time of at least 100 normal total diagnostic colonoscopy procedures (i.e., no lesions were detected) performed by that colonoscopist as recorded in the database as previously described[18].
The adenoma size was measured by the colonoscopist during the colonoscopy using the opening aperture of a biopsy forceps (6 mm as a cut-off value), or after resection and recorded in the colonoscopy reports. The locations of adenomas were defined as the right colon (including the cecum, ascending colon, transverse colon, and hepatic flexure), the left colon (including the sigmoid colon, descending colon and splenic flexure) and the rectum. Adenoma location was estimated using anatomic landmarks and insertion distances. The Japanese Research For Cancer of Colon and Rectum Classification was applied to classify lesions as protruding and flat including flat elevated and flat depressed) lesions[3]. Flat elevated lesions were defined as those with a height less than half of the lesion in diameter[19]. Flat depressed lesions were defined as those with a central distinct depression[20]. Two experienced endoscopists verified the morphology from the photo documentation for a representative group of adenomas that were randomly selected from the analyzed samples. Pathological classifications were interpreted by pathologists using the WHO and Vienna criteria for colorectal adenoma[21]. The serrated adenomas including sessile serrated adenomas/polyps and traditional serrated adenomas were diagnosed as recommended by the WHO[22], and intraepithelial dysplasia was defined as low and high grade, depending on the glandular complexity, extent of nuclear stratification, and severity of nuclear morphology[23]. Advanced adenomas were defined as tubular adenomas of at least 10 mm in diameter (including serrated adenomas) or as adenomas containing villous or tubulovillous histological characteristics, high grade dysplasia, or any combination thereof[24].
Continuous variables are expressed as mean ± SD, and categorical variables are expressed as proportion or percentage. Comparisons among multiple groups with continuous variables were performed by analysis of variance. The χ2 test was used to determine the association between potential risk factors and miss of adenoma. The Bonferroni test was used for multiple comparisons. The multivariate logistic regression analysis was used to determine the independent risk factors for flat adenoma. To establish a group of missed adenomas and a group of diagnosed adenomas, one of the adenomas missed at the first colonoscopy was selected from each of patients with missed adenoma(s) and one of the adenomas detected at the first colonoscopy selected from each of patients without any missed adenoma, by using a simple random sampling method. Then, a multivariate analysis model was developed to determine independent risk factors associated with an increased AMR for colorectal flat adenoma. The Spearman correlation analysis was used to analyze the correlation between the “per-adenoma” AMR for overall adenomas and that for flat adenomas, as previously used for determination of the correlation between the detection rate for overall adenomas and that for flat adenomas by Reinhart et al[25]. A P value < 0.05 was considered statistically significant. SPSS 17.0 (IBM, United States) statistical software was employed in this study.
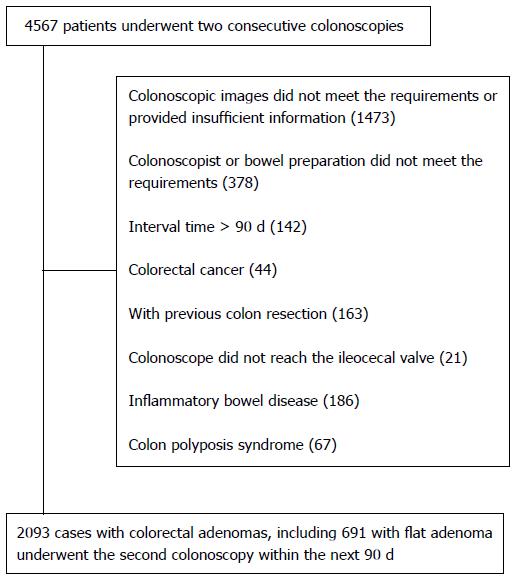
Overall, 4567 patients who underwent two consecutive colonoscopies were screened from the data system, of which 2474 were ineligible for the study, and thus 2093 patients with colorectal adenomas were included in the study (Figure 1). Among these 2093 patients, 691 (33.0%) were diagnosed with flat adenoma(s), including 177 without protruding adenoma and 514 with protruding adenoma. The remaining 1402 (67%) cases were diagnosed with protruding adenomas without flat adenomas. Among the 177 patients only with flat adenomas and those with flat adenomas and concomitant protruding adenomas, males accounted for 51.4% and 64%, respectively. Among the 514 patients with flat adenomas and concomitant protruding adenomas, 222 (43.2%) had more than three adenomas at the first colonoscopy, which was significantly greater than that in other groups (Table 1).
| Patients | Total(n = 2093) | Flat adenoma(n = 177) | Flat and protruding adenoma (n = 514) | Protruding adenoma(n = 1402) | P value |
| Sex | |||||
| Male | 1369 | 91 (51.4) | 329 (64.0) | 949 (67.7) | < 0.001 |
| Female | 724 | 86 (48.6) | 185 (34.0) | 453 (32.3) | < 0.001 |
| Age (mean ± SD, yr) | 55.91 ± 12.68 | 59.30 ± 12.47 | 54.78 ± 13.24 | < 0.001 | |
| Alarm symptoms | 1621 | 134 (75.7) | 388 (75.5) | 1099 (78.4) | 0.341 |
| Diverticulosis | 123 | 10 (5.6) | 27 (5.3) | 86 (6.1) | 0.761 |
| Family history of CRC | 131 | 11 (6.2) | 32 (6.2) | 88 (6.3) | 0.999 |
| History of adenomas | 475 | 40 (22.6) | 139 (27.0) | 296 (21.1) | 0.023 |
| History of abdominal surgery | 149 | 3 (1.7) | 35 (6.8) | 111 (7.9) | 0.010 |
| Cases with adenomas ≥ 3 at first colonoscopy | 458 | 8 (4.5) | 222 (43.2) | 228 (16.3) | < 0.001 |
In total, 4632 adenomas were detected, including 3665 detected at the first colonoscopy and 967 detected at the second colonoscopy but missed at the first colonoscopy. There were 916 flat adenomas (19.8%) and 3716 protruding adenomas (80.2%). Compared with the protruding colorectal adenomas, the flat adenomas were mainly localized in the right colon (410/916; 44.8%), and smaller in size [most (744/916, 81.2%) were less than 10 mm] (Table 2). Pathologically, the majority of flat adenomas (745/916; 81.3%) were tubular adenomas, followed by tubulovillous or villous adenoma (151/916; 16.3%) and serrated adenomas (20/916; 2.2%). The proportion of a villous structure in patients with flat adenomas was more than that in protruding adenoma (16.5% vs 13.9%, P = 0.041). Compared with the protruding adenomas, the flat adenomas were more associated with high grade dysplasia adenoma (7.5% vs 5.2%, P = 0.006) (Table 2). Among the 916 flat adenomas, 906 (98.9%) were classified as flat elevated and 10 (1.1%) as flat depressed adenomas. Flat elevated and flat depressed adenomas were mainly located at the right colon (44.7% vs 50.0%). However, more flat depressed adenomas had an adenoma size of 6-9 mm (70.0% vs 42.4%), a pathologically tubulovillous and villous type (50.0% vs 16.1%) and a high-grade dysplasia (40.0% vs 7.2%), compared with flat elevated adenomas. However, due to the small number of cases with flat depressed adenomas, no further analysis was conducted to compare the two types of flat adenomas.
| Characteristic | Total (n = 4632) | Flat (n = 916) | Protruding (n = 3716) | χ2test | P value |
| Location | 61.902 | < 0.001 | |||
| Rectum | 1003 | 184 (20.1) | 819 (22.0) | ||
| Left colon | 2054 | 322 (35.2) | 1732 (46.6) | ||
| Right colon | 1575 | 410 (44.8) | 1165 (31.4) | ||
| Size | 95.954 | < 0.001 | |||
| < 6 mm | 1223 | 353 (38.5) | 870 (23.4) | ||
| 6-9 mm | 2159 | 391 (42.7) | 1768 (47.6) | ||
| ≥ 10 mm | 1250 | 172 (18.8) | 1078 (29.0) | ||
| Pathological classification | 13.775 | 0.001 | |||
| Tubular | 3910 | 745 (81.3) | 3165 (85.2) | ||
| Tubulovillous and villous | 666 | 151 (16.5) | 515 (13.9) | ||
| Serrated | 56 | 20 (2.2) | 36 (1.0) | ||
| Dysplasia | 7.534 | 0.006 | |||
| Low | 4370 | 847 (92.5) | 3523 (94.8) | ||
| High | 262 | 69 (7.5) | 193 (5.2) | ||
| Advanced adenoma | 19.606 | < 0.001 | |||
| No | 3322 | 711 (77.6) | 2611 (70.3) | ||
| Yes | 1310 | 205 (22.4) | 1105 (29.7) |
Among the 2093 patients, “missed” adenomas were observed in 560, and thus the overall “per-patient” AMR was 26.8%. Accordingly, the “per-patient” AMR were 43.3% (299/691) in patients with flat adenomas; the rates were 54.3% (279/514) and 11.3% (20/177), respectively, for those with concomitant protruding adenomas and those without (OR = 9.320, 95%CI: 5.672-15.314, χ2 = 99.084, P < 0.001). The “per-patient” AMR was 18.6% (261/1402) for those with only protruding adenomas, which was significantly lower than that for those with flat adenomas (OR = 0.300, 95%CI: 0.245-0.367, χ2 = 143.566, P < 0.001).
Among the 4632 adenomas, 967 were missed at the first colonoscopy, and thus the overall “per-adenoma” AMR was 20.9%. Accordingly, the “per-adenoma” AMRs were 44.3% (406/916) and 15.1% (561/3716), respectively, for flat and protruding adenomas (OR = 4.477, 95%CI: 3.822-5.245, χ2 = 380.002, P < 0.001).
In addition, a total of 205 advanced flat adenomas were diagnosed in 200 patients at the first and second examinations; 24 were missed in 22 patients. Thus, the “per-patient” and “per-adenoma” AMRs for advanced flat adenomas were 11.0% and 11.7%, respectively.
Associations of potential risk factors related to patients, adenomas, and procedures are summarized in Table 3.
| Risk factor | Total(n = 916) | Diagnosed(n = 510) | Missed(n = 406) | Univariate analysis | Multivariate analysis | ||
| χ2test | P value | OR (95%CI) | P value | ||||
| Patient-related | |||||||
| Age | 9.021 | 0.003 | |||||
| < 60 (yr) | 493 | 297 (60.2) | 196 (39.8) | 1 | |||
| ≥ 60 (yr) | 423 | 213 (50.4) | 210 (49.6) | 2.062 (1.390-3.061) | < 0.001 | ||
| Sex | 1.586 | 0.208 | |||||
| Male | 559 | 302 (54.0) | 257 (46.0) | ||||
| Female | 357 | 208 (58.3) | 149 (41.7) | ||||
| Anesthesia | 0.197 | 0.657 | |||||
| Yes | 239 | 136 (56.9) | 103 (43.1) | ||||
| No | 677 | 374 (55.2) | 303 (44.8) | ||||
| History of adenomas | |||||||
| Yes | 243 | 129 (53.1) | 114 (46.9) | 0.899 | 0.343 | ||
| No | 673 | 381 (56.6) | 292 (43.4) | ||||
| Previous surgery | 0.105 | 0.746 | |||||
| Yes | 45 | 24 (53.3) | 21 (46.7) | ||||
| No | 871 | 486 (55.8) | 385 (44.2) | ||||
| Diverticulosis | 0.013 | 0.909 | |||||
| Yes | 51 | 28 (54.9) | 23 (45.1) | ||||
| No | 865 | 482 (55.7) | 383 (44.3) | ||||
| Numbers at first colonoscopy | 0.163 | 0.686 | |||||
| < 3 | 580 | 320 (55.2) | 260 (44.8) | ||||
| ≥ 3 | 336 | 190 (56.5) | 146 (43.5) | ||||
| Concomitance with protruding adenoma(s) at first colonoscopy | 127.154 | < 0.001 | |||||
| No | 280 | 234 (83.6) | 46 (16.4) | 1 | |||
| Yes | 636 | 276 (43.4) | 360 (56.6) | 7.759 (4.420-13.618) | < 0.001 | ||
| Bowel preparation | 45.773 | < 0.001 | |||||
| Good | 757 | 460 (60.8) | 297 (39.2) | 1 | |||
| Poor | 159 | 50 (31.4) | 109 (68.6) | 4.389 (2.314-8.352) | < 0.001 | ||
| Adenoma-related | |||||||
| Size | 122.706 | < 0.001 | |||||
| ≥ 10 mm | 172 | 159 (92.4) | 13 (7.6) | 1 | |||
| 6-9 mm | 391 | 202 (51.7) | 189 (48.3) | 9.239 (4.306-19.824) | < 0.001 | ||
| < 6 mm | 353 | 149 (42.2) | 204 (57.8) | 19.613 (8.984-42.822) | < 0.001 | ||
| Location | 74.571 | < 0.001 | |||||
| Rectum | 184 | 152 (82.6) | 32 (17.4) | 6.81 | 0.0091 | 1 | |
| Left colon | 322 | 175 (54.3) | 147 (45.7) | 2.866 (1.623-5.062) | < 0.001 | ||
| Right colon | 410 | 183 (44.6) | 227 (55.4) | 3.259 (1.819-5.838) | < 0.001 | ||
| Advanced adenoma | 113.850 | < 0.001 | |||||
| Yes | 205 | 181 (88.3) | 24 (11.7) | ||||
| No | 711 | 329 (46.3) | 382 (53.7) | ||||
| Pathologic classification | 74.745 | < 0.001 | |||||
| Tubular | 745 | 366 (49.1) | 379 (50.9) | ||||
| Tubulovillous and villous | 151 | 132 (87.4) | 19 (12.6) | ||||
| Serrated | 20 | 12 (60.0) | 8 (40.0) | ||||
| Procedure-related | |||||||
| Proficiency | 28.18 | < 0.001 | |||||
| > 1000 cases | 585 | 363 (62.1) | 222 (37.9) | 1 | |||
| 500-1000 cases | 234 | 109 (46.6) | 125 (53.4) | 2.219 (1.397-3.525) | 0.001 | ||
| < 500 cases | 97 | 38 (39.2) | 59 (60.8) | 3.003 (1.568-5.754) | 0.001 | ||
| Specialty | 6.86 | 0.009 | |||||
| Gastroenterologist | 739 | 427 (57.8) | 312 (42.2) | ||||
| Non-gastroenterologist | 177 | 83 (46.9) | 94 (53.1) | ||||
| Operative mode | 4.862 | 0.027 | |||||
| One-person technique | 721 | 415 (57.6) | 306 (42.4) | ||||
| Two-person technique | 195 | 95 (48.7) | 100 (51.3) | ||||
| Withdrawal time | 6.069 | 0.014 | |||||
| ≥ 6 min | 254 | 158 (62.2) | 96 (37.8) | 1 | |||
| < 6 min | 662 | 352 (53.2) | 310 (46.8) | 1.958 (1.276-3.006) | 0.020 | ||
In univariate analysis, older age, presence of concomitant protruding adenomas, poor bowel preparation, smaller size of adenoma, location at the right colon, tubular type, non-advanced adenoma, insufficient experience of the colonoscopist, double operative mode and withdrawal time < 6 min were associated with an increased “per-adenoma” AMR for flat adenomas (Table 3). In the multivariate analysis, all above factors, except for pathological type of adenomas, status of advanced adenoma, non-gastroenterology specialty and double operative mode, were identified to be independently associated with an increased “per-adenoma” AMR for flat adenomas (Table 3).
In univariate analysis, an adenoma size less than 10 mm, the location at the right colon, poor bowel preparation, and non-gastroenterology specialty were significantly associated with an increased AMR for advanced flat adenomas. Due to the small number of advanced flat adenomas, multivariate analysis was not performed (Table 4).
| Factor | Total (n = 205) | Diagnosed (n = 181) | Missed (n = 24) | χ2test | P value |
| Size | 17.796 | < 0.001 | |||
| < 10 mm | 33 | 22 (66.7) | 11 (33.3) | ||
| ≥ 10 mm | 172 | 159 (92.4) | 13 (7.6) | ||
| Location | 11.838 | 0.003 | |||
| Rectum | 68 | 67 (98.5) | 1 (1.5) | 1.127 | 0.2881 |
| Left colon | 36 | 32 (88.9) | 4 (11.1) | ||
| Right colon | 101 | 82 (81.2) | 19 (18.8) | ||
| Pathologic classification | 0.025 | 0.874 | |||
| Tubular | 57 | 50 (87.7) | 7 (12.3) | ||
| Tubulovillous and villous | 148 | 131 (88.5) | 17 (11.5) | ||
| Age (yr) | 1.157 | 0.282 | |||
| < 60 | 98 | 89 (90.8) | 9 (9.2) | ||
| ≥ 60 | 107 | 92 (86.0) | 15 (1.4) | ||
| Bowel preparation | 13.815 | < 0.001 | |||
| Good | 162 | 150 (92.6) | 12 (7.4) | ||
| Poor | 43 | 31 (72.1) | 12 (27.9) | ||
| Proficiency | 1.078 | 0.583 | |||
| > 1000 cases | 135 | 120 (88.9) | 15 (11.1) | ||
| 500-1000 cases | 55 | 49 (89.1) | 6 (10.9) | ||
| < 500 cases | 15 | 12 (80.0) | 3 (20.0) | ||
| Specialty | 6.167 | 0.013 | |||
| Gastroenterologist | 156 | 146 (93.6) | 10 (6.4) | ||
| Non- gastroenterologist | 49 | 35 (71.4) | 14 (28.6) | ||
| Operative mode | 2.272 | 0.132 | |||
| One-person technique | 161 | 145 (90.1) | 16 (9.9) | ||
| Two-person technique | 44 | 36 (81.8) | 8 (18.2) | ||
| Withdrawal time | |||||
| ≥ 6 min | 145 | 129 (89.0) | 16 (11.0) | 0.217 | 0.641 |
| < 6 min | 60 | 52 (86.7) | 8 (13.3) | ||
The median “per-adenoma” AMRs for overall and flat adenomas obtained by different colonoscopists were 22.3% (interquartile range, 18.37%-26.35%) and 45.65% (interquartile range, 34.48%-60.83%). There was a moderate correlation between the total miss rates for overall adenomas and the miss rates for flat adenomas. The correlation coefficient was 0.516 (P < 0.001).
The present study revealed that colorectal flat adenomas are very common in patients undergoing colonoscopy. Moreover, flat adenomas are more frequently seen in the Oriental population than in the Western population[26]. However, this type of adenoma is frequently missed in clinical practice, especially when the flat adenoma is concomitant with a protruding adenoma(s). In the present study, the “per-adenoma” flat adenoma miss rate (44.3%) is significantly higher than the overall adenoma miss rate (20.9%). It is also significantly higher than that of the protruding adenoma (15.1%). These results are in line with those of previous reports[6,27].
Moreover, multivariate logistic regression analysis showed that many factors including patient-related, adenoma-related, and procedure-related ones influenced the miss rates for colorectal flat adenomas. In the present study, age was shown to be an independent risk factor affecting the detection of flat adenomas. Patients older than 60 years had a much higher miss rate than those younger than 60 years. This finding is consistent with a previous study, which showed that miss rate was higher in patients with older age in both univariate and multivariate analyses[16].
The quality of bowel preparation also affects the detection of flat adenomas. Sufficient bowel preparation is a prerequisite for a better view of flat adenomas[28]. Poor bowel preparation places more impacts on the detection of flat adenomas than protruding adenomas[7]. Our present study showed that poor bowel preparation was closely correlated with a higher miss rate for flat adenomas, which was as high as 68.6%. Correspondingly, the miss rate for advanced flat adenomas also increased significantly with poor preparation. When the bowel is poorly prepared, mucus and chymes released from the small intestine could easily accumulate in the cecum and ascending colon, and thus, it is even more difficult to clean out these parts of the colon than the left half of the colon. Previous studies have shown that the quality of bowel preparation, especially in the right colon, can be improved by changing the cleaning methods so as to increase the flat adenoma detection rates[7,29].
It has been known that protruding adenomas frequently co-exist with flat adenomas[4]. In the present study, co-existence was observed in approximately one fourth (514/2093) of patients with colorectal adenomas. Previous studies have demonstrated that co-existence of concomitant protruding adenomas is an independent risk factor affecting the detection rate for flat adenomas[30]. The present study demonstrated that the miss rate in patients with flat adenomas and concomitant protruding adenomas was significantly higher than those with flat adenomas only or those with protruding adenomas only. For the first time, co-existence with concomitant protruding adenomas was identified as an independent risk factor affecting the miss rate for flat adenomas. We postulate that once the protruding adenomas are detected, the less apparent flat adenomas could be easily neglected by the colonoscopist because the detection of the protruding adenoma represents a positive endoscopic diagnosis, which is usually considered by the colonoscopist to be the cause of the indication (e.g., clinical symptoms or signs) for colonoscopy. Also, the numbers of adenomas were more than three in most patients with flat and concomitant protruding adenomas in a previous observation[4]. Our study showed that 44.3% patients with flat and concomitant protruding adenomas had more than three adenomas at the first colonoscopy, which is in agreement with the previous observation.
It has been suggested that the number of colorectal adenomas identified at the initial colonoscopy is positively associated with the miss rates for overall adenomas[6,27]. However, in the present study, there was no significant association between the numbers of adenomas detected at the first colonoscopy and the miss rates for flat adenomas in both univariate and multivariate analyses. The discrepancy between the present study and previous studies suggests the difference in the risk factors for the miss rates between flat adenomas and overall adenomas, in terms of the number of adenomas identified at the initial colonoscopy. In other words, the adenoma numbers at the first colonoscopy is associated with the miss rate for overall adenomas, but not with the miss rate for flat adenomas. Specifically, the adenoma number of ≤ three at the first colonoscopy is associated with a lower miss rate for overall adenomas, compared with the number of more than three[6,26]. However, there was no association between the adenoma number at the first colonoscopy and AMR for flat adenomas. Even if the adenoma number at the first colonoscopy is less than three, AMR for flat adenomas is still very high and similar to that in those patients with more than three adenomas at the first colonoscopy.
The present study revealed that adenoma location and size significantly affected the miss rates for this type of adenoma. We also found that the miss rate was much higher for adenomas < 10 mm than those ≥ 10 mm and for flat adenomas in the right colon than those in the left colon, which is different from the miss rate for overall adenoma observed in a previous study[6,16]. The reasons for the discrepancy are considered as follows: (1) Unlike protruding adenomas, flat adenomas are majorly localized at the right colon[13,31]; and (2) the colonic pouch at the right colon is deep and big, and thus it is difficult to detect the flat adenoma inside the deep colonic pouch. Therefore, it is essential to take measures such as training in fold exploration, specification of right colon withdrawal time, and examination of the right colon twice to improve the detection rate for adenomas at the right colon. The miss rates for flat adenomas in the left and right colon were significantly higher than that in the rectum, clearly indicating that the miss rate for flat adenoma in the colon is much higher than that in the rectum. Our study also revealed that the proportions of histologically villous adenomas and high-grade dysplastic adenoma in patients with flat adenomas were significantly higher than those in patients with protruding adenomas, which was consistent with previous observations[4,5,31]. In the present study, we reported an incidence (1.1%) of flat depressed adenomas, which is similar to that (1.4%) observed by Nicolás-Pérez et al[30], indicating that the incidence of flat depressed adenomas is very low. Based on previous findings[4,5,30], it is conceivable that the flat depressed adenomas may have a great tendency to develop dysplasia or even neoplasia. Indeed, flat depressed adenomas appeared to be more associated with high-grade dysplasia as observed in the present study. However, the number of cases with this type of adenomas in the present study was small and thus further investigation with a larger number of cases with this particular type of adenoma is needed. In addition, we found that the miss rate for advanced adenomas was slightly higher, albeit not statistically significantly, in the right colon than that in the left colon. This finding is in agreement with previous observations that flat adenomas including those located at the right colon have a higher tendency to become malignant than the protruding adenoma[4,5,31]. Thus, reduction of the miss rate for flat adenomas will help to decrease the occurrence of the colon cancer, especially in the right colon.
In the present study, proficiency of colonoscopists and the withdrawal time of colonoscopy were identified as independent risk factors affecting the miss rates for flat adenomas, which is consistent with previous observations on the miss rates for the overall adenoma[32-34]. Specifically, the miss rates significantly decreased in patients undergoing colonoscopy performed by more experienced and knowledgeable operators. It has been shown that the staining endoscopy technique is very helpful in improving the detection of flat adenomas by conventional white light colonoscopy[13,35]. Therefore, we recommend that less-experienced endoscopists learn this technique as soon as possible. Currently, the withdrawal time is required to be kept for more than six minutes according to the colonoscopy guidelines in order to guarantee the quality of colonoscopy[34], as the withdrawal time of more than six minutes can carefully observe colonic mucous and effectively reduce the miss rate for flat adenomas. Our study further supports that the withdrawal time of more than 6 min can reduce the miss rate and improve the detection of flat adenomas.
The background of colonoscopists may affect the quality of colonoscopy. Bressler and colleagues[36] suggested that colonoscopy by an internist or family physician was an independent risk factor for new or missed CRC. In addition, the protective effects of colonoscopy on the right and left colon when colonoscopy was performed by gastroenterologists are similar. However, the protective effect against colon cancer was less on the right colon than on the left colon if colonoscopy was performed by non-gastroenterologists[37]. In the present study, univariate analysis showed that flat adenomas, as well as advanced flat adenomas, in cases operated by gastroenterologists were less likely missed than those by non-gastroenterologists. However, multivariate analysis did not reveal this finding. Thus, we postulate that the impact of colonoscopy by non-gastroenterologists on the miss rate for flat adenomas may be demolished if the colonoscopists are well-trained and the quality of colonoscopy improved. In addition, our study showed that there was no difference in the miss rate between colonoscopies performed by one person and those by two persons, in the multivariate analysis, which is consistent with a previous observation that the operative mode of colonoscopy performed by either single or double operators had no significant influence on the quality of colonoscopy[38].
We further analyzed the association between the potential risk factors and the miss rates for advanced flat adenomas, and found that the proficiency of endoscopists, mode of operation, and withdrawal time were not associated with the miss rates for advanced flat adenomas. The main reasons are that the advanced adenomas are usually large, or the villous structure on the colonic surface is more easily recognized. Therefore, the miss rates could be low even if the endoscopists lack experience, and the withdrawal time is shorter than the conventional standards. It should be stated that only univariate analysis was used to determine the association due to a limited number of patients with advanced flat adenomas, and thus studies with large sample sizes are needed to further determine the risk factors for advanced flat adenomas.
Kahi et al[39] suggested that it would be reasonable to consider that improved detection of nonpolypoid lesions by thorough and high-quality colonoscopy will result in improved overall adenoma detection rates. On the contrary, in the largest study of flat adenoma detection, Reinhart et al[25] demonstrated only a poor correlation between the overall adenoma detection rate and the detection rate for flat adenomas (r = 0.24). Thus, they did not support the recommendation of adding the flat adenoma detection rate to the widely accepted adenoma detection rate in clinical practice[40]. So far, there has been no report on the correlation between the miss rate for the overall adenomas and that for flat adenomas. The present study, from the perspective of AMR analysis, showed a better correlation (r = 0.516), compared with Reinhart’s study regarding the adenoma detection rates. Therefore, we postulate that, on the routine colonoscopy, the missed adenomas are proportional to the missed flat adenomas, and thus, measures should be taken to avoid missing flat adenomas.
There were some limitations in this study. For example, due to the retrospective nature of the study, the withdrawal time was counted by the average time of an endoscopist to perform a negative colonoscopy instead of the actual time in each patient; nevertheless, this method has been applied previously[18]. In addition, some auxiliary techniques such as chromoscopy and high-definition colonoscopy were excluded from this study. Studies have shown that applications of these techniques are able to reduce the miss rate for flat adenomas[10,14]. However, for the routine colonoscopy, these techniques may not be required as previous studies have shown similar miss rates for colorectal adenomas[9,15]. It should be mentioned that the endoscopic morphologic classification of flat adenomas may be important in predicting post resection recurrence. However, the present study did not focus on this issue and, due to the small number of flat depressed adenomas (only 10 out of 916 flat adenomas), any analyzed results based on this number may not be clinically meaningful, and thus a well-designed study specifically targeting this issue is warranted.
In conclusion, the miss rate for flat adenomas during colonoscopy is high. Patient’s age, concomitant protruding adenomas, bowel preparation, size and location of adenomas, proficiency of the colonoscopist, and withdrawal time are factors affecting the “per-adenoma” AMR for flat adenomas. These findings have significant clinical implications in helping clinicians to detect and treat flat adenomas and thus prevent the development of CRC, and reduce the incidence, morbidity and mortality of CRC.
We would like to thank Dr. Bo Jiang who is the director of Guangdong Provincial Key Laboratory of Gastroenterology, Department of Gastroenterology, Nanfang Hospital, Southern Medical University, for support and assistance. We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Colorectal cancer (CRC) is the most common malignant tumor and the second leading cause of cancer-related deaths in the world. CRC mainly originates from colorectal adenomas. It has been reported that flat colorectal adenomas have a greater tendency to develop into severe dysplasia and carcinoma than protruding adenomas
Flat adenomas are frequently missed during colonoscopy. However, the risk factors that influence their miss rates are unclear.
The study aimed to determine the miss rate for colorectal flat adenomas during colonoscopy and the risk factors. The “per-patient” and “per-adenoma” adenoma miss rates (AMR) for overall adenomas and flat adenomas, and patient-, adenoma-, and procedure-related risk factors potentially associated with the “per-adenoma” AMR for flat adenomas were determined. Chromoscopy and high-definition colonoscopy were not taken under consideration in the study.
This is an interesting paper on the risk factors associated with missed flat adenomas, which provides a contribution to the current knowledge of this topic.
P- Reviewer: Pescatori M, Souza JLS S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8283] [Cited by in F6Publishing: 8159] [Article Influence: 509.9] [Reference Citation Analysis (0)] |
| 2. | Kim EC, Lance P. Colorectal polyps and their relationship to cancer. Gastroenterol Clin North Am. 1997;26:1-17. [PubMed] [Cited in This Article: ] |
| 3. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 4. | Parra-Blanco A, Gimeno-García AZ, Nicolás-Pérez D, García C, Medina C, Díaz-Flores L, Grosso B, Jiménez A, Quintero E. Risk for high-grade dysplasia or invasive carcinoma in colorectal flat adenomas in a Spanish population. Gastroenterol Hepatol. 2006;29:602-609. [PubMed] [Cited in This Article: ] |
| 5. | Hurlstone DP, Cross SS, Adam I, Shorthouse AJ, Brown S, Sanders DS, Lobo AJ. A prospective clinicopathological and endoscopic evaluation of flat and depressed colorectal lesions in the United Kingdom. Am J Gastroenterol. 2003;98:2543-2549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 7. | Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, Grosso B, Jimenez A, Ortega J, Quintero E. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006;12:6161-6166. [PubMed] [Cited in This Article: ] |
| 8. | Church JM, Muto T, Appau K. Flat lesions of the colorectal mucosa: differences in recognition between Japanese and American endoscopists. Dis Colon Rectum. 2004;47:1462-1466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Kahi CJ, Anderson JC, Waxman I, Kessler WR, Imperiale TF, Li X, Rex DK. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterol. 2010;105:1301-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 13. | Lasisi F, Rex DK. Improving protection against proximal colon cancer by colonoscopy. Expert Rev Gastroenterol Hepatol. 2011;5:745-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Hirata I, Nakagawa Y, Ohkubo M, Yahagi N, Yao K. Usefulness of magnifying narrow-band imaging endoscopy for the diagnosis of gastric and colorectal lesions. Digestion. 2012;85:74-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, Sharma VK. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363-370; quiz 371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Kim JH, Kim YS, Cheon JH, Lee SK, Kim TI, Myoung S, Kim WH. Influence of the insertion time and number of polyps on miss rate in colonoscopy. Scand J Gastroenterol. 2011;46:634-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, Nickerson C, McNally RJ, Patnick J, Rees CJ. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 18. | Simmons DT, Harewood GC, Baron TH, Petersen BT, Wang KK, Boyd-Enders F, Ott BJ. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24:965-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Sawada T, Hojo K, Moriya Y. Colonoscopic management of focal and early colorectal carcinoma. Baillieres Clin Gastroenterol. 1989;3:627-645. [PubMed] [Cited in This Article: ] |
| 20. | Kudo S, Tamura S, Hirota S, Sano Y, Yamano H, Serizawa M, Fukuoka T, Mitsuoka H, Nakajima T, Kusaka H. The problem of de novo colorectal carcinoma. Eur J Cancer. 1995;31A:1118-1120. [PubMed] [Cited in This Article: ] |
| 21. | Huang SF. [The World Health Organization and the Vienna classification of gastrointestinal epithelial neoplasia]. Zhonghua Binglixue Zazhi. 2005;34:540-541. [PubMed] [Cited in This Article: ] |
| 22. | Aust DE, Baretton GB, Members of the Working Group GIPotGSoP. Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria. Virchows Arch. 2010;457:291-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Cooper H. Pathology of the gastrointestinal tract. 2nd ed. Baltimore: William & Willkins 1999; . [Cited in This Article: ] |
| 24. | Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959-2967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Reinhart K, Bannert C, Dunkler D, Salzl P, Trauner M, Renner F, Knoflach P, Ferlitsch A, Weiss W, Ferlitsch M. Prevalence of flat lesions in a large screening population and their role in colonoscopy quality improvement. Endoscopy. 2013;45:350-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Fiche M, Bonvin R, Bosman F. Microscopes and computers in small-group pathology learning. Med Educ. 2006;40:1138-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 878] [Cited by in F6Publishing: 868] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 28. | Soetikno RM, Kahng LS, Ono A, Fujii T. Flat and depressed colorectal neoplasms. Curr Opin Gastroenterol. 2003;19:69-75. [PubMed] [Cited in This Article: ] |
| 29. | Park JS, Sohn CI, Hwang SJ, Choi HS, Park JH, Kim HJ, Park DI, Cho YK, Jeon WK, Kim BI. Quality and effect of single dose versus split dose of polyethylene glycol bowel preparation for early-morning colonoscopy. Endoscopy. 2007;39:616-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Nicolás-Pérez D, Parra-Blanco A, Gimeno-García AZ, Ortega-Sánchez JA, Carrillo-Palau M, Jiménez-Sosa A, Quintero-Carrion E. Risk factors associated with colorectal flat adenoma detection. Eur J Gastroenterol Hepatol. 2013;25:302-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Kil Lee S, Il Kim T, Kwan Shin S, Ho Kim W, Kim H, Kyu Kim N. Comparison of the clinicopathologic features between flat and polypoid adenoma. Scand J Gastroenterol. 2008;43:1116-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Munroe CA, Lee P, Copland A, Wu KK, Kaltenbach T, Soetikno RM, Friedland S. A tandem colonoscopy study of adenoma miss rates during endoscopic training: a venture into uncharted territory. Gastrointest Endosc. 2012;75:561-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Millan MS, Gross P, Manilich E, Church JM. Adenoma detection rate: the real indicator of quality in colonoscopy. Dis Colon Rectum. 2008;51:1217-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA, Kirk LM, Litlin S, Lieberman DA, Waye JD. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 700] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 35. | Adler A, Pohl H, Papanikolaou IS, Abou-Rebyeh H, Schachschal G, Veltzke-Schlieker W, Khalifa AC, Setka E, Koch M, Wiedenmann B. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut. 2008;57:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 36. | Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 37. | Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 38. | Hoff G, Volker M, Bretthauer M, Aabakken L, Høie O, Delange T, Berset I, Kjellevold Ø, Glomsaker T, Huppertz-Hauss G. Gastronet survey on the use of one- or two-person technique for colonoscopy insertion. BMC Gastroenterol. 2011;11:73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Kahi CJ, Hewett DG, Rex DK. Relationship of non-polypoid colorectal neoplasms to quality of colonoscopy. Gastrointest Endosc Clin N Am. 2010;20:407-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16-S28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |