Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5235
Revised: December 11, 2013
Accepted: January 8, 2014
Published online: May 14, 2014
Helicobacter pylori (H. pylori) is a flagellated, spiral-shaped, microaerophilic Gram-negative bacillus that colonises the gastric mucosa of more than 50% of the human population. Infection is a risk factor for gastritis, ulcer disease and stomach cancer. Immunity against H. pylori is mainly related to Th1/Th17 skewing, and the activation of regulatory T cells is the main strategy used to limit inflammatory responses, which can result in the pathogen persistence and can lead to chronic gastrointestinal diseases, including cancer. Furthermore, host genetic factors that affect cytokines may determine differences in the susceptibility to many diseases. In this review, we present the cytokine profiles and the main cytokine gene polymorphisms associated with resistance/susceptibility to H. pylori and discuss how such polymorphisms may influence infection/disease outcomes.
Core tip: In this review, we present the cytokine profiles of infection and the main cytokine gene polymorphisms associated with resistance/susceptibility to Helicobacter pylori. We also discuss how such polymorphisms may influence infection/disease outcomes.
-
Citation: Figueiredo CA, Marques CR, Costa RDS, Silva HBFD, Alcantara-Neves NM. Cytokines, cytokine gene polymorphisms and
Helicobacter pylori infection: Friend or foe? World J Gastroenterol 2014; 20(18): 5235-5243 - URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5235
Helicobacter pylori (H. pylori) is a flagellated, spiral-shaped, microaerophilic Gram-negative bacillus that colonises the gastric mucosa, causing gastroduodenal diseases such as chronic gastritis, ulcer disease and gastric cancer[1]. H. pylori infection is the major environmental risk factor for non-cardia gastric adenocarcinoma. Although it is estimated that 50% of the population worldwide is infected by this bacterium, only a subset of 1% to 2% of those individuals will develop gastric malignancies[2,3]. The prevalence of H. pylori infection may vary depending on the studied population. Furthermore, researchers have associated the increased prevalence of H. pylori infection with socioeconomic indicators, as the incidence of infection is higher in developing countries[4].
H. pylori infection is associated with poor hygiene conditions[5]. Although the transmission mechanism of H. pylori has not been completely elucidated, evidence suggests that human-to-human spread via oral-oral or faecal-oral routes are important mechanisms[6]. The main mechanism of H. pylori survival in the stomach is due to the action of urease, which hydrolyses the urea present in the stomach into CO2 and ammonia, which then neutralise stomach acidity. These components are essential for neutralising gastric acid and promoting a favourable environment for H. pylori survival in the stomach[7,8].
H. pylori starts colonisation of the gastric mucosa by crossing the gastric mucus layer and adhering to the gastric epithelium, where it obtains the necessary nutrients and simultaneously avoids attack by the host immune system[1]. The inflammation caused by H. pylori infection is generated by multiple pathways from both the gastric epithelial cells, which are the host first line of defence against this organism, and the circulating immune cells recruited to the site of infection[9].
Several mechanisms of H. pylori pathogenicity have been described, including modification on host gene expression, decreased gastric acid secretion, infection-induced cell proliferation, epithelial cell elongation, degradation of cell-cell junctions and the production of systemic and mucosal IgA and IgG antibodies. However, the effect of antibody production on bacterial colonisation has not been completely elucidated[10].
The major pathogenicity factors of H. pylori include the virulence components vacuolating toxin (VacA), cytotoxin-associated gene A product (CagA) and γ-glutamyltranspeptidase (GGT), in addition to pathogen-associated molecular patterns (PAMPs) including lipopolysaccharide (LPS) and flagella[11]. The CagA+ strains are more potent in causing gastric mucosal damage than the CagA- strains[12]. Several studies have shown the importance of CagA in H. pylori-induced inflammation and suggest that CagA plays a role in nuclear factor kappa-B (NF-κB) activation and interleukin-8 (IL-8) production[2]. Moreover, the ectopic expression of CagA induces NF-κB nuclear translocation and IL-8 production in gastric epithelial cells[12].
Another crucial element required for the persistence of H. pylori infection is related to the secretion of the VacA protein, which is reported to inhibit both T cell proliferation and the production of the transcription factors necessary for IL-2 production[13]. VacA also induces the production of pro-inflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α), macrophage-inflammatory protein-1α, IL-1β, IL-6, IL-10 and IL-13 in a dose-dependent manner without causing the degranulation of mast cells[14]. Thus, H. pylori virulence factors play an important role in the infection and act by down-modulating immunity against the bacterium.
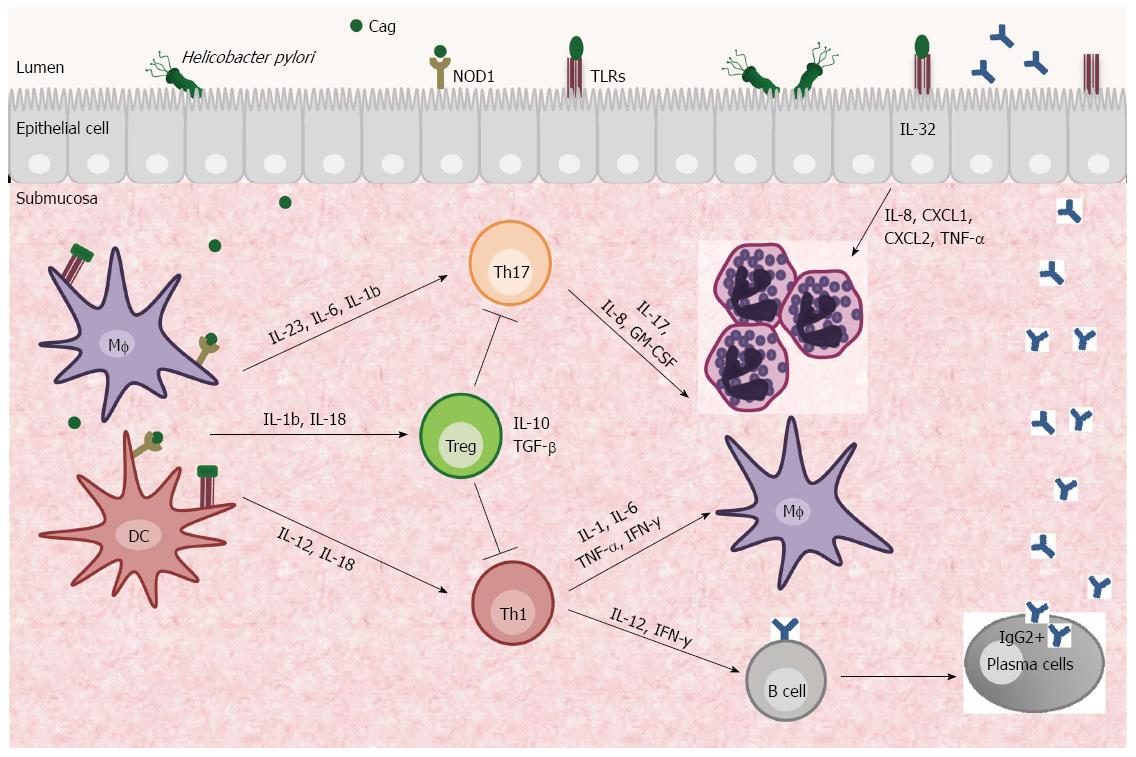
Colonization of the stomach by H. pylori elicits an inflammatory response and recruits neutrophils, lymphocytes, macrophages and dendritic cells (DCs) to the gastric mucosa (Figure 1)[15]. The molecular mechanisms by which H. pylori triggers and maintains the local immune response are complex, but there is evidence that cytokines produced by both innate and adaptive immune systems can lead to the development of ulcer disease, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue lymphoma[13].
The initial interaction between H. pylori and the innate host immune response is mediated through surface receptors expressed on gastric epithelial cells and antigen-presenting cells (APCs - DCs and macrophages). Integrins may recognise virulence factors excreted by the bacterium, and pattern recognition receptors (PRRs) such as Toll-like receptors (TLR) and Nucleotide-binding oligomerisation domain receptor 1 (NOD1) interact with bacterial PAMPs[14,16].
The activation of human gastric DCs by H. pylori directs naïve CD4+ T cells to Th1 differentiation through IL-12 production and enables these cells to secrete cytokines such as IL-1, IL-6, TNF-α and IFN-γ by activation of the transcription factors T-bet (T box expressed in T cells) and Stat 4 (signal transducer and activation of transcription factor 4)[17]. This differentiation is dependent on IL-18, which is a Th1-related cytokine that promotes IFN-γ production by T cells and natural killer (NK) cells in the presence of IL-12 and is correlated with gastric inflammation[18,19]. The initial Th1 response aims to eradicate the H. pylori infection. However, in some individuals Th1 cell-producing cytokines sustain the mucosal inflammation and contribute to the development of the infection-associated gastric preneoplastic immunopathology that manifests histologically as atrophic gastritis, epithelial hyperplasia and intestinal metaplasia[17].
Although it is expected that Th2 immune responses are required for protection against extracellular bacteria, Th2 cells are weakly associated with H. pylori infection[20]. However, a predominant Th2 immune response is characterised by IL-13 secreting cells. A Th2 response has been observed in patients with H. pylori-related intestinal metaplasia and intestinal-type gastric cancer, suggesting that Th2 cells could be involved in different outcomes of H. pylori infection[21].
In infected individuals, a Th2 response induces IgG1 production while a Th1 response contributes to significant increase in the overall levels of IgG2 through IL-2 and IFN-γ production. IgG2 titres are higher than IgG1 titres in H. pylori-infected patients, particularly those with ulcer disease[22]. Studies have reported that gastric cancer risk is positively correlated with higher serum antibody levels against H. pylori[23]. However, it has also been shown that weak serum IgG antibody responses against CagA may be associated with the risk of gastric cancer development, suggesting that CagA might play a role in carcinogenesis[24].
In addition to Th1/Th2 immune responses, Th17 cells also contribute to mucosal host defence by functioning as mediators of the inflammation associated with the production of IL-17, IL-8 and granulocyte macrophage colony-stimulating factor (GM-CSF), which are used to attract granulocytes. An increased IL-17 response depends on IL-6, IL-23 and CCL20 expression by H. pylori-stimulated macrophages and is regulated by Stat3 and the NF-κB pathway[25]. H. pylori-stimulated DCs are potent inducers of Th17 cells by regulating IL-1β/IL-23 production, and the virulence factor CagA may play an important role in mediating this process[26].
H. pylori-specific Th17 cells persist in the blood and gastric mucosa of individuals after H. pylori eradication and can be associated with the persistence of gastric inflammation following H. pylori eradication observed in both animal models and humans[27]. Th17 persistence has been shown to be a consequence of IL-1β levels, which remain elevated in the gastric mucosa and might favour the low-level proliferation of Th17 cells previously recruited into the gastric mucosa during active H. pylori infection[28].
In contrast to potent pro-inflammatory responses, neither Th1 nor Th17 cells are by themselves absolutely essential for the spontaneous control of Helicobacter infections. These data suggest that both Th subsets act synergistically or that there might be other Th subsets involved in the process[29]. Recently, it was shown that the expression of the intracellular pro-inflammatory cytokine IL-32 is induced in gastric epithelial cells by H. pylori in a Cag Pathogenicity Island (PAI)-dependent manner. IL-32 activates NF-κB and stimulates the production of cytokines and chemokines, including IL-8, CXCL1, CXCL2, and TNF-α; IL-32 high levels are also related to human gastritis and gastric cancer[30]. Further studies are necessary to clarify the role of IL-32 in H. pylori-infected gastric mucosa.
Despite inciting inflammation, the clearance of H. pylori is often incomplete due to the activation of CD4+CD25hi forkhead box protein 3 (Foxp3+) regulatory T cells (Tregs). Treg cells are potent suppressors of T cell effector function and regulate the balance between immunity and infection[31]. Treg cells have been implicated in limiting inflammatory responses to H. pylori by producing anti-inflammatory cytokines such as IL-10 and TGF-β that, in some circumstances, result in pathogen persistence to limit tissue injury. This process is positively correlated with chronic gastritis and gastric adenocarcinoma[31,32].
H. pylori infection is known to suppress host immune responses. However, the mechanism of this suppression is poorly understood, although it is hypothesised that the suppression is caused by efficient DC re-programming toward a tolerance-promoting phenotype via IL-1β and IL-18. These DC cells have the capacity to induce Treg cell production through mucosal expression of transforming growth factor-beta 1 (TGF-β1) and IL-10[33-35]. This regulation seems to be dependent on the CagA virulence factor, and only CagA+ H. pylori promotes IL-10 expression, inhibiting DC maturation and inducing DCs to have a tolerogenic phenotype which can suppress the proliferation of CD4+ and CD8+ effector T cells[36]. The functional outcome is the suppression of H pylori-specific Th17 and Th1 immunity and chronic colonization of the stomach[36,37].
Previous reports have demonstrated that the gastric mucosa of H. pylori-infected children contained higher numbers of Treg Foxp3+ cells and increased levels of IL-10, TGFβ1 and IL-6 compared to infected adults[38,39]. Thus, reduced gastric inflammation, including diminished neutrophil accumulation, in H. pylori-infected children compared to infected adults is likely due to the downregulation of gastric Th17 and Th1 responses caused by enhanced mucosal regulatory T-cell activity in children[39,40]. The down-regulated Th17 responses in the gastric mucosa of H. pylori-infected children might account for the susceptibility of children to the infection and to the persistence of infection. Additionally, there may be important implications in the development of an effective anti-H. pylori vaccine because IL-17 has been described as a key mediator of vaccine-induced reduction of H. pylori infection[39,41].
The enhanced Treg response induced by H. pylori chronic infection has been shown to modulate coexisting inflammatory disease by conferring protection against allergies, asthma and inflammatory bowel diseases[42]. An epidemiological study revealed an inverse association of asthma with seropositivity to H. pylori that was CagA-dependent. This result demonstrates the importance of the CagA virulence factor in inducing immune regulation[43].
The way certain individuals respond to H. pylori infection and how the immune cells are recruited to H. pylori may determine their susceptibility/resistance immune profiles to the bacterium. Furthermore, cytokine polymorphisms may play important roles in H. pylori pathogenicity.
The exon sequences of genes encoding cytokines and cytokine receptors are generally conserved. However, mutations in other gene regions that do not affect the amino acid sequence can be found. These polymorphisms may affect protein expression in various ways, including levels of gene transcription, splicing, stability and levels of mRNA translation[44]. Polymorphisms in cytokine genes may influence the development of several diseases[45]. Susceptibility to many diseases is associated with a particular ‘‘pro-inflammatory’’ profile that can be explained by individual genetic determinants. Host genetic factors that affect cytokine polymorphisms may determine why some individuals infected with H. pylori develop gastric cancer while others do not[46].
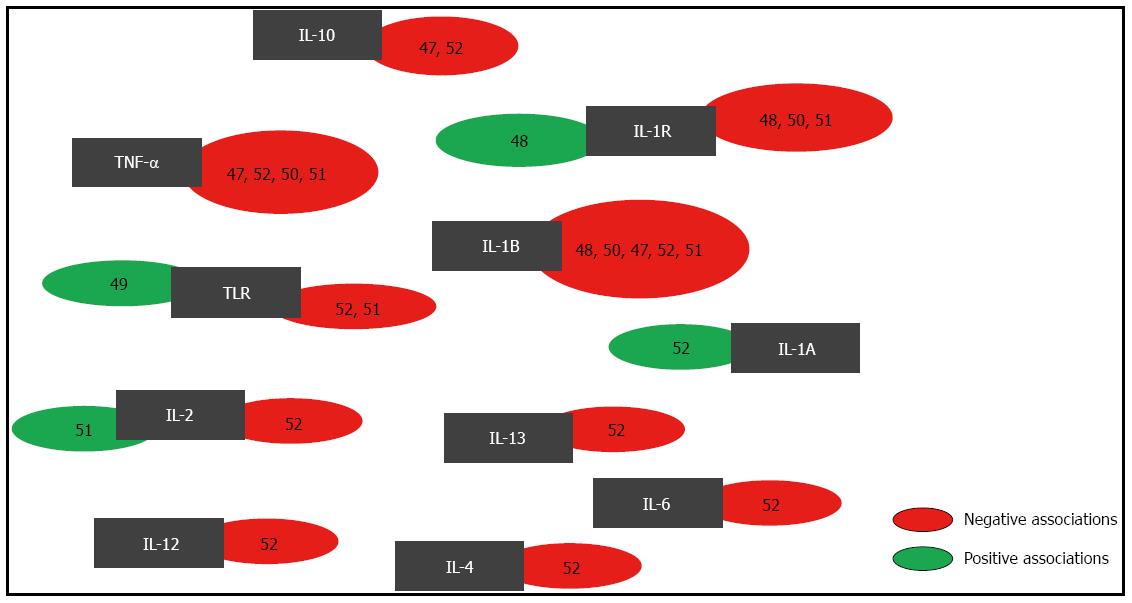
Several studies have evaluated the direct association of H. pylori infection with cytokine gene polymorphisms. Figure 2 presents the gene polymorphisms that were previously evaluated in association studies with risk for H. pylori infection. These genes include the following: IL-1R, IL-1B, IL-1A, TNF-α, IL-6, IL-4, IL-13, IL-12, IL-2 and IL-10[47-52]. To generate Figure 2, a systematic literature search was performed using Web of Science and PubMed. We searched for articles published from March 2000 until May 2013 using the following keywords: “H. pylori infection”, “H. pylori and polymorphism”, and “H. pylori and cytokine polymorphism and association”. We excluded the articles that had no full text available. Our results indicate that statistically significant associations were observed for IL-1R, IL-1A, IL-2 and TLR polymorphisms.
Many studies have examined the potential modulatory effect of H. pylori infection on the association between genetic polymorphisms and the risk of gastric cancer, ulcer disease, gastritis and lymphoid follicle lymphoma development[53-60]. Several gene polymorphisms have been reported; the most frequently studied are in the IL-1, IL-10 and TNF-α genes.
There are three members of the interleukin IL-1 gene family: IL-1A, IL-1B, and IL-1RN, which encode the pro-inflammatory cytokines IL-1α, IL-1β and the endogenous anti-inflammatory cytokine IL-1ra, respectively. The IL-1 gene cluster is located on chromosome 2q and contains three related genes within a 430-kb region[61]. The IL-1RN gene has a penta-allelic 86-bp variable number tandem repeat in intron 2. The IL-1RN gene is associated with a wide range of chronic inflammatory diseases and enhanced IL-1β secretion[62].
Tseng et al[52] investigated the relationship between the risk of H. pylori antibody seropositivity and cytokine gene polymorphisms among Jamaican children. They observed a negative association between IL-1A -889 allelic polymorphisms and the presence of H. pylori antibodies. Hartland et al[48] reported a positive association for IL-1R +1622 and H. pylori infection. However, no association was observed for IL-1RN polymorphisms[48,50] or the IL-1R +1498 polymorphism[48] and the risk of H. pylori infection.
Two diallelic polymorphisms at positions -511 and -31 in IL-1B have been extensively studied in several diseases. El-omar et al[46] reported that interleukin-1 gene cluster polymorphisms are associated with an increased risk of both hypochlorhydria induced by H. pylori and gastric cancer. Based on their results, several studies have examined the relationship of IL-1 polymorphisms with H. pylori infection in gastrointestinal diseases[63-66].
Studies have shown that gastric adenocarcinoma and ulcer disease have distinct effects on gastric secretions. Gastric cancer is associated with low gastric acid production, whereas ulcer disease is associated with high levels of gastric acid secretion[67,68]. IL-1β is also a potent inhibitor of gastric acid secretion[69,70]. IL-1β is upregulated in the presence of H. pylori and plays an important role in the initiation and amplification the inflammatory responses to infection[71-73].
The IL-10 gene is located on chromosome 1 (1q31-1q32). Many single-nucleotide polymorphisms (SNPs) have been identified in the IL-10 gene promoter region. Three polymorphic promoter variants in IL-10 are located at positions -1082, -819 and -592. These SNPs have been frequently studied, and gene variants are associated with increased IL-10 production[74,75].
Our group recently submitted a study that evaluates IL-10 gene polymorphisms and the risk of H. pylori infection. We identified associations for 4 SNPs that were risk factors for H. pylori infection (data not shown). Furthermore, 8 SNPs were associated with spontaneous IL-10 production, suggesting that IL-10 polymorphisms can modulate the levels of cytokine production and can thus modify the risk of H. pylori infection. Interestingly, statistically significant associations were identified between the IL-10 polymorphisms at positions -1082[57] and -592[63] and gastric cancer risk only in individuals infected by H. pylori. An increased risk of gastric cancer was observed in patients who carry these polymorphisms and are infected by H. pylori, suggesting a synergistic effect for the combination of host genotype and the presence of the bacterium.
The TNF-α gene is located within the human major histocompatibility complex (MHC) on chromosome 6p21.3. An important SNP that has been studied is located at position -308 in the promoter region. One mechanism regulating the expression of TNF-α occurs at the transcriptional level. Studies evaluating the relationship between TNF-α polymorphisms and H. pylori infection have found no associations[47,52]. However, many studies suggest that TNF-α polymorphisms are potential determinants of gastric disease susceptibility[76]. In contrast, Chakravorty et al[54] observed a negative association between the TNF-α -1031 polymorphism and ulcer disease only in patients infected by H. pylori.
The SNP TLR -113T/A was negatively associated with H. pylori seroprevalence[49]. However, no association with H. pylori seroprevalence was found with SNP TLR +636[52]. Furthermore, there were no significant associations between SNPs on TLR2 Arg677Trp, TLR2 Arg753Gln, TLR4 Asp299Gly and TLR5 392STOP and the risk of H. pylori infection[51].
No significant associations were found for SNPs IL-6 -597 G/A, IL-6 -572G/C, IL-6 -174 G/C, IL-4 -524 T/C and IL-13 -1069C/T[52]. These results suggest that more studies are needed to elucidate the roles of these polymorphisms as risk factors for H. pylori infection.
In the IL-2 gene, the T330G polymorphism was negatively associated with H. pylori infection and was responsible for increasing the serum IL-2 levels in H. pylori-positive adults and children[51]. The same polymorphism in the IL-2 gene was not an important contributor to the pathogenesis of ulcer disease, gastric cancer and ulcer disease in Korean patients[65]. This result suggests that genetic variations between ethnicities could influence H. pylori susceptibility and infection outcomes.
Sequencing of the IL-12 p40 gene revealed a TaqI restriction fragment length polymorphism in the 3′-untranslated region at position 1188 (A>C). Although there are inter-individual variations in IL-12 production levels, this cytokine is not involved in defining the genetic basis for peptic ulcer susceptibility[77,78]. Several studies have evaluated the impact of IL-12 gene SNPs in H. pylori-infected patients. Three SNPs tested in IL-12A (IVS2 -798 A>T, IVS2 -701 A>C and Ex7 +277 A>G) were not related to gastric cancer risk[79], while the other 2 SNPs (IL-12A 2504 and IL-12B +15485) were correlated with non-cardia gastric adenocarcinoma[80].
These studies support the hypothesis that a combination of host genotype and the presence of H. pylori could be crucial for the development of gastric diseases. More studies are necessary to explain the consequences of genetic polymorphisms at the cytokine level and their functions in and impacts on H. pylori susceptibility. Additional studies may provide support for new strategies of vaccine production against this infection.
P- Reviewers: Baryshnikova NV, Kato J, Yi JM S- Editor: Wen LL L- Editor: A E- Editor: Ma S
| 1. | Ruggiero P. Helicobacter pylori and inflammation. Curr Pharm Des. 2010;16:4225-4236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Lamb A, Chen LF. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J Cell Biochem. 2013;114:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Wu MS, Chen CJ, Lin JT. Host-environment interactions: their impact on progression from gastric inflammation to carcinogenesis and on development of new approaches to prevent and treat gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1878-1882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Azevedo NF, Huntington J, Goodman KJ. The epidemiology of Helicobacter pylori and public health implications. Helicobacter. 2009;14 Suppl 1:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Dattoli VC, Veiga RV, da Cunha SS, Pontes-de-Carvalho LC, Barreto ML, Alcântara-Neves NM. Seroprevalence and potential risk factors for Helicobacter pylori infection in Brazilian children. Helicobacter. 2010;15:273-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16 Suppl 1:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 232] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 7. | Schoep TD, Fulurija A, Good F, Lu W, Himbeck RP, Schwan C, Choi SS, Berg DE, Mittl PR, Benghezal M. Surface properties of Helicobacter pylori urease complex are essential for persistence. PLoS One. 2010;5:e15042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Voland P, Weeks DL, Marcus EA, Prinz C, Sachs G, Scott D. Interactions among the seven Helicobacter pylori proteins encoded by the urease gene cluster. Am J Physiol Gastrointest Liver Physiol. 2003;284:G96-G106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | McGee DJ, Mobley HL. Pathogenesis of Helicobacter pylori infection. Curr Opin Gastroenterol. 2000;16:24-31. [PubMed] [Cited in This Article: ] |
| 10. | Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:237-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 428] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 12. | Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18:479-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Caruso R, Pallone F, Monteleone G. Emerging role of IL-23/IL-17 axis in H pylori-associated pathology. World J Gastroenterol. 2007;13:5547-5551. [PubMed] [Cited in This Article: ] |
| 14. | Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, Chiba T, Fuss IJ, Kitani A, Strober W. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest. 2010;120:1645-1662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 15. | Suzuki T, Kato K, Ohara S, Noguchi K, Sekine H, Nagura H, Shimosegawa T. Localization of antigen-presenting cells in Helicobacter pylori-infected gastric mucosa. Pathol Int. 2002;52:265-271. [PubMed] [Cited in This Article: ] |
| 16. | Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Müller-Hermelink HK, Eck M. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Bimczok D, Clements RH, Waites KB, Novak L, Eckhoff DE, Mannon PJ, Smith PD, Smythies LE. Human primary gastric dendritic cells induce a Th1 response to H. pylori. Mucosal Immunol. 2010;3:260-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Shimada M, Ando T, Peek RM, Watanabe O, Ishiguro K, Maeda O, Ishikawa D, Hasegawa M, Ina K, Ohmiya N. Helicobacter pylori infection upregulates interleukin-18 production from gastric epithelial cells. Eur J Gastroenterol Hepatol. 2008;20:1144-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Yamauchi K, Choi IJ, Lu H, Ogiwara H, Graham DY, Yamaoka Y. Regulation of IL-18 in Helicobacter pylori infection. J Immunol. 2008;180:1207-1216. [PubMed] [Cited in This Article: ] |
| 20. | Taylor JM, Ziman ME, Canfield DR, Vajdy M, Solnick JV. Effects of a Th1- versus a Th2-biased immune response in protection against Helicobacter pylori challenge in mice. Microb Pathog. 2008;44:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Marotti B, Rocco A, De Colibus P, Compare D, de Nucci G, Staibano S, Tatangelo F, Romano M, Nardone G. Interleukin-13 mucosal production in Helicobacter pylori-related gastric diseases. Dig Liver Dis. 2008;40:240-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Martínez-Becerra F, Castillo-Rojas G, Ponce de León S, López-Vidal Y. IgG subclasses against Helicobacter pylori isolates: an important tool for disease characterization. Scand J Immunol. 2012;76:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Watanabe M, Kato J, Inoue I, Yoshimura N, Yoshida T, Mukoubayashi C, Deguchi H, Enomoto S, Ueda K, Maekita T. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer. 2012;131:2632-2642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Suzuki G, Cullings H, Fujiwara S, Hattori N, Matsuura S, Hakoda M, Akahoshi M, Kodama K, Tahara E. Low-positive antibody titer against Helicobacter pylori cytotoxin-associated gene A (CagA) may predict future gastric cancer better than simple seropositivity against H. pylori CagA or against H. pylori. Cancer Epidemiol Biomarkers Prev. 2007;16:1224-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Zhuang Y, Shi Y, Liu XF, Zhang JY, Liu T, Fan X, Luo J, Wu C, Yu S, Chen L. Helicobacter pylori-infected macrophages induce Th17 cell differentiation. Immunobiology. 2011;216:200-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Khamri W, Walker MM, Clark P, Atherton JC, Thursz MR, Bamford KB, Lechler RI, Lombardi G. Helicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytes. Infect Immun. 2010;78:845-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Serelli-Lee V, Ling KL, Ho C, Yeong LH, Lim GK, Ho B, Wong SB. Persistent Helicobacter pylori specific Th17 responses in patients with past H. pylori infection are associated with elevated gastric mucosal IL-1β. PLoS One. 2012;7:e39199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106:7119-7124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 426] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 29. | Hitzler I, Kohler E, Engler DB, Yazgan AS, Müller A. The role of Th cell subsets in the control of Helicobacter infections and in T cell-driven gastric immunopathology. Front Immunol. 2012;3:142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Sakitani K, Hirata Y, Hayakawa Y, Serizawa T, Nakata W, Takahashi R, Kinoshita H, Sakamoto K, Nakagawa H, Akanuma M. Role of interleukin-32 in Helicobacter pylori-induced gastric inflammation. Infect Immun. 2012;80:3795-3803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Cheng HH, Tseng GY, Yang HB, Wang HJ, Lin HJ, Wang WC. Increased numbers of Foxp3-positive regulatory T cells in gastritis, peptic ulcer and gastric adenocarcinoma. World J Gastroenterol. 2012;18:34-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Chang LL, Wang SW, Wu IC, Yu FJ, Su YC, Chen YP, Wu DC, Kuo CH, Hung CH. Impaired dendritic cell maturation and IL-10 production following H. pylori stimulation in gastric cancer patients. Appl Microbiol Biotechnol. 2012;96:211-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Kandulski A, Wex T, Kuester D, Peitz U, Gebert I, Roessner A, Malfertheiner P. Naturally occurring regulatory T cells (CD4+, CD25high, FOXP3+) in the antrum and cardia are associated with higher H. pylori colonization and increased gene expression of TGF-beta1. Helicobacter. 2008;13:295-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Mitchell PJ, Afzali B, Fazekasova H, Chen D, Ali N, Powell N, Lord GM, Lechler RI, Lombardi G. Helicobacter pylori induces in-vivo expansion of human regulatory T cells through stimulating interleukin-1β production by dendritic cells. Clin Exp Immunol. 2012;170:300-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, Maxeiner J, Hansson M, Taube C, Quiding-Järbrink M. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 36. | Tanaka H, Yoshida M, Nishiumi S, Ohnishi N, Kobayashi K, Yamamoto K, Fujita T, Hatakeyama M, Azuma T. The CagA protein of Helicobacter pylori suppresses the functions of dendritic cell in mice. Arch Biochem Biophys. 2010;498:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, Eaton KA, Zou W, Berndt BE, Cole TS. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138:1046-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 38. | Freire de Melo F, Rocha AM, Rocha GA, Pedroso SH, de Assis Batista S, Fonseca de Castro LP, Carvalho SD, Bittencourt PF, de Oliveira CA, Corrêa-Oliveira R. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes Infect. 2012;14:341-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Serrano C, Wright SW, Bimczok D, Shaffer CL, Cover TL, Venegas A, Salazar MG, Smythies LE, Harris PR, Smith PD. Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children. Mucosal Immunol. 2013;6:950-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Peña A, Rollán A, Viviani P, Guiraldes E. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134:491-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Velin D, Favre L, Bernasconi E, Bachmann D, Pythoud C, Saiji E, Bouzourene H, Michetti P. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology. 2009;136:2237-2246.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Arnold IC, Hitzler I, Müller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol. 2012;2:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI, Blaser MJ. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;3:e4060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Bidwell JL, Wood NA, Morse HR, Olomolaiye OO, Laundy GJ. Human cytokine gene nucleotide sequence alignments, 1998. Eur J Immunogenet. 1998;25:83-265. [PubMed] [Cited in This Article: ] |
| 45. | Bidwell JL, Wood NA, Morse HR, Olomolaiye OO, Keen LJ, Laundy GJ. Human cytokine gene nucleotide sequence alignments: supplement 1. Eur J Immunogenet. 1999;26:135-223. [PubMed] [Cited in This Article: ] |
| 46. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1690] [Cited by in F6Publishing: 1614] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 47. | Ando T, El-Omar EM, Goto Y, Nobata K, Watanabe O, Maeda O, Ishiguro K, Minami M, Hamajima N, Goto H. Interleukin 1B proinflammatory genotypes protect against gastro-oesophageal reflux disease through induction of corpus atrophy. Gut. 2006;55:158-164. [PubMed] [Cited in This Article: ] |
| 48. | Hartland S, Newton JL, Griffin SM, Donaldson PT. A functional polymorphism in the interleukin-1 receptor-1 gene is associated with increased risk of Helicobacter pylori infection but not with gastric cancer. Dig Dis Sci. 2004;49:1545-1550. [PubMed] [Cited in This Article: ] |
| 49. | Mayerle J, den Hoed CM, Schurmann C, Stolk L, Homuth G, Peters MJ, Capelle LG, Zimmermann K, Rivadeneira F, Gruska S. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA. 2013;309:1912-1920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 50. | Queiroz DM, Rocha AM, Melo FF, Rocha GA, Teixeira KN, Carvalho SD, Bittencourt PF, Castro LP, Crabtree JE. Increased gastric IL-1β concentration and iron deficiency parameters in H. pylori infected children. PLoS One. 2013;8:e57420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Queiroz DM, Saraiva IE, Rocha GA, Rocha AM, Gomes LI, Melo FF, Bittencourt PF. IL2-330G polymorphic allele is associated with decreased risk of Helicobacter pylori infection in adulthood. Microbes Infect. 2009;11:980-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Tseng FC, Brown EE, Maiese EM, Yeager M, Welch R, Gold BD, Owens M, Cranston B, Hanchard B, El-Omar E. Polymorphisms in cytokine genes and risk of Helicobacter pylori infection among Jamaican children. Helicobacter. 2006;11:425-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Achyut BR, Tripathi P, Ghoshal UC, Moorchung N, Mittal B. Interleukin-10 (-819 C/T) and tumor necrosis factor-alpha (-308 G/A) gene variants influence gastritis and lymphoid follicle development. Dig Dis Sci. 2008;53:622-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Chakravorty M, Datta De D, Choudhury A, Santra A, Roychoudhury S. Association of specific haplotype of TNFalpha with Helicobacter pylori-mediated duodenal ulcer in eastern Indian population. J Genet. 2008;87:299-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Chen CC, Yang SY, Liu CJ, Lin CL, Liaw YF, Lin SM, Lee SD, Chen PJ, Chen CJ, Yu MW. Association of cytokine and DNA repair gene polymorphisms with hepatitis B-related hepatocellular carcinoma. Int J Epidemiol. 2005;34:1310-1318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Gatti LL, Burbano RR, Zambaldi-Tunes M, de-Lábio RW, de Assumpção PP, de Arruda Cardoso-Smith M, Marques-Payão SL. Interleukin-6 polymorphisms, Helicobacter pylori infection in adult Brazilian patients with chronic gastritis and gastric adenocarcinoma. Arch Med Res. 2007;38:551-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631-636. [PubMed] [Cited in This Article: ] |
| 58. | Perri F, Piepoli A, Bonvicini C, Gentile A, Quitadamo M, Di Candia M, Cotugno R, Cattaneo F, Zagari MR, Ricciardiello L. Cytokine gene polymorphisms in gastric cancer patients from two Italian areas at high and low cancer prevalence. Cytokine. 2005;30:293-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Sugimoto M, Furuta T, Shirai N, Nakamura A, Kajimura M, Sugimura H, Hishida A. Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol. 2007;22:1443-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | Togawa S, Joh T, Itoh M, Katsuda N, Ito H, Matsuo K, Tajima K, Hamajima N. Interleukin-2 gene polymorphisms associated with increased risk of gastric atrophy from Helicobacter pylori infection. Helicobacter. 2005;10:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095-2147. [PubMed] [Cited in This Article: ] |
| 62. | Rad R, Prinz C, Neu B, Neuhofer M, Zeitner M, Voland P, Becker I, Schepp W, Gerhard M. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. J Infect Dis. 2003;188:272-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 63. | Con SA, Takeuchi H, Con-Chin GR, Con-Chin VG, Yasuda N, Con-Wong R. Role of bacterial and genetic factors in gastric cancer in Costa Rica. World J Gastroenterol. 2009;15:211-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Santos JC, Ladeira MS, Pedrazzoli J, Ribeiro ML. Relationship of IL-1 and TNF-α polymorphisms with Helicobacter pylori in gastric diseases in a Brazilian population. Braz J Med Biol Res. 2012;45:811-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Shin WG, Jang JS, Kim HS, Kim SJ, Kim KH, Jang MK, Lee JH, Kim HJ, Kim HY. Polymorphisms of interleukin-1 and interleukin-2 genes in patients with gastric cancer in Korea. J Gastroenterol Hepatol. 2008;23:1567-1573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 66. | Xuan J, Deguchi R, Watanabe S, Ozawa H, Urano T, Ogawa Y, Fukuda R, Kijima H, Koga Y, Takagi A. Relationship between IL-1beta gene polymorphism and gastric mucosal IL-1beta levels in patients with Helicobacter pylori infection. J Gastroenterol. 2005;40:796-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997;113:15-24. [PubMed] [Cited in This Article: ] |
| 68. | Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983-1991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 251] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 69. | Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 221] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 70. | Wallace JL, Cucala M, Mugridge K, Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol. 1991;261:G559-G564. [PubMed] [Cited in This Article: ] |
| 71. | Basso D, Scrigner M, Toma A, Navaglia F, Di Mario F, Rugge M, Plebani M. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2. Int J Clin Lab Res. 1996;26:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. 1997;12:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 348] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 74. | Suárez A, Castro P, Alonso R, Mozo L, Gutiérrez C. Interindividual variations in constitutive interleukin-10 messenger RNA and protein levels and their association with genetic polymorphisms. Transplantation. 2003;75:711-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1254] [Cited by in F6Publishing: 1327] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 76. | Yea SS, Yang YI, Jang WH, Lee YJ, Bae HS, Paik KH. Association between TNF-alpha promoter polymorphism and Helicobacter pylori cagA subtype infection. J Clin Pathol. 2001;54:703-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Seegers D, Zwiers A, Strober W, Peña AS, Bouma G. A TaqI polymorphism in the 3’UTR of the IL-12 p40 gene correlates with increased IL-12 secretion. Genes Immun. 2002;3:419-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | García-González MA, Lanas A, Wu J, Benito R, Santolaria S, Crusius B, Peña S. Lack of association of IL-12 p40 gene polymorphism with peptic ulcer disease. Hum Immunol. 2005;66:72-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Hou L, El-Omar EM, Chen J, Grillo P, Rabkin CS, Baccarelli A, Yeager M, Chanock SJ, Zatonski W, Sobin LH. Polymorphisms in Th1-type cell-mediated response genes and risk of gastric cancer. Carcinogenesis. 2007;28:118-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Navaglia F, Basso D, Zambon CF, Ponzano E, Caenazzo L, Gallo N, Falda A, Belluco C, Fogar P, Greco E. Interleukin 12 gene polymorphisms enhance gastric cancer risk in H pylori infected individuals. J Med Genet. 2005;42:503-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |