VASCULAR ANATOMY
In embryogenesis segmental arteries regress until three major visceral vessels remain: the celiac artery, the superior mesenteric artery (SMA) and the inferior mesenteric artery (IMA). Hereby, frequent variants with complex anatomy may develop. In health, the visceral vascular supply is highly collateralized to ensure sufficient visceral blood supply. Retroduodenal and retropancreatic collaterals shunt blood from the coeliac artery to the SMA and vice versa[1]. The SMA supplies the small bowel and parts of the colon. It originates only 1.5 to 2 cm below the coeliac artery, crosses the horizontal part of the duodenum and then enters the mesenteric root. At the splenic colon flexure the marginal artery of Drummond represents an important collateral which anastomoses the middle colic artery (MCA) with the left colic artery. The riolan anastomosis is another vascular arcade present in the colonic mesentery that connects the proximal MCA artery with the proximal IMA. The IMA supplies the descending colon, the sigmoid colon and the upper rectum. Here multiple collaterals are preformed with the hypogastric artery via the medial rectal artery[2].
These interconnections between the celiac artery, the SMA and the IMA may easily compensate if stenotic lesions in one major visceral vessel develops. Therefore chronic mesenteric ischemia (CMI) with intestinal malperfusion is rarely seen in clinical practice but represents a serious and complex vascular disorder.
EPIDEMIOLOGY
In a population-based study, 553 healthy elderly persons underwent duplex sonography of the visceral arteries. Relevant stenosis was detected in 17.5% of the study cohort. The majority had isolated celiac disease. SMA stenosis and celiac artery occlusion demonstrated a significant and independent association with weight loss and concurrent renal artery disease[3]. Among the patients with relevant stenosis of visceral arteries only few develop symptoms. In a natural history study of 980 patients who received consecutive aortograms to identify significant mesenteric stenoses, eighty-two patients (8%) were found to have 50% stenosis of at least one mesenteric artery. Sixty of these patients (6%) had significant occlusions involving one, two, or all three mesenteric arteries. Mesenteric ischemia developed in only four patients (0.4%). Each of these four patients had significant three-vessel disease[4].
CLINICAL PRESENTATION
The majority (over 70%) of CMI-patients are woman. Classically the symptoms include abdominal angina which presents as postprandial pain resulting in significant weight loss. Patients may develop a fear to eat, therefore among the differential diagnosis malignant diseases and functional disorders have to be considered. Atherosclerosis is by the most common etiology of CMI. Other etiologies causing this clinical entity include fibromuscular dysplasia, Buerger disease, and aortic dissection. In these patients atherosclerosis will often have manifested in other vascular beds by a history of myocardial infarction, stroke, or intermittent claudication. Atypical symptoms include diarrhea, constipation, vomiting and lower gastrointestinal bleeding which is associated with ischemic colitis or ischemic gastropathy. Endoscopic findings are unspecific and therefore subjective to inter observer variance. Previous research studies have suggested a possible role for tonometry, laser doppler, magnetic resonance (MR) oximetry, and MR measurement of mesenteric venous blood flow and spectroscopic oxymetric devices in the assessment of intestinal ischemia[5]. All of these are still under investigation and their clinical usefulness has not been adequately established. In parallel to peripheral artery disease which may progress from claudication to tissue loss CMI may also progress from post-prandial pain to mesenteric infarction[6]. Evidence of significant occlusion of two or more of these vessels is often found when classic symptoms and endoscopy suggest bowel ischemia. Single-vessel disease, usually of the SMA, has also been described as a cause of symptomatic CMI, particularly if collateral connections have been disrupted[7].
DIAGNOSIS
Established non-invasive diagnostic means are duplex ultrasound or computed tomography (CT)- and MR-angiography. Especially duplex ultrasound is fast and readily available. Diagnosis is based on morphology and flow velocities. The largest series correlated angiograms and duplex sonography of 153 patients[8]. Peak systolic velocity (PSV), end diastolic velocity, and SMA or colic artery/aortic PSV ratio were used to detect ≥ 50% and ≥ 70% stenosis. PSV threshold value for detecting ≥ 50% SMA stenosis was ≥ 295 cm/s (sensitivity 87%, specificity 89% and OA 88%); and for detecting ≥ 70% SMA ≥ 400 cm/s. PSV and ratio measurements were less reliable. Duplex ultrasound of the mesenteric artery requires a high level of technical expertise. It also depends on the fasting status of the patients. In ideal patients even higher sensitivity and specificity have been reported[9]. There is also the tendency to measure higher flow velocities in stented arteries as compared to non-stented vessels. Therefore, the degree of in-stent-stenosis may be overestimated[10]. Native CT-scans without the application of a contrast agent already provide valuable information on the calcification status of the aorta. This is necessary to plan a revascularisation. In multiplanar reconstruction the complete aorta with all visceral branches can be visualized. Hereby vascular diameters, collateral formation and vascular variants can be imaged. Therefore, therapy should be planned on the basis of a 3-D reconstructed CT and on an invasive digital subtraction angiography[11]. Magnetic resonance angiograph plays no role in the diagnosis of chronic mesenteric ischemia. The digital subtraction angiography should show both lateral and frontal projections of the aorta and all major vessels. Furthermore the individual major branches should be imaged to gain information on collateralisation (i.e., the patency of the Drummond artery or the arc of Riolan).
TREATMENT
With stenoses exceeding 70% in one or more visceral arteries patients may develop symptoms of mesenteric insufficiency. In these cases revascularisation is strongly recommended. While analgesia and parenteral nutrition may be used for optimal preparation of the patient for open surgery or endovascular intervention, conservative treatment will not slow disease progression. Therefore the clinical decision has to be made between endovascular treatment or conventional vascular revascularisation.
ENDOVASCULAR TREATMENT
In the majority of patients endovascular treatment of a mesenteric stenosis is feasible. The choice of vascular access depends on the patient´s vascular anatomy. If the right femoral or iliac artery is heavily calcified, occluded or severely kinked the left femoral artery or the brachial artery may be used. Brachial access is also advisable in patients that show a steep angle between the SMA and the aorta. The regular transfemoral vascular access is achieved by puncture or cut down. A 7 F sheath normally allows the introduction of appropriately sized stents. Via sidewinder catheter the SMA is catheterized and a stiff wire with a long intervention sheath is placed into the SMA. Any wire manipulation has to be confirmed by angiography to avoid vessel injury. In the case of ostial calcifications balloon expandable stents should be used which are placed a few millimetres into the lumen of the aorta. Flexible nitinol stents may be used in the distal SMA. All patients should be heparinised to lower the risk of thromboembolism and/or stroke. Retrograde cannulation and stent placement of the SMA during laparotomy is another alternative approach which combines endovascular and open revascularisation of the occluded vessels.
CONVENTIONAL REVASCULARISATION
Open surgery requires a medial laparotomy since both the supracolic and the infracolic segments of the aorta have to be dissected. The celiac artery and the SMA are most frequently implicated in the disease process, and their involvement may result in chronic ischemia of the small intestine (Figure 1). Stenosis or occlusion of the IMA may lead to ischemic colitis. If both the celiac artery and the mesenteric arteries are involved, all arteries should be reconstructed to ensure long term prognosis[12]. After mobilisation of the left hepatic lobe and transsection of the right crus of the diaphragm the aorta may be tangentially clamped and longitudinal aortio-coeliac or aortomesenteric bypasses may be constructed using vein or prosthetic material (> 7 mm) (Figure 2). Bypass conduction to the mesenteric artery should follow a retropancreatic route to enter the mesenteric root. Here, a tunnel is formed left of the aorta. For a better hemodynamic approach to the SMA, bypasses are conducted curved around the left renal vein. Bypasses should be created tension free and kink-resistant. If longer clamping times are necessary, perfusion catheters may lengthen ischemic tolerance. For short ostial stenosis either transaortic endarterectomy with patch plasty or orthotopic short vein bypasses may be used.
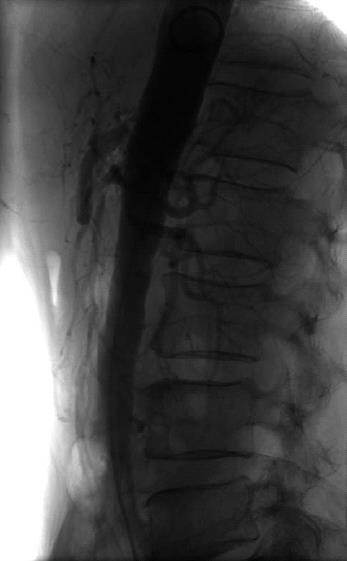
Figure 1 Conventional angiography of a 68 year-old patient who presented with weight loss and abdominal angina.
The angiography shows an ostial stenosis of the celiac artery and a 1 cm stenosis of the superior mesenteric artery.
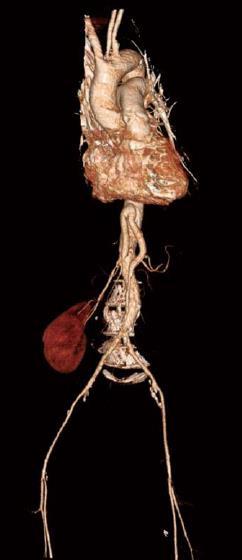
Figure 2 3-D reconstruction of a postoperative computed tomography.
It shows the successful revscularisation of the common hepatic artery and the superior mesenteric artery by a ninfradiaphracmatic bifurcation prothesis.
EVIDENCE
So far no level 1 evidence governs the decision whether patients should receive endovascular or open revascularisation in chronic mesenterial ischemia. Early series showed a similar survival at 2 years between patients who received open revascularisation and patients who received a stent pta. No difference in the incidence of symptomatic or radiographic recurrence was found. However, primary and assisted patency was significantly lower in the percutaneous transluminal angioplasty (PTA)/stent groups compared to OR[13,14]. At the Cleveland clinics (United States) angioplasty and stenting was performed in 28 patients with chronic mesenteric ischemia. This cohort was compared to 85 patients who received open revascularisation[15]. While no significant differences were found in terms of mortality, morbidity and restenosis rates, long term symptom recurrence was significantly higher in the endovascular group. In a large retrospective study major complications occurred in 7% of patients who underwent mesenteric artery stenting and resulted in higher mortality, morbidity, and longer hospital length of stay. The use of antiplatelet therapy reduced the risk of distal embolization or vessel thrombosis[16].
A retrospective comparison at the Mayo clinic which involved 229 patients found a higher morbidity and longer hospital stay in patients after open surgery. Mortality was not different between the groups. Restenosis was five times more common in the endovascular group and symptom recurrence seven times more common than after open surgery[17]. Restenosis within the stents occurs in nearly 40% of patients after stent placement in the mesenteric artery within the first 29 mo. Half of these patients require reintervention because of symptom recurrence or progression to an asymptomatic preocclusive lesion[18]. This “in stent stenosis” is the result of intimal hyperplasia as a reaction to manipulation and trauma within the lumen of the artery. It involves apoptosis and the invasion of inflammatory cells. Vascular smooth muscle cells are recruited into the subendothelial space via signalling cascades involving tyrosin kinase receptors and the ras-pathway[19]. The same pathways will be induced once more if a restenosis is treated by an endovascular approach. Furthermore endovascular interventions are associated with risk of access site and arterial complications from catheter and wire manipulation, balloon dilation, and stent placement. These risks are higher in the case of a reintervention on the SMA[18]. In the mesenteric arteries, dissection, thrombosis, embolization, or perforation may result in bowel ischemia or bleeding, necessitating additional “bail-out” manoeuvres, including emergency conversion to open repair. These complications can be fatal or result in significant morbidity and prolonged hospitalization if not recognized immediately. It is therefore advisable to change to open revascularisation especially in those patients in which endovascular treatment has already failed and who developed “in stent stenoses”.
CLINICAL PRACTISE
Today PTA/stent of the mesenteric artery has surpassed open bypass as the most frequently used option for mesenteric revascularistation, rendering open surgery as method of choice for these with unsuitable anatomy. Schermerhorn et al[20] have reported on a 7 fold increase on mesenteric interventions. This was accompanied by a decrease in mortality from 15% with open bypass surgery to 4% with endovascular treatment. Kougias et al[21] compared outcomes of endovascular treatment in a retrospective analysis which included 48 patients (58 vessels) in the endovascular group while open repair was performed in 96 patients (157 vessels). Endovascular treatment offered shorter hospitalization. Both groups had similar morbidity and mortality rates. Patients treated with surgical reconstruction were more likely to experience long-term symptomatic relief compared to endovascular cohorts, possibly due to higher incidence of two-vessel surgical revascularization[21]. The enthusiasm for endovascular procedures is particularly surprising since no improvement of patency rates has been reported in the literature in the past 20 years[22].
CONCLUSION
The durability and efficacy of open surgical repair are convincing over time. Therefore, open surgical revascularisation remains the treatment of choice for patients who are fit or whose fitness could be improved before surgery. Endovascular therapy has been proposed for patients at high risk for surgery and post surgical corrections. For these unfit patients, or those with short life expectancy, endovascular treatment is preferable owing to its minimally invasive nature and reduced postoperative mortality and morbidity. All others should be treated by conventional reconstruction especially if endovascular treatment has failed before. Randomized controlled studies or patient registries are needed to compare the long-term durability and efficacy of both procedures
P- Reviewers Butterworth J, Sakata N, Naito Y S- Editor Gou SX L- Editor A E- Editor Li JY