Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.797
Revised: January 9, 2013
Accepted: January 18, 2013
Published online: February 14, 2013
Percutaneous ablation using thermal or chemical methods has been widely used in the treatment of hepatocellular carcinoma (HCC). Nowadays, contrast-enhanced imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and contrast-enhanced ultrasound (CEUS) are widely used to evaluate local treatment response after ablation therapies. CEUS is gaining increasing attention due to its characteristics including real-time scanning, easy performance, lack of radiation, wide availability, and lack of allergy reactions. Several studies have documented that CEUS is comparable to CT or MRI in evaluating local treatment efficacy within 1 mo of treatment. However, little information is available regarding the role of CEUS in the follow-up assessment after first successful ablation treatment. Zheng et al found that in comparison with contrast-enhanced computed tomography (CECT), the sensitivity, specificity, positive predictive value, negative predictive value and overall accuracy of CEUS in detecting local tumor progression (LTP) were 67.5%, 97.4%, 81.8%, 94.4% and 92.3%, respectively, and were 77.7%, 92.0%, 92.4%, 76.7% and 84.0%, respectively for the detection of new intrahepatic recurrence. They concluded that the sensitivity of CEUS in detecting LTP and new intrahepatic recurrence after ablation is relatively low in comparison with CECT, and CEUS cannot replace CECT in the follow-up assessment after percutaneous ablation for HCC. These results are meaningful and instructive, and indicated that in the follow-up period, the use of CEUS alone is not sufficient. In this commentary, we discuss the discordance between CT and CEUS, as well as the underlying mechanisms involved. We propose the combined use of CT and CEUS which will reduce false positive and negative results in both modalities. We also discuss future issues, such as an evidence-based ideal imaging follow-up scheme, and a cost-effectiveness analysis of this imaging follow-up scheme.
- Citation: Liu LN, Xu HX, Zhang YF, Xu JM. Hepatocellular carcinoma after ablation: The imaging follow-up scheme. World J Gastroenterol 2013; 19(6): 797-801
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/797.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.797
We read with great interest the recent article by Zheng et al[1] evaluating the usefulness of contrast-enhanced ultrasound (CEUS) in the follow-up of patients with hepatocellular carcinoma (HCC) who had undergone local ablation therapies.
As a minimal invasive and safe treatment method, percutaneous ablation using thermal or chemical methods has been widely used in the treatment of early HCC, recurrent HCC, and even advanced HCC[2-11]. Percutaneous ablation such as radiofrequency ablation (RFA), microwave ablation and ethanol ablation (EA), is regarded as the curative treatment method for early HCC[12,13]. In contrast to surgical resection where the tumor is removed, the tumor is not eradicated from the body but is deactivated by ablation therapy, therefore, it is of paramount importance to evaluate the efficacy of this treatment to determine follow-up treatment and strategy. Currently, the use of contrast-enhanced imaging to detect residual viable tumor or recurrent HCC is widely accepted[14-19]. The underlying mechanism of this imaging method relates to the fact that viable tumor tissue shows arterial hypervascularity (i.e., hyper-enhancement), whereas destroyed tumor shows absence of vascularity (i.e., non-enhancement), thus the distinction between them is achievable. However, this method is not ideal as imaging studies may fail to detect tiny viable tumor tissue, especially when neoangiogenesis is not obvious. Percutaneous biopsy may be another option, however, this technique has significant sample error and it is not ethical and practical to sample all the lesion after ablation. Consequently, contrast-enhanced imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and CEUS are currently widely used to evaluate local treatment response after ablation therapies. Although CT and MRI are accepted as the reference standards, the newly introduced CEUS is also gaining increasing attention due to its characteristics including real-time scanning, easy performance, lack of radiation, wide availability, and lack of allergic reactions[20-38]. Several studies have documented that CEUS is comparable to CT or MRI in evaluating local treatment efficacy within one mo of treatment (Table 1)[18,39-41]. Kim et al[42] also reported that they were in favor of CEUS as it had the advantage of being able to detect lesions < 2 cm; therefore, CEUS has been used effectively in diagnostic algorithms for small 1-2 cm newly detected nodules in HCC patients. However, little information is available regarding the role of CEUS in the follow-up assessment after first successful ablation treatment. In the follow-up period, the patient may develop local tumor progression (LTP) or new intrahepatic recurrence, and imaging modalities are used to successfully detect these lesions.
In the study by Zheng et al[1], 141 patients with HCCs who underwent percutaneous ablation therapy were assessed by paired follow-up CEUS and contrast-enhanced computed tomography (CECT). Using CECT as the reference standard, the ability of CEUS to detect LTP or new intrahepatic recurrence during follow-up was evaluated. They found that 33 LTP and 131 new intrahepatic recurrent foci were detected on CEUS, whereas 40 and 183 were detected on CECT, respectively (both P < 0.05). Compared with CECT, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy of CEUS in detecting LTP were 67.5%, 97.4%, 81.8%, 94.4% and 92.3%, respectively, and were 77.7%, 92.0%, 92.4%, 76.7% and 84.0%, respectively for the detection of new intrahepatic recurrence. They concluded that the sensitivity of CEUS in detecting LTP and new intrahepatic recurrence after ablation was relatively low compared with CECT, and that CEUS can not replace CECT in the follow-up assessment after percutaneous ablation for HCC. Their results are interesting and meaningful, and we agree with the authors regarding the role of CEUS in the follow-up of HCC patients after ablation therapy.
The discordance in imaging features between CT and CEUS is well recognized, and is largely due to the difference in pharmacokinetics between CT contrast medium and ultrasound contrast agent[35,43]. The CT contrast medium diffuses into the interstitial space, while the ultrasound contrast agent is a pure blood pool tracer. The CEUS characteristic of real-time scanning is helpful in detecting subtle lesions with transient arterial hyper-enhancement which is hard to visualize on CT. Some lesions may show arterial iso-enhancement on CT and hyper-enhancement on CEUS due to the limited time window in CT scanning[44-49]. On the other hand, CEUS also has its shortcomings as shown by Zheng et al[1] who found that image quality was affected by lesions located near the liver dome, and obscuration due to gas from the lung or intestine. The development of new foci may be located in different lobes of the liver, and the arterial hyper-enhancement on CEUS only lasts for several seconds, thus it is difficult to detect all hypervascular lesions in one scanning procedure. Most importantly, in comparison with CT/MRI, there is universal bias in readers’ minds with regards to ultrasound images. The quality of the procedure and subsequent results are largely operator-dependent, thus less uniformity is encountered in clinical practice (Table 2).
| CEUS | CECT | |
| Pharmacokinetics of the contrast | Early vascular phase; followed by diffusion into the interstitial space | Pure blood pool tracer; without diffusion into the interstitial space |
| Strong points | Real-time scanning, easy to perform, no radiation, wide availability, and no allergic reactions | High image quality; operator-independent; panoramic imaging; easy to interpret |
| Weak points | Image quality is apt to be affected by lesions located near the liver dome, and obscuration by gas from the lung or intestine; | Inferior temporal resolution; allergic reaction to the contrast-medium; unsuitable for patients with kidney function impairment; radiation; inferior availability |
| Inability to imaging multiple lesions in one procedure; operator-dependent |
The data in the above-mentioned article are detailed and reasonable results have been obtained. However, there is still controversy over the role of CEUS vs CECT in the diagnosis of HCC after ablation, which is largely dependent on individual opinion and familiarity with the techniques. Frieser et al[39] concluded that CEUS is equal to CECT in evaluating treatment response. Gallotti et al[46] found that CEUS was excellent in evaluating treatment response after RFA, whereas it was inadequate for evaluating treatment response after EA. It should be noted that despite the vast number of published studies on the subject, a unanimous consensus has not been achieved.
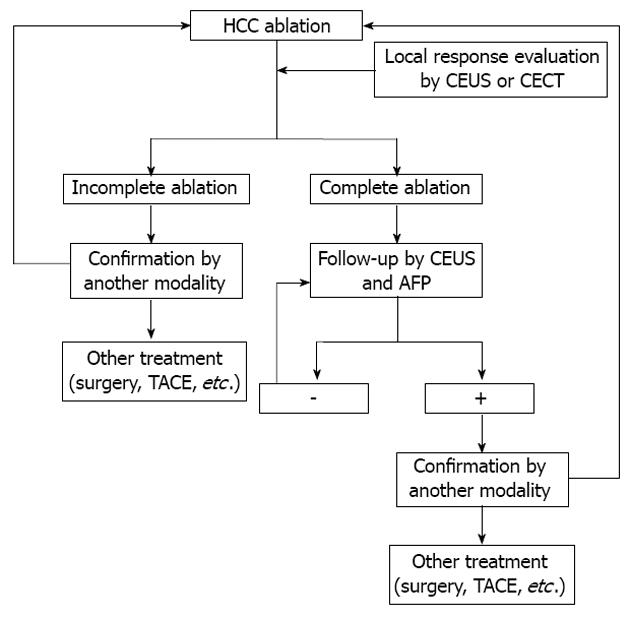
There are a number of unsolved issues regarding the use of CEUS and CECT including the following: (1) The authors should recommend the combined use of CT and CEUS in clinical practice when CEUS is available, which will reduce the number of false positive and negative findings in both modalities[50]. In a study of liver lesion characterization, although no follow-up assessment after ablation was carried out, the authors found that combined assessment using CEUS/CT provided higher sensitivity (97%, both readers) than separate assessment using CEUS (88% reader 1; 87% reader 2) and CT (74% reader 1; 71% reader 2; P < 0.05), while no change in specificity was observed using the combined analysis. The combined assessment of hepatocellular nodule vascularity using CT and CEUS improved diagnostic sensitivity of malignancy in patients with liver cirrhosis[50]. However, the study by Zheng et al[1] was retrospective, thus it is difficult to assess the value of the combined diagnostic procedures in clinical practice, which has prompted a future prospective study to evaluate this issue. Fusion imaging may be another solution which combines the virtues of both modalities[51]; (2) The ideal imaging follow-up scheme is not yet available. Future evidence-based studies are necessary to establish this scheme. Every practicing clinician must make a decision on the most accurate, cost-effective radiologic test to be used in the follow-up of liver lesions after local treatment. A reasonable algorithm is proposed and is shown in Figure 1, however, further study is mandatory to evaluate its efficacy; (3) A cost-effectiveness analysis should be performed to select the best imaging follow-up scheme; and (4) Long-term follow-up studies are needed to help guide our approach and therapy.
P- Reviewers Koch TR, Adinolfi LE, Tischendorf J, Marin JJG S- Editor Wen LL L- Editor Webster JR E- Editor Zhang DN
| 1. | Zheng SG, Xu HX, Lu MD, Xie XY, Xu ZF, Liu GJ, Liu LN. Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J Gastroenterol. 2013;6:855-865. [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85:1078-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Ohmoto K, Yamamoto S. Comparison between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. Clin Radiol. 2006;61:800-801; author reply 801-802. [PubMed] [Cited in This Article: ] |
| 4. | Xu HX, Lu MD, Xie XY, Yin XY, Kuang M, Chen JW, Xu ZF, Liu GJ. Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol. 2005;60:1018-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Xu HX, Xie XY, Lu MD, Chen JW, Yin XY, Xu ZF, Liu GJ. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol. 2004;59:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Yin XY, Xie XY, Lu MD, Kuang M, Liu GJ, Xu ZF, Xu HX, Wang Z. Percutaneous ablative therapies of recurrent hepatocellular carcinoma after hepatectomy: proposal of a prognostic model. Ann Surg Oncol. 2012;19:4300-4306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Kuang M, Lu MD, Xie XY, Xu HX, Xu ZF, Liu GJ, Yin XY, Huang JF, Lencioni R. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 2009;253:552-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, Liu GJ, Liang JY, Lau WY. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914-1923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Lu MD, Yin XY, Xie XY, Xu HX, Xu ZF, Liu GJ, Kuang M, Zheng YL. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg. 2005;92:1393-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, Xu ZF, Liu GJ, Zheng YL. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005;40:1054-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Liu LN, Xu HX, Lu MD, Xie XY. Percutaneous ultrasound-guided thermal ablation for liver tumor with artificial pleural effusion or ascites. Chin J Cancer. 2010;29:830-835. [PubMed] [Cited in This Article: ] |
| 12. | Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 13. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6340] [Article Influence: 487.7] [Reference Citation Analysis (1)] |
| 14. | Xu HX, Lu MD, Xie XH, Xie XY, Kuang M, Xu ZF, Liu GJ, Wang Z, Chen LD, Lin MX. Treatment response evaluation with three-dimensional contrast-enhanced ultrasound for liver cancer after local therapies. Eur J Radiol. 2010;76:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Xu HX, Lu MD, Xie XH, Xie XY, Xu ZF, Chen LD, Liu GJ, Liang JY, Lin MX, Wang Z. Three-dimensional contrast-enhanced ultrasound of the liver: experience of 92 cases. Ultrasonics. 2009;49:377-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Xu HX. Era of diagnostic and interventional ultrasound. World J Radiol. 2011;3:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Xu HX. Contrast-enhanced ultrasound: The evolving applications. World J Radiol. 2009;1:15-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Lu MD, Yu XL, Li AH, Jiang TA, Chen MH, Zhao BZ, Zhou XD, Wang JR. Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol. 2007;33:1736-1749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Ippolito D, Bonaffini PA, Capraro C, Leni D, Corso R, Sironi S. Viable residual tumor tissue after radiofrequency ablation treatment in hepatocellular carcinoma: evaluation with CT perfusion. Abdom Imaging. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Xu HX, Lu MD, Liu LN, Zhang YF, Guo LH, Liu C, Wang S. Imaging Features of Intrahepatic Biliary Cystadenoma and Cystadenocarcinoma on B-Mode and Contrast-Enhanced Ultrasound. Ultraschall Med. 2012;33:E241-E249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Liu LN, Xu HX, Lu MD, Xie XY, Wang WP, Hu B, Yan K, Ding H, Tang SS, Qian LX. Contrast-enhanced ultrasound in the diagnosis of gallbladder diseases: a multi-center experience. PLoS One. 2012;7:e48371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Xu HX, Lu MD, Liu LN, Zhang YF, Guo LH, Xu JM, Liu C. Discrimination between neoplastic and non-neoplastic lesions in cirrhotic liver using contrast-enhanced ultrasound. Br J Radiol. 2012;85:1376-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Xu HX, Lu MD. The current status of contrast-enhanced ultrasound in China. J Med Ultrason. 2010;37:97-106. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Xu ZF, Xu HX, Xie XY, Liu GJ, Zheng YL, Lu MD. Renal cell carcinoma and renal angiomyolipoma: differential diagnosis with real-time contrast-enhanced ultrasonography. J Ultrasound Med. 2010;29:709-717. [PubMed] [Cited in This Article: ] |
| 25. | Xu ZF, Xu HX, Xie XY, Liu GJ, Zheng YL, Liang JY, Lu MD. Renal cell carcinoma: real-time contrast-enhanced ultrasound findings. Abdom Imaging. 2010;35:750-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Xu HX, Xie XY, Xie XH, Kuang M, Xu ZF, Liu GJ, Chen LD, Lin MX, Lu MD. Imaging features of hepatic angiomyolipomas on real-time contrast-enhanced ultrasound. Br J Radiol. 2010;83:411-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, Wang Z, Lin MX, Lu MD. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2010;20:743-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Xie XH, Xu HX, Xie XY, Lu MD, Kuang M, Xu ZF, Liu GJ, Wang Z, Liang JY, Chen LD. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol. 2010;20:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Lin MX, Xu HX, Lu MD, Xie XY, Chen LD, Xu ZF, Liu GJ, Xie XH, Liang JY, Wang Z. Diagnostic performance of contrast-enhanced ultrasound for complex cystic focal liver lesions: blinded reader study. Eur Radiol. 2009;19:358-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 30. | Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Zheng YL, Liang JY, Chen LD. Contrast-enhanced sonography in the diagnosis of small hepatocellular carcinoma < or = 2 cm. J Clin Ultrasound. 2008;36:257-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Liang JY, Chen LD. Unusual benign focal liver lesions: findings on real-time contrast-enhanced sonography. J Ultrasound Med. 2008;27:243-254. [PubMed] [Cited in This Article: ] |
| 32. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med. 2006;25:349-361. [PubMed] [Cited in This Article: ] |
| 33. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, Liang JY. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med. 2006;25:23-33. [PubMed] [Cited in This Article: ] |
| 35. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver - Update 2012. Ultraschall Med. 2013;34:11-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 479] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 36. | Wong GL, Xu HX, Xie XY. Detection of focal liver lesions in cirrhotic liver using contrast-enhanced ultrasound. World J Radiol. 2009;1:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Xu HX. Contrast-enhanced ultrasound in the biliary system: Potential uses and indications. World J Radiol. 2009;1:37-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Xu HX, Chen LD, Liu LN, Zhang YF, Guo LH, Liu C. Contrast-enhanced ultrasound of intrahepatic cholangiocarcinoma: correlation with pathological examination. Br J Radiol. 2012;85:1029-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Frieser M, Kiesel J, Lindner A, Bernatik T, Haensler JM, Janka R, Hahn EG, Strobel D. Efficacy of contrast-enhanced US versus CT or MRI for the therapeutic control of percutaneous radiofrequency ablation in the case of hepatic malignancies. Ultraschall Med. 2011;32:148-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Ricci P, Cantisani V, Drudi F, Pagliara E, Bezzi M, Meloni F, Calliada F, Erturk SM, D’Andrea V, D’Ambrosio U. Is contrast-enhanced US alternative to spiral CT in the assessment of treatment outcome of radiofrequency ablation in hepatocellular carcinoma? Ultraschall Med. 2009;30:252-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Salvaggio G, Campisi A, Lo Greco V, Cannella I, Meloni MF, Caruso G. Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging. 2010;35:447-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Kim TK, Lee KH, Khalili K, Jang HJ. Hepatocellular nodules in liver cirrhosis: contrast-enhanced ultrasound. Abdom Imaging. 2011;36:244-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Chen LD, Xu HX, Xie XY, Lu MD, Xu ZF, Liu GJ, Liang JY, Lin MX. Enhancement patterns of intrahepatic cholangiocarcinoma: comparison between contrast-enhanced ultrasound and contrast-enhanced CT. Br J Radiol. 2008;81:881-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Wiggermann P, Zuber-Jerger I, Zausig Y, Loss M, Scherer MN, Schreyer AG, Stroszczynski C, Jung EM. Contrast-enhanced ultrasound improves real-time imaging of ablation region during radiofrequency ablation: preliminary results. Clin Hemorheol Microcirc. 2011;49:43-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Liu F, Yu X, Liang P, Cheng Z, Han Z, Dong B. Contrast-enhanced ultrasound-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional ultrasound. Int J Hyperthermia. 2011;27:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Gallotti A, D’Onofrio M, Ruzzenente A, Martone E, De Robertis R, Guglielmi A, Pozzi Mucelli R. Contrast-enhanced ultrasonography (CEUS) immediately after percutaneous ablation of hepatocellular carcinoma. Radiol Med. 2009;114:1094-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Chen MH, Wu W, Yang W, Dai Y, Gao W, Yin SS, Yan K. The use of contrast-enhanced ultrasonography in the selection of patients with hepatocellular carcinoma for radio frequency ablation therapy. J Ultrasound Med. 2007;26:1055-1063. [PubMed] [Cited in This Article: ] |
| 48. | Chen MH, Yang W, Yan K, Dai Y, Wu W, Fan ZH, Callstrom MR, Charboneau JW. The role of contrast-enhanced ultrasound in planning treatment protocols for hepatocellular carcinoma before radiofrequency ablation. Clin Radiol. 2007;62:752-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51 Suppl:S19-S23. [PubMed] [Cited in This Article: ] |
| 50. | Quaia E, Alaimo V, Baratella E, Medeot A, Midiri M, Cova MA. The added diagnostic value of 64-row multidetector CT combined with contrast-enhanced US in the evaluation of hepatocellular nodule vascularity: implications in the diagnosis of malignancy in patients with liver cirrhosis. Eur Radiol. 2009;19:651-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Xu HX, Lu MD, Liu LN, Guo LH. Magnetic navigation in ultrasound-guided interventional radiology procedures. Clin Radiol. 2012;67:447-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |