Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2482
Revised: January 7, 2014
Accepted: February 17, 2014
Published online: March 14, 2014
Irritable bowel syndrome (IBS) is a common condition characterized by abdominal pain or discomfort, bloating, and altered stool form and passage. Small intestinal bacterial overgrowth (SIBO) is a condition in which there is overgrowth of bacteria in small bowel in excess of 105 colony forming units per milliliter on culture of the upper gut aspirate. Frequency of SIBO varied from 4%-78% among patients with IBS and from 1%-40% among controls. Higher frequency in some studies might be due to fallacious criteria [post-lactulose breath-hydrogen rise 20 PPM above basal within 90 min (early-peak)]. Glucose hydrogen breath test (GHBT) has a low sensitivity to diagnose SIBO. Hence, studies based on GHBT might have under-estimated frequency of SIBO. Therefore, it is important to analyze these studies carefully to evaluate whether the reported association between IBS and SIBO is over or under-projected. This review evaluates studies on association between SIBO and IBS, discordance between different studies, their strength and weakness including methodological issues and evidence on therapeutic manipulation of gut flora on symptoms of IBS.
Core tip: Irritable bowel syndrome (IBS) has been conventionally thought to be a disorder without an organic basis. However, recently data are emerging to show that it may have organic basis at least in a subset of patients. Though several studies reported an association between small intestinal bacterial overgrowth (SIBO) and IBS, the frequency of SIBO reported to vary between 4% and 78%. The current review suggests that the association between SIBO and IBS is definite, but the studies reporting high prevalence of SIBO in IBS over-estimated its frequency due to use of fallacious diagnostic methods. Better test to diagnose SIBO in patients with IBS is highly needed.
- Citation: Ghoshal UC, Srivastava D. Irritable bowel syndrome and small intestinal bacterial overgrowth: Meaningful association or unnecessary hype. World J Gastroenterol 2014; 20(10): 2482-2491
- URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2482
Irritable bowel syndrome (IBS) is a common condition characterized by abdominal pain or discomfort, bloating, associated with altered stool form (such as diarrhea, constipation) and passage. World-wide, 4%-30% of subjects suffer from IBS[1-7]. Small intestinal bacterial overgrowth (SIBO), which has been conventionally described in patients with anatomical abnormalities in the gut such as ileo-transverse anastomosis, stricture, fistula, slow motility and reduced gut defence, is also characterized by abdominal pain or discomfort, bloating, flatulence and loose motion[8-10]. Recently, it has been realized that SIBO may occur in the absence of apparent anatomical abnormalities[11]. These patients, however, may be wrongly diagnosed as IBS.
Small intestinal bacterial overgrowth is currently defined as presence of bacteria in excess of 105 colony forming units per milliliter on culture of the upper gut aspirate[12,13]. Since this is an invasive test, several non-invasive methods including lactulose and glucose hydrogen breath tests (LHBT and GHBT) have been popularly used to diagnose SIBO[14-18]. This condition is being increasingly recognized among patients with IBS. In different studies, frequency of SIBO among patients presenting with IBS varied from 4% to 78% (Table 1), more so among patients with diarrhea-predominant IBS[12,14,18,19]. Not only quantitative increase (SIBO) but qualitative change in the gut bacteria (dysbiosis) has been reported among patients with IBS[20]. These studies led to a paradigm shift in understanding pathogenesis of IBS and have led to increasing debate on therapeutic manipulation of gut microbiota to treat this enigmatic chronic disorder using antibiotics, probiotics and lately fecal transplantation[21-23]. However, it is important to recognize the wide-variability in frequency of SIBO among patients with IBS in different studies; such wide-variability in frequency may suggest that it is important to analyze these studies carefully to evaluate whether the reported association between IBS and SIBO is over or under-projected in some of the earlier studies?
| Ref. | Type of the study | Frequency of SIBO in cases | Frequency of SIBO in controls | Methane producers in cases | Methane producers in controls | Method for diagnosis |
| Park et al[86] | Prospective (Case-control) | 34/76 (44.7) | 16/40 (40) | 19/76 (25) | 10/40 (25) | LHBT |
| Scarpellini et al[88] | Prospective (Case-control) | 28/43 (65) | 4/56 (7) | 4/43 (9.3) | 0 | LHBT |
| Carrara et al[14] | Prospective | 55/127 (43) | NCG | ND | ND | LHBT |
| Mann and Limoges-Gonzales[87] | Prospective | 89/258 (34.5) | NCG | ND | ND | LHBT |
| Nucera et al[89] | Prospective | 64/98 (65) | NCG | ND | ND | LHBT |
| Pimentel et al[17] | Prospective | 157/202 (78) | NCG | ND | ND | LHBT |
| Sachdeva et al[18] | Prospective (Case-control) | 14/59 (23.7) | 1/37 (2.7) | 5/59 (8.5) | 9/37 (24.3) | GHBT |
| Reddymasu et al[90] | Prospective | 35/98 (36) | NCG | Data NA | ND | GHBT |
| Lombardo et al[91] | Prospective (Case-control) | 49/200 (24.5) | 3/50 (6) | ND | ND | GHBT |
| Ford et al[74] | Meta-analysis | 595/1921 (31) | NCG | ND | ND | GHBT |
| Parodi et al[92] | Prospective (Case-control) | 21/130 (16.1) | 3/70 (4.2) | 34/130 (26) | Data NA | GHBT |
| Rana et al[93] | Prospective (Case-control) | 25/225 (11.1) | 1/100 (1) | ND | ND | GHBT |
| Majewski et al[94] | Prospective (Case-control) | 93/204 (46) | NCG | 27/204 (13.2) | ND | GHBT |
| Cuoco and Salvangnini[75] | Retrospective | 44/96 (45.8) | NCG | ND | ND | GHBT |
| Lupascu et al[95] | Prospective (Case-control) | 20/65 (31) | 4/102 (4) | ND | ND | GHBT |
| Ghoshal et al[16] | Prospective (Case-control) | 11/129 (8.5) | 1/51(2) | ND | ND | GHBT |
| Posserud et al[12] | Prospective (Case-control) | 6/162 (4) | 1/26 (4) | ND | ND | Hydrogen breath test and culture of small bowel aspirate |
We hereby review the studies on association between SIBO and IBS, discordance between different studies, their strength and weakness including methodological issues and evidence on therapeutic manipulation of gut flora on symptoms of IBS.
Table 1 summarizes the results from studies on SIBO among patients with IBS. As can be noted from this table, the frequency of SIBO among patients with IBS varied from 4% to 78% and that among controls from 1% and 40%. In most studies, frequency of SIBO among patients with IBS was higher than that among controls. Therefore, it can be concluded that SIBO is associated with IBS. But it is important to critically evaluate the reasons for such a wide variability in frequency of SIBO in different studies.
IBS is a heterogeneous condition. The sub-types may be diarrhea or constipation-predominant or may be alternating. Patients with diarrhea-predominant IBS more often have organic cause including SIBO than other sub-types of IBS. In a study on 129 patients with non-diarrheal IBS, 73 with chronic non-specific diarrhea including diarrhea-predominant IBS, and 51 healthy controls, frequency of SIBO using GHBT was 11 (8.5%), 16 (22%) and 1 (2%), respectively[16]. Similar findings were reported in other studies as well[18,24]. Diarrheal IBS, therefore, should be particularly evaluated for SIBO than other types of IBS. Moreover, studies which included larger proportion of patients with diarrhea-predominant IBS are likely to show higher frequency of SIBO.
Bloating is a frequent symptom reported by patients with IBS. Frequency of bloating has been reported to vary from 26% to 83% in studies on IBS from Asia[3,25]. The pathogenesis of bloating may be related to increased amount of gas in the gut, its abnormal distribution and increased visceral perception in response to distension of the gut[26,27]. Patients with SIBO may have increased amount of gas inside the gut, hence, it is logical to believe that IBS patients with marked bloating are expected to have SIBO[16]. However, there are limited data on this issue. Several studies showed that both fasting as well as post-substrate (e.g., glucose, lactulose) breath hydrogen is higher among patients with IBS than controls[16,28]. Probiotics and antibiotics, which are known to reduce gas inside the gut, are known to relieve abdominal bloating among patients with IBS[17,29,30]. It has been reported that successful treatment with antibiotics causes the hydrogen breath test to revert to normal[17]. Hence, patients with IBS with marked bloating and flatulence should be particularly evaluated for SIBO. More studies, however, are needed on this issue.
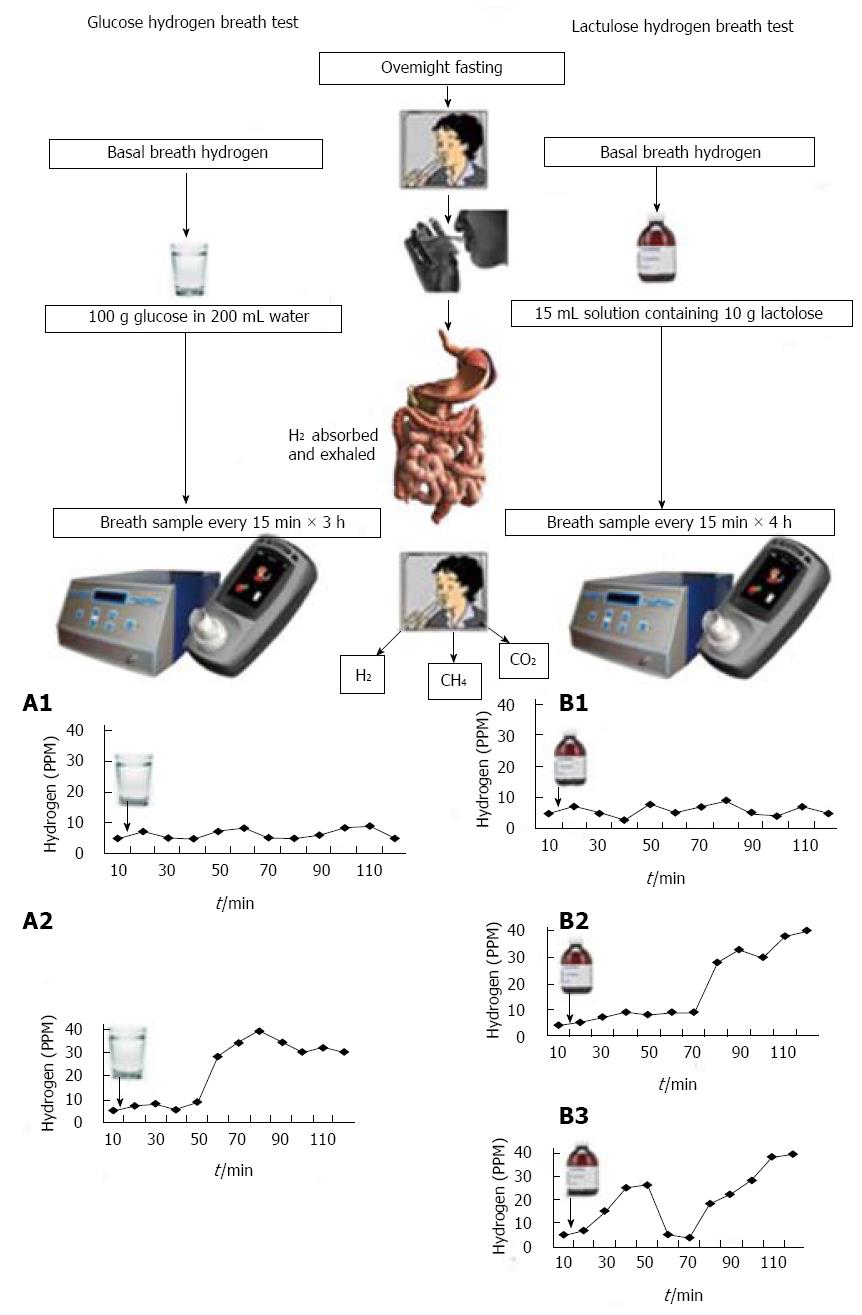
Several techniques have been used to diagnose SIBO; these include LHBT, GHBT, 14C xylose breath test, and quantitative culture of jejunal aspirate[12,15,31,32]. Principle of hydrogen breath tests is summarized in Figure 1. Dietary carbohydrates, unabsorbed in the small intestine, produce hydrogen in the large intestine by bacterial fermentation[33]. In patients with SIBO, the bacteria ferment these carbohydrates in the small bowel itself producing hydrogen, which gets absorbed and is exhaled in the breath[33].
Hydrogen breath test involves giving patients a load of carbohydrate (usually in the form of glucose and lactulose) and measuring expired hydrogen concentrations over a period of time. Diagnosis of SIBO using hydrogen breath test is based on the physiological principle that in patients with SIBO, glucose would be fermented by bacteria in the small bowel resulting in production of hydrogen gas that is absorbed and exhaled in expired air (Figure 1, A1)[31,33]. In contrast, lactulose, which is a non-absorbable disaccharide, will produce an early peak due to fermentation in small bowel (typically within 90-min) or double peak (first due to small bowel fermentation and second from colon), if SIBO is present (Figure 1, B2 and B3)[33]. There are several limitations in hydrogen breath test for diagnosis of SIBO. There may be similarities in the pattern of gas production in patients with SIBO and subjects with rapid intestinal transit, thus making distinction difficult[34]. An early peak is often false positive in people with fast gut transit time. For example, in a study from India, median oro-cecal transit time was 65 min (range 40-110 min) in healthy subjects[35]. In another study from Taiwan, mean oro-cecal transit time was 85 min[36]. This has also been substantiated in Western population recently by simultaneously using LHBT and radio-nuclide method to assess gut transit[37-39]. Double peak criteria for diagnosis of SIBO using LHBT is quite insensitive[15,33]. Sensitivity of GHBT to diagnose SIBO is 44% considering quantitative culture of upper gut aspirate as gold standard[15]. Hence, it is expected that the authors who used an early peak criteria in LHBT would get a high frequency of SIBO among patients with IBS as well as controls. In contrast, those who would use either GHBT or double peak criterion in LHBT would get a low frequency of SIBO both in patients with IBS and controls. It is worth noting from the Table 1 that the frequency of SIBO among patients with IBS and controls on LHBT (early peak criteria) varied from 34.5% to 78% and 7%-40%, respectively; in contrast, the frequency on GHBT varied from 8.5%-46% and 2%-18%, respectively.
Fifteen percent of people may have methanogenic flora in the gut[34,40]. Methanobrevibacter smithii, Methanobrevibacter stadmanae and possibly some of the coliform bacteria are methanogens[41]. In these subjects, only hydrogen breath tests may not diagnose SIBO, estimation of methane is also needed (Figure 1). Table 1 shows that 8.5%-26% of IBS patients and 0%-25% of controls exhaled methane in their breath. Therefore, SIBO could not have been diagnosed if methane was not estimated in them. As summarized in Table 1, in a large proportion of studies, methane was not estimated, which could have resulted in underestimation of frequency of SIBO in these studies. Excessive methane production is associated with constipation[42]. Hence, methane estimation in breath, which is not available in several commercially available hydrogen breath test machines, is particularly important in patients with constipation-predominant IBS. Some individuals may have slow transit through the small intestine making prolonged testing up to five hours necessary and many individuals may not like to undergo such prolonged testing[43,44]. However, a shorter period of testing for them may miss the diagnosis of SIBO.
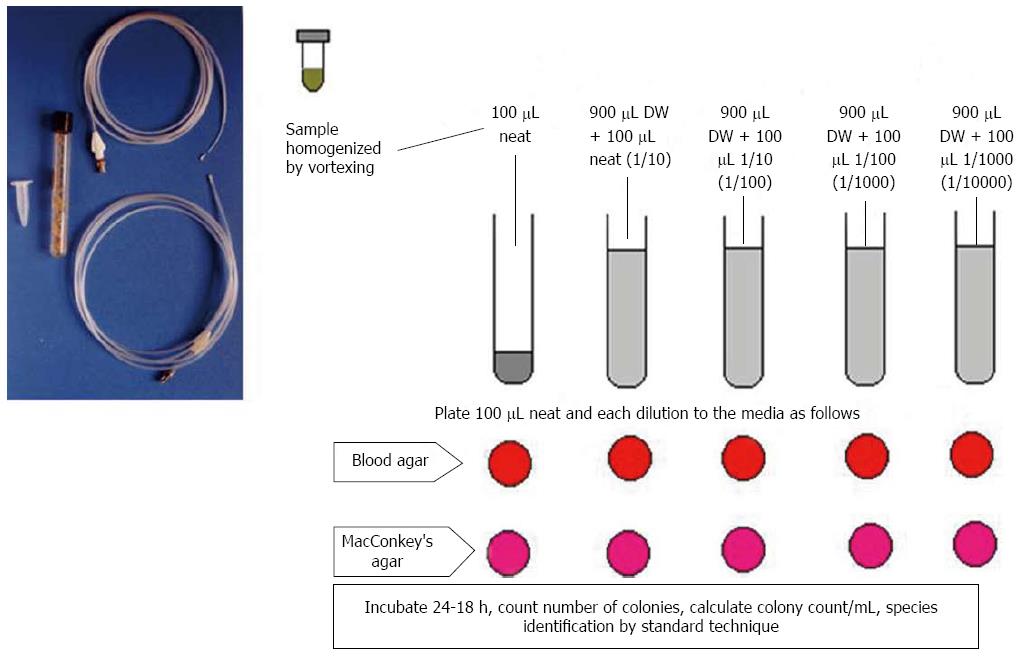
The jejunal aspirate culture has traditionally been used as the gold standard to diagnose SIBO (Figure 2)[15,45]. However, the limitations of this test include invasiveness and the challenges posed by attempting to culture all strains and species[46]. In fact, use of air during endoscopy can lead to a false negative result as anaerobes do not grow once these are exposed to oxygen present in the air[13]. Also, a large proportion of bacteria are not cultured[47,48]. In contrast, single lumen catheter passed through the nose or through the biopsy channel of endoscope, may lead to contamination by oro-pharyngeal flora giving false positive result[13]. Therefore, we designed a double-lumen catheter to prevent such oro-pharyngeal contamination (Figure 2)[13]. Studies on SIBO among patients with IBS using quantitative culture of small bowel aspirate are scanty (Table 1). A study by Posserud et al[12] reported a frequency of SIBO of 4% among patients with IBS. Considering the result of other studies using GHBT, which has sensitivity of 44% to diagnose SIBO considering upper gut aspirate as gold standard, the former study appears to have falsely under-estimated the frequency of SIBO among patients with IBS[15]. More studies are needed on this issue.
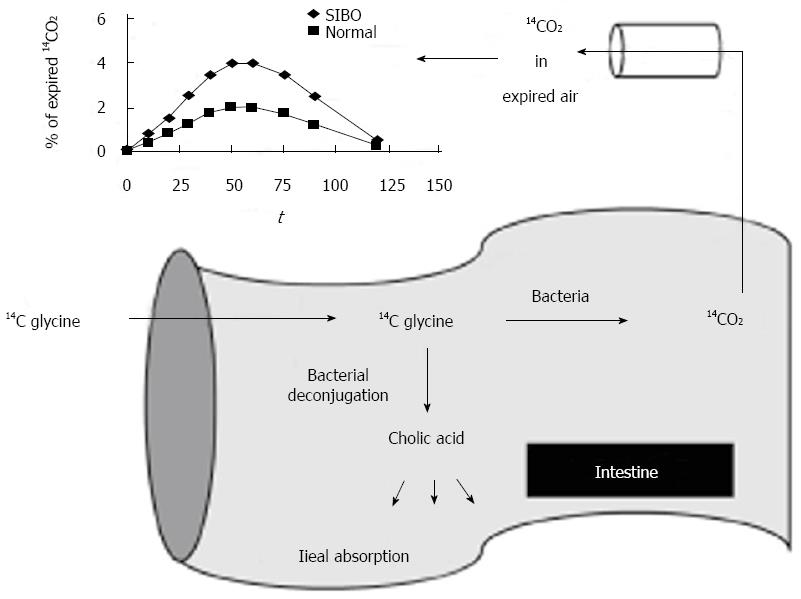
13C and 14C based tests have also been developed based on the bacterial metabolism of D-xylose (Figure 3)[32]. Bacterial de-conjugation of bile acids containing 13C and 14C can also be used to diagnose SIBO[49]. The glycocholic acid breath test involves the administration of the bile acid 14C glycocholic acid, and the detection of 14CO2, which would be elevated in SIBO (Figure 3)[50,51].
Bacteria in gut lumen play important role in modulating multiple intestinal functions. Quantitative change in luminal bacteria in the small intestine (SIBO) disrupts digestion and absorption. Gut bacteria are also important for immune activation[52]. Immune mediated cytokines could have multiple actions including altered epithelial secretion, exaggerated nociceptive signalling and abnormal motility[52,53]. Together, these changes may lead to IBS like symptoms. It has also been proposed that this mechanism could account for overlap syndromes, such as fibromyalgia[54,55]. There has been renewed interest in gut flora recently, as there are recent developments on relationship between gut flora and intestinal function, pathogenesis of various diseases and potential value of probiotics, and other means of modifying gut flora as therapeutic modalities.
In patients with SIBO, bacteria ferment ingested carbohydrates in the small intestine causing increased gas production[15]. Accumulation of this gas in the intestine results in bloating and flatulence[56,57]. Excessive luminal distension may even cause abdominal pain or discomfort[57]. 15% of the population produces methane instead of hydrogen gas[13,34]. Evidence suggest that excessive methane produced by overgrowth of methanogenic flora causes constipation[42]. Reduction in breath methane by therapeutic manipulation of gut flora improves constipation[58].
Bacteria in the intestine may produce toxic by-products after fermentation, which may damage the inner lining of the small intestine and colon[59]. A study on small bowel biopsies in patients with SIBO revealed thinning of the mucosa and crypts and increased intra-epithelial lymphocytes[60]. This may cause osmotic load in the intestine resulting into diarrhea. Studies have revealed that patients with IBS having SIBO more often have diarrhea-predominant disease[11,12,14,18,19]. Bacteria also de-conjugates bile salts present in the intestine. These de-conjugated bile salts can stimulate colonic water secretion causing diarrhea. Thereafter, free bile acids, which are toxic to the intestinal mucosa may cause mucosal inflammation and release of pro-inflammatory cytokines[61,62]. SIBO is known to be associated with increased IL-8 levels (pro-inflammatory cytokine)[63].
Pathophysiology of IBS includes altered motility, visceral hypersensitivity and abnormal brain-gut interaction. Bacteria affect the sensori-motor functions of the gut[64]. Bacterial chemotactic peptides, such as formyl-methionyl-leucyl-phenylalanine, stimulate the enteric nervous system and afferent nerves, while endotoxins (lipopolysaccharides) may affect gut motility[65]. Bacteria in the small intestine in patients with SIBO produce short-chain fatty acids (SCFA) such as butyrate, acetate, and propionate. Colonic motility is increased due to acidification by SCFA[66,67]. In contrast, SCFA causes reduction in motility of proximal intestine due to release of peptide YY, neurotensin and glucagon-like peptide-1 in the ileum[68]. Therefore, alteration in gut flora may cause altered motility and predispose to IBS like symptoms.
Gut flora of IBS patients is different from that of healthy subjects, resulting in more gas production due to bacterial fermentation[69-71]. Evidence suggest that therapeutic manipulation of gut flora either with antibiotics or probiotics may lead to relief in symptoms of IBS[72,73]. Several meta-analysis have reported the presence of SIBO in a subset of IBS patients[14,17,74]. Recent studies have revealed that treatment of SIBO relieves symptoms of IBS[17,75]. In a study, eradication of SIBO by open label antibiotic treatment resolved symptoms of IBS to the extent of Rome I criteria turning negative in 48% of patients[17]. SIBO is more often found in diarrheal IBS than other subtypes[16]. Treatment of patients with diarrhea-predominant IBS with antibiotics, which is the primary treatment of SIBO, may lead to relief in symptoms including bloating, abdominal pain and loose stools. Rifaximin, a non-absorbable broad spectrum antibiotic, has been widely used for treatment of SIBO[76]. In a phase 3, double-blind, placebo-controlled trial on IBS patients without constipation, treatment with rifaximin provided significant relief in bloating, abdominal pain, and loose or watery stools as compared to placebo[77].
Methane produced by methanogenic flora is shown to cause slow transit constipation, which is associated with IBS[78]. Recently, rifaximin is found to reduce methane gas and improve gut transit[58]. A combination of rifaximin and neomycin is more effective in treating methane-producing IBS patients as compared to treatment with neomycin and rifaximin as single agents (87% vs 33% and 28%, respectively)[79].
Some studies support the use of probiotics to be as effective as antibiotics in relieving IBS related symptoms[80,81]. A study showed that treatment with probiotics was effective in reducing symptoms of abdominal pain, bowel frequency, urgency and distension in patients with chronic diarrhea[82]. Lactobacilli are less gas producing than other bacteria[70]. Therefore, administration of Lactobacilli in patients with IBS was associated with reduced gas-related symptoms[83]. In a single blinded randomized control trial, IBS patients randomized to receive Lactobacillus acidophilus, Lactobacillus helveticus, and Bifidobacterium showed significant improvement in pain and bloating as compared to those who received placebo[84]. Another study showed Bacillus subtilis and Streptococcus faecium to be effective in reducing abdominal pain as compared to placebo in patients with diarrhea or alternating type of IBS[85]. However, more studies are needed to know the best strains of probiotic bacteria, their dose and duration for treatment of patient with IBS.
Association between IBS and SIBO is definite. In fact, controversy exists whether patients presenting with IBS but found to have SIBO on further testing should be diagnosed as IBS or should be considered as SIBO as symptoms of IBS and SIBO are similar. However, in the current diagnostic algorithm of Rome Foundation, IBS is diagnosed by symptom-based criteria. Exclusion of SIBO by appropriate testing is not essential before diagnosing IBS. In future iteration on IBS diagnostic algorithm by Rome Foundation, it may be important that consideration is given to exclude SIBO before a diagnosis of IBS is made, at least in a subset of patients with higher probability of SIBO. However, as evident from the review of the existing data, we conclude that whereas in some studies, the frequency of SIBO was over-estimated, in others it was under-estimated. Studies that used an early-peak criteria on LHBT showed higher frequency of SIBO than those that used other methods to diagnose. In the context of recent studies that showed that early-peak criteria on LHBT if often false positive, it is likely that all these studies over-estimated frequency of SIBO and therefore, created an un-necessary hype[14,17,86,87]. GHBT has a low sensitivity to diagnose SIBO[15]. Therefore, studies based on GHBT might have under-estimated frequency of SIBO. Though jejunal aspirate culture is considered as gold standard for diagnosis of SIBO, this has limitations. Most importantly, this is invasive and hence, not acceptable to the patients. Therefore, there is urgent need to know the clinical predictors for considering diagnosis of SIBO in patients presenting as IBS and better diagnostic techniques to confirm this.
P- Reviewers: Gwee KA, Hauser G, Shoaran M S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Ballou SK, Keefer L. Multicultural considerations in the diagnosis and management of irritable bowel syndrome: a selective summary. Eur J Gastroenterol Hepatol. 2013;25:1127-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 2. | Gonzales Gamarra RG, Ruiz Sánchez JG, León Jiménez F, Cubas Benavides F, Díaz Vélez C. [Prevalence of irritable bowel syndrome in the adult population of the city of Chiclayo in 2011]. Rev Gastroenterol Peru. 2012;32:381-386. [PubMed] [Cited in This Article: ] |
| 3. | Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 129] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 4. | Krogsgaard LR, Engsbro AL, Bytzer P. The epidemiology of irritable bowel syndrome in Denmark. A population-based survey in adults ≤ 50 years of age. Scand J Gastroenterol. 2013;48:523-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Ibrahim NK, Battarjee WF, Almehmadi SA. Prevalence and predictors of irritable bowel syndrome among medical students and interns in King Abdulaziz University, Jeddah. Libyan J Med. 2013;8:21287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Lee YY, Waid A, Tan HJ, Chua AS, Whitehead WE. Rome III survey of irritable bowel syndrome among ethnic Malays. World J Gastroenterol. 2012;18:6475-6480; discussion p. 6479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Ghoshal UC, Abraham P, Bhatt C, Choudhuri G, Bhatia SJ, Shenoy KT, Banka NH, Bose K, Bohidar NP, Chakravartty K. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol. 2008;27:22-28. [PubMed] [Cited in This Article: ] |
| 8. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [PubMed] [Cited in This Article: ] |
| 9. | Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol. 2006;4:11-20. [PubMed] [Cited in This Article: ] |
| 10. | Bouhnik Y, Alain S, Attar A, Flourié B, Raskine L, Sanson-Le Pors MJ, Rambaud JC. Bacterial populations contaminating the upper gut in patients with small intestinal bacterial overgrowth syndrome. Am J Gastroenterol. 1999;94:1327-1331. [PubMed] [Cited in This Article: ] |
| 11. | Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y). 2007;3:112-122. [PubMed] [Cited in This Article: ] |
| 12. | Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802-808. [PubMed] [Cited in This Article: ] |
| 13. | Ghoshal U, Ghoshal UC, Ranjan P, Naik SR, Ayyagari A. Spectrum and antibiotic sensitivity of bacteria contaminating the upper gut in patients with malabsorption syndrome from the tropics. BMC Gastroenterol. 2003;3:9. [PubMed] [Cited in This Article: ] |
| 14. | Carrara M, Desideri S, Azzurro M, Bulighin GM, Di Piramo D, Lomonaco L, Adamo S. Small intestine bacterial overgrowth in patients with irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2008;12:197-202. [PubMed] [Cited in This Article: ] |
| 15. | Ghoshal UC, Ghoshal U, Das K, Misra A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J Gastroenterol. 2006;25:6-10. [PubMed] [Cited in This Article: ] |
| 16. | Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503-3506. [PubMed] [Cited in This Article: ] |
| 18. | Sachdeva S, Rawat AK, Reddy RS, Puri AS. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. J Gastroenterol Hepatol. 2011;26 Suppl 3:135-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Park H. The role of small intestinal bacterial overgrowth in the pathophysiology of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:3-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Ghoshal UC, Shukla R, Ghoshal U, Gwee KA, Ng SC, Quigley EM. The gut microbiota and irritable bowel syndrome: friend or foe? Int J Inflam. 2012;2012:151085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Brown AC. Ulcerative colitis, Crohn’s disease and irritable bowel syndrome patients need fecal transplant research and treatment. J Crohns Colitis. 2014;8:179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Sampath K, Levy LC, Gardner TB. Fecal transplantation: beyond the aesthetic. Gastroenterology. 2013;145:1151-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Grace E, Shaw C, Whelan K, Andreyev HJ. Review article: small intestinal bacterial overgrowth--prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther. 2013;38:674-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Yakoob J, Abbas Z, Khan R, Hamid S, Awan S, Jafri W. Small intestinal bacterial overgrowth and lactose intolerance contribute to irritable bowel syndrome symptomatology in Pakistan. Saudi J Gastroenterol. 2011;17:371-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Gwee KA, Bak YT, Ghoshal UC, Gonlachanvit S, Lee OY, Fock KM, Chua AS, Lu CL, Goh KL, Kositchaiwat C. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Lacy BE, Gabbard SL, Crowell MD. Pathophysiology, evaluation, and treatment of bloating: hope, hype, or hot air? Gastroenterol Hepatol (N Y). 2011;7:729-739. [PubMed] [Cited in This Article: ] |
| 27. | Harder H, Serra J, Azpiroz F, Passos MC, Aguadé S, Malagelada JR. Intestinal gas distribution determines abdominal symptoms. Gut. 2003;52:1708-1713. [PubMed] [Cited in This Article: ] |
| 28. | Kumar S, Misra A, Ghoshal UC. Patients with irritable bowel syndrome exhale more hydrogen than healthy subjects in fasting state. J Neurogastroenterol Motil. 2010;16:299-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Hungin AP, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G, Winchester C, de Wit N. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | Attar A, Flourié B, Rambaud JC, Franchisseur C, Ruszniewski P, Bouhnik Y. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: a crossover, randomized trial. Gastroenterology. 1999;117:794-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Marcelino RT, Fagundes-Neto U. [Hydrogen test (H2) in the air expired for the diagnosis of small bowel bacterial overgrowth]. Arq Gastroenterol. 1995;32:191-198. [PubMed] [Cited in This Article: ] |
| 32. | Santavirta J. Lactulose hydrogen and [14C]xylose breath tests in patients with ileoanal anastomosis. Int J Colorectal Dis. 1991;6:208-211. [PubMed] [Cited in This Article: ] |
| 33. | Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 34. | Yang CY, Chang CS, Chen GH. Small-intestinal bacterial overgrowth in patients with liver cirrhosis, diagnosed with glucose H2 or CH4 breath tests. Scand J Gastroenterol. 1998;33:867-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Ghoshal UC, Ghoshal U, Ayyagari A, Ranjan P, Krishnani N, Misra A, Aggarwal R, Naik S, Naik SR. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J Gastroenterol Hepatol. 2003;18:540-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Lu CL, Chen CY, Chang FY, Lee SD. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: an Oriental study. Clin Sci (Lond). 1998;95:165-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Sciarretta G, Furno A, Mazzoni M, Garagnani B, Malaguti P. Lactulose hydrogen breath test in orocecal transit assessment. Critical evaluation by means of scintigraphic method. Dig Dis Sci. 1994;39:1505-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, Scott MS, Simren M, Soffer E, Szarka L. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 39. | Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 40. | Rana SV, Sharma S, Sinha SK, Kaur H, Sikander A, Singh K. Incidence of predominant methanogenic flora in irritable bowel syndrome patients and apparently healthy controls from North India. Dig Dis Sci. 2009;54:132-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One. 2009;4:e7063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 42. | Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Lunia MK, Sharma BC, Sachdeva S. Small intestinal bacterial overgrowth and delayed orocecal transit time in patients with cirrhosis and low-grade hepatic encephalopathy. Hepatol Int. 2013;7:268-273. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Resmini E, Parodi A, Savarino V, Greco A, Rebora A, Minuto F, Ferone D. Evidence of prolonged orocecal transit time and small intestinal bacterial overgrowth in acromegalic patients. J Clin Endocrinol Metab. 2007;92:2119-2124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Hamilton I, Worsley BW, Cobden I, Cooke EM, Shoesmith JG, Axon AT. Simultaneous culture of saliva and jejunal aspirate in the investigation of small bowel bacterial overgrowth. Gut. 1982;23:847-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Corazza GR, Sorge M, Strocchi A, Benati G, Di Sario A, Treggiari EA, Brusco G, Gasbarrini G. Non-absorbable antibiotics and small bowel bacterial overgrowth. Ital J Gastroenterol. 1992;24:4-9. [PubMed] [Cited in This Article: ] |
| 47. | Kuwahara T, Ogura Y, Oshima K, Kurokawa K, Ooka T, Hirakawa H, Itoh T, Nakayama-Imaohji H, Ichimura M, Itoh K. The lifestyle of the segmented filamentous bacterium: a non-culturable gut-associated immunostimulating microbe inferred by whole-genome sequencing. DNA Res. 2011;18:291-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Beumer RR, de Vries J, Rombouts FM. Campylobacter jejuni non-culturable coccoid cells. Int J Food Microbiol. 1992;15:153-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 132] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Fromm H, Sarva RP, Ravitch MM, McJunkin B, Farivar S, Amin P. Effects of jejunoileal bypass on the enterohepatic circulation of bile acids, bacterial flora in the upper small intestine, and absorption of vitamin B12. Metabolism. 1983;32:1133-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Yoshida T, McCormick WC, Swell L, Vlahcevic ZR. Bile acid metabolism in cirrhosis. IV. Characterization of the abnormality in deoxycholic acid metabolism. Gastroenterology. 1975;68:335-341. [PubMed] [Cited in This Article: ] |
| 51. | Bjørneklett A, Fausa O, Midtvedt T. Bacterial overgrowth in jejunal and ileal disease. Scand J Gastroenterol. 1983;18:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Vanner S. The small intestinal bacterial overgrowth. Irritable bowel syndrome hypothesis: implications for treatment. Gut. 2008;57:1315-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Kinross JM, von Roon AC, Holmes E, Darzi A, Nicholson JK. The human gut microbiome: implications for future health care. Curr Gastroenterol Rep. 2008;10:396-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Clauw DJ. Fibromyalgia: an overview. Am J Med. 2009;122:S3-S13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 55. | Pimentel M, Wallace D, Hallegua D, Chow E, Kong Y, Park S, Lin HC. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 99] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 56. | Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 273] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 57. | Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, Gasbarrini G. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25:237-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Ghoshal UC, Srivastava D, Verma A, Misra A. Slow transit constipation associated with excess methane production and its improvement following rifaximin therapy: a case report. J Neurogastroenterol Motil. 2011;17:185-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Bala L, Ghoshal UC, Ghoshal U, Tripathi P, Misra A, Gowda GA, Khetrapal CL. Malabsorption syndrome with and without small intestinal bacterial overgrowth: a study on upper-gut aspirate using 1H NMR spectroscopy. Magn Reson Med. 2006;56:738-744. [PubMed] [Cited in This Article: ] |
| 60. | Haboubi NY, Lee GS, Montgomery RD. Duodenal mucosal morphometry of elderly patients with small intestinal bacterial overgrowth: response to antibiotic treatment. Age Ageing. 1991;20:29-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Shindo K, Machida M, Koide K, Fukumura M, Yamazaki R. Deconjugation ability of bacteria isolated from the jejunal fluid of patients with progressive systemic sclerosis and its gastric pH. Hepatogastroenterology. 1998;45:1643-1650. [PubMed] [Cited in This Article: ] |
| 62. | Wanitschke R, Ammon HV. Effects of dihydroxy bile acids and hydroxy fatty acids on the absorption of oleic acid in the human jejunum. J Clin Invest. 1978;61:178-186. [PubMed] [Cited in This Article: ] |
| 63. | Shanab AA, Scully P, Crosbie O, Buckley M, O’Mahony L, Shanahan F, Gazareen S, Murphy E, Quigley EM. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56:1524-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | Ghoshal UC, Park H, Gwee KA. Bugs and irritable bowel syndrome: The good, the bad and the ugly. J Gastroenterol Hepatol. 2010;25:244-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560-2568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Cherbut C, Aubé AC, Blottière HM, Galmiche JP. Effects of short-chain fatty acids on gastrointestinal motility. Scand J Gastroenterol Suppl. 1997;222:58-61. [PubMed] [Cited in This Article: ] |
| 67. | Ramakrishna BS, Roediger WE. Bacterial short chain fatty acids: their role in gastrointestinal disease. Dig Dis. 1990;8:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology. 1998;139:3780-3786. [PubMed] [Cited in This Article: ] |
| 69. | Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185-194. [PubMed] [Cited in This Article: ] |
| 70. | Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 358] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 71. | Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 950] [Cited by in F6Publishing: 859] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 72. | Camilleri M. Probiotics and irritable bowel syndrome: rationale, mechanisms, and efficacy. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 1:S123-S125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Spiller R. Probiotics: an ideal anti-inflammatory treatment for IBS? Gastroenterology. 2005;128:783-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 276] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 75. | Cuoco L, Salvagnini M. Small intestine bacterial overgrowth in irritable bowel syndrome: a retrospective study with rifaximin. Minerva Gastroenterol Dietol. 2006;52:89-95. [PubMed] [Cited in This Article: ] |
| 76. | Di Stefano M, Corazza GR. Treatment of small intestine bacterial overgrowth and related symptoms by rifaximin. Chemotherapy. 2005;51 Suppl 1:103-109. [PubMed] [Cited in This Article: ] |
| 77. | Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 651] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 78. | Hwang L, Low K, Khoshini R, Melmed G, Sahakian A, Makhani M, Pokkunuri V, Pimentel M. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci. 2010;55:398-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 79. | Low K, Hwang L, Hua J, Zhu A, Morales W, Pimentel M. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol. 2010;44:547-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Bengmark S. Colonic food: pre- and probiotics. Am J Gastroenterol. 2000;95:S5-S7. [PubMed] [Cited in This Article: ] |
| 81. | Quigley EM, Quera R. Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics. Gastroenterology. 2006;130:S78-S90. [PubMed] [Cited in This Article: ] |
| 82. | Xiao SD, Zhang DZ, Lu H, Jiang SH, Liu HY, Wang GS, Xu GM, Zhang ZB, Lin GJ, Wang GL. Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Adv Ther. 2003;20:253-260. [PubMed] [Cited in This Article: ] |
| 83. | O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. [PubMed] [Cited in This Article: ] |
| 84. | Tsuchiya J, Barreto R, Okura R, Kawakita S, Fesce E, Marotta F. Single-blind follow-up study on the effectiveness of a symbiotic preparation in irritable bowel syndrome. Chin J Dig Dis. 2004;5:169-174. [PubMed] [Cited in This Article: ] |
| 85. | Kim YG, Moon JT, Lee KM, Chon NR, Park H. [The effects of probiotics on symptoms of irritable bowel syndrome]. Korean J Gastroenterol. 2006;47:413-419. [PubMed] [Cited in This Article: ] |
| 86. | Park JS, Yu JH, Lim HC, Kim JH, Yoon YH, Park HJ, Lee SI. [Usefulness of lactulose breath test for the prediction of small intestinal bacterial overgrowth in irritable bowel syndrome]. Korean J Gastroenterol. 2010;56:242-248. [PubMed] [Cited in This Article: ] |
| 87. | Mann NS, Limoges-Gonzales M. The prevalence of small intestinal bacterial vergrowth in irritable bowel syndrome. Hepatogastroenterology. 2009;56:718-721. [PubMed] [Cited in This Article: ] |
| 88. | Scarpellini E, Giorgio V, Gabrielli M, Lauritano EC, Pantanella A, Fundarò C, Gasbarrini A. Prevalence of small intestinal bacterial overgrowth in children with irritable bowel syndrome: a case-control study. J Pediatr. 2009;155:416-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 89. | Nucera G, Gabrielli M, Lupascu A, Lauritano EC, Santoliquido A, Cremonini F, Cammarota G, Tondi P, Pola P, Gasbarrini G. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;21:1391-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 90. | Reddymasu SC, Sostarich S, McCallum RW. Small intestinal bacterial overgrowth in irritable bowel syndrome: are there any predictors? BMC Gastroenterol. 2010;10:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010;8:504-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 92. | Parodi A, Dulbecco P, Savarino E, Giannini EG, Bodini G, Corbo M, Isola L, De Conca S, Marabotto E, Savarino V. Positive glucose breath testing is more prevalent in patients with IBS-like symptoms compared with controls of similar age and gender distribution. J Clin Gastroenterol. 2009;43:962-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Rana SV, Sinha SK, Sikander A, Bhasin DK, Singh K. Study of small intestinal bacterial overgrowth in North Indian patients with irritable bowel syndrome: a case control study. Trop Gastroenterol. 2008;29:23-25. [PubMed] [Cited in This Article: ] |
| 94. | Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139-142. [PubMed] [Cited in This Article: ] |
| 95. | Lupascu A, Gabrielli M, Lauritano EC, Scarpellini E, Santoliquido A, Cammarota G, Flore R, Tondi P, Pola P, Gasbarrini G. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:1157-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |