Published online Feb 7, 2013. doi: 10.3748/wjg.v19.i5.755
Revised: December 6, 2012
Accepted: December 15, 2012
Published online: February 7, 2013
AIM: To investigate the feasibility of laparoscopy-assisted total gastrectomy (LATG) using trans-orally inserted anvil (OrVilTM) in terms of operative characteristics and short term outcomes.
RESULTS: Characteristics of 27 patients with gastric cancer who underwent LATG from October 2009 to October 2012 in the Foshan Affiliated Hospital of South Medical University were retrospectively reviewed. Among these patients, six were reconstructed by mini-laparotomy and 21 by OrVilTM. The clinicopathological characteristics, total operation time, total blood loss, abdominal incision and complications of anastomosis including stenosis and leakage, were compared between the groups undergoing LATG with OrVilTM and the group undergoing mini-laparotomy.
RESULTS: The operations were successfully performed on all the patients without intraoperative complications or conversion to open surgery. Two (10%) patients received palliative procedure under laparoscope who were prepared for LATG preoperatively. One case had hepatic metastatic carcinoma and 1 case had tumor recurrence near the anastomosis 8 mo after surgery. The mean follow-up duration was 10 mo (range, 2-24 mo). Operation time was significantly reduced by the use of OrVilTM (198.42 ± 30.28 min vs 240.83 ± 8.23 min). The postoperative course with regard to occurrence of stenosis and leakage was not different between the two groups. There were no significant differences in estimated blood loss. The upper abdominal incision was smaller in OrVilTM group than in mini-laparotomy group (4.31 ± 0.45 cm vs 6.43 ± 0.38 cm).
CONCLUSION: LATG using OrVilTM is a technically feasible surgical procedure with sufficient lymph node dissection, less operation time and acceptable morbidity.
- Citation: Liao GQ, Ou XW, Liu SQ, Zhang SR, Huang W. Laparoscopy-assisted total gastrectomy with trans-orally inserted anvil (OrVilTM): A single institution experience. World J Gastroenterol 2013; 19(5): 755-760
- URL: https://www.wjgnet.com/1007-9327/full/v19/i5/755.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i5.755
Laparoscopic gastrectomy (LG) including laparoscopy-assisted gastrectomy (LAG) and totally LG with regional lymph node dissection as an alternative surgical treatment for gastric cancer has become increasingly common worldwide, especially in Asia[1-3]. However, laparoscopy-assisted total gastrectomy (LATG) has not been accepted as widely as other LGs because of the low incidence of gastric cancer requiring LATG and because it is particularly difficult even for some experienced surgeons to perform[4,5]. Nevertheless, comparing with the conventional open total gastrectomy, laparoscopic surgery as an advanced procedure offers the advantages of less invasiveness and the same curability if surgeons are adroit at performing LATG[6,7].
Since 2009, our institution has adopted laparoscopic modalities for both the early and advanced stage gastric cancer patients, including 27 cases of LATG. Herein, we review our experience with LATG and analyze the results of LATG in terms of operative characteristics and short-term outcomes.
We retrospectively reviewed a series of 27 patients who underwent LATG between October 2009 and October 2012. Twenty-one of them were reconstructed by trans-orally inserted anvil (OrVilTM) and six by mini-laparotomy. The mini-laparotomy was performed in relatively early period and set for the comparisons of short-term outcomes.
Preoperative tumor node metastasis (TNM) stage was determined in all the patients according to the International Union Against Cancer (UICC, 7th edition) and based on endoscopic biopsy and abdominal computed tomography. The indication for LATG in gastric cancer was limited to preoperative stage T1-4N0-3M0. Patients whose NRS 2002 score was more than 4 received more than 1-wk nutrition therapy before operation. Patients suitable for endoscopic submucosal dissection or had surgical contraindications were excluded. Written informed consent was signed by each patient who agreed to undergo LATG.
All patients were placed in relaxed dorsal lithotomy position. The surgeon usually stood on the left side of the patient, the first assistant surgeon on the right, and the second assistant surgeon holding the camera stood between the patient’s legs. At the beginning, five trocars were introduced into the right upper quadrant (5 mm), right middle quadrant (5 mm), subumbilical (10 mm; camera port), left middle quadrant (5 mm), and left upper quadrant (12 mm) regions of the abdomen. The intraperitoneal pressure was maintained as 12 mmHg with carbon dioxide.
Total gastrectomy with complete omentectomy and extended lymphadenectomy (D2) was performed in all the patients. After sufficient mobilization of the duodenum near the pylorus ring and abdominal esophagus, the duodenum and esophagus were transected using EndoGIATM Universal stapler (60 mm; Covidien). The stomach was bagged in an isolation pocket and pulled out extracorporeally through a 4-6 cm upper midline incision. In the next step, the gastrointestinal continuity was restored in a Roux-en-Y mode extracorporeally through the incision.
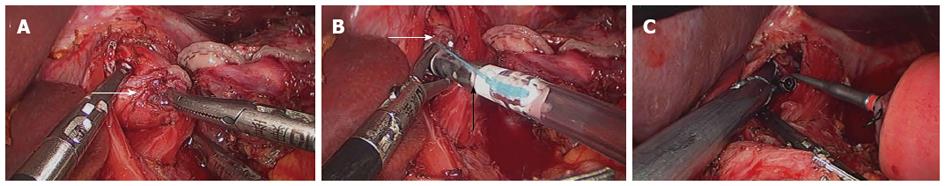
The OrVilTM orogastric tube was transorally introduced into the esophagus. The orogastric tube was then used to make a small hole on the middle of the abdominal esophageal stump. The tube was pulled out into the abdominal cavity through the hole until the anvil reached the esophageal stump. The orogastric tube was disconnected from the anvil and taken out of the esophagus. Subsequently, intracorporeal stapling esophagojejunostomy was performed and the jejunal stump was intracorporeally sutured with EndoGIATM Universal (Figure 1). The intraperitoneal chemohyperthermia was performed and two drains were placed around the esophagojejunal anastomosis and pelvic cavity, respectively.
The theory of fast track surgery is prevalent in our institution, but we adopt a conservative approach for the LATG patients postoperatively. The preoperatively inserted nasogastric tube for air decompression was removed at the end of surgery. A soft diet commenced orally on postoperative day (POD) 4, and abdominal drain tube was removed after 1 or 2 d when the drainage was less than 30 mL per 24 h. After a meglumine diatrizoate meal examination of esophago-intestinal tract was performed to evaluate anastomotic leakage and stenosis on PODs 8 to 10, patients were discharged on PODs 10 to 13.
Data were analyzed by the SPSS statistical software (SPSS 13.0). Quantitative variables were compared using the Student’s t test and were expressed as means ± SD. P values were considered to be statistically significant at 0.05.
Patient characteristics including age, gender, body mass index (kg/m2), history of abdominal surgery, NRS 2002 score and comorbidities are listed in Table 1. The operations were successfully performed in all the patients, without intraoperative complications or conversion to open surgery. Two (10%) cases received palliative procedure under laparoscope who were prepared for LATG preoperatively. One case developed hepatic metastatic carcinoma and 1 case had tumor recurrence near the anastomosis 8 mo after surgery. Mean follow-up duration was 10 mo (range, 2-24 mo).
| Case | Gender | Age (yr) | NRS score | BMI | Previous abdominal operation | Comorbidity |
| 1 | Female | 46 | 5 | 27.2 | No | Urinary lithiasis |
| 2 | Male | 77 | 7 | 24.1 | No | No |
| 3 | Male | 65 | 8 | 21.4 | Appendectomy | No |
| 4 | Male | 61 | 4 | 26.4 | No | No |
| 5 | Male | 62 | 5 | 22.1 | Cholecystotomy | No |
| 6 | Female | 71 | 8 | 20.9 | No | Gout; high blood pressure |
| 7 | Male | 48 | 5 | 24.4 | No | No |
| 9 | Male | 62 | 5 | 23.7 | Cesarean section | Hepatic cyst; high blood pressure; cholecystolithiasis |
| 10 | Male | 60 | 2 | 23.8 | No | No |
| 11 | Female | 55 | 2 | 23.2 | No | No |
| 12 | Female | 52 | 8 | 17.4 | Cesarean section | No |
| 13 | Male | 70 | 2 | 21.5 | No | No |
| 14 | Male | 54 | 3 | 20.5 | No | Urinary lithiasis; urinary infection |
| 15 | Male | 42 | 7 | 18.8 | Gastrectomy | |
| 16 | Male | 72 | 3 | 24.8 | No | |
| 17 | Male | 61 | 6 | 22.1 | No | High blood pressure |
| 18 | Female | 63 | 2 | 20.8 | Appendectomy | |
| 19 | Male | 66 | 5 | 19.2 | No | |
| mean | NA | NA | NA |
Table 2 shows the surgical outcomes and postoperative complications. All patients underwent LATG with antecolic type Roux-en-Y esophagojejunostomy and D2 lymph node dissection. One case received combined spleen and pancreatic tail resection. Operation time was significantly reduced by the use of OrVilTM (198.42 ± 30.28 min vs 240.83 ± 8.23 min, P < 0.05). The postoperative course with regard to stenosis and leakage did not differ between the two groups. There were no significant differences in estimated blood loss (130.57 ± 65.17 mL vs 140.83 ± 78.41 mL, P > 0.05). The upper abdominal incision was smaller in OrVilTM group than in mini-laparoscopy group (4.31 ± 0.45 cm vs 6.43b ± 0.38 cm, P < 0.05).
| OrVilTM group (n = 21) | Mini-laparotomy group (n = 6) | P value | |
| Total operation time (min) | 198 (180-320) | 240 (230-290) | 0.018 |
| Total blood loss (mL) | 130 (100-400) | 140 (100-300) | 0.211 |
| Abdominal incision (cm) | 4.3 (4-6) | 6.4 (5.5-7.0) | 0.022 |
| Complications of anastomosis | 2 | 0 | 1.000 |
| Stenosis | 1 | 0 | 1.000 |
| Leakage | 1 | 0 | 1.000 |
The mean time to first oral intake and postoperative hospital stay were 3.2 d (range, 2-5 d) and 12.5 d (range, 10-19 d). Anastomotic stenosis and major leakage occurred in one case, respectively. All the patients were evaluated at over stage I and received adjuvant chemotherapy.
Histologically, 13 patients had poorly differentiated carcinoma and 3 patients had signet ring cell carcinoma. The mean tumor size was 4.5 cm (range, 3.2-7 cm). The location of the tumor was the upper body in 7 patients and the mid body in 11 patients. Esophageal invasion was detected in 1 patient and double lesions were detected in 1 patient who had a mid-body cancer. The mean length of proximal resection margin was 4.7 cm (range, 2.2-6.1 cm) and the distal one was 6.2 cm (range, 3.1-9 cm). TNM staging according to the 7th UICC identified stage IIA in 2, stage IIB in 7, stage IIIA in 6, stage IIIB in 5 and stage IIIC in 1 patient. The mean number of retrieved lymph nodes was 22.4 (range, 16-42). Multiple lymph node metastases were detected, 1-2 lymph nodes in 2 patients, 3-6 in 8 patients and more than 7 in 11 patients.
Since the first report of LG in 1992[8], LAG has been carried out not only in distal and proximal gastrectomy, but also in total gastrectomy which was more often used in advanced gastric cancer[9-11]. Although performance of LATG for gastric cancer has been increasing worldwide, especially in Asia, it remains controversial if laparoscopic D2 dissection is equivalent to open surgery for advanced gastric cancer (AGC). In our cases, the dissection of more than 15 lymph nodes was performed and the final cutting edge negative rate was 100%. Some recent studies focus on the outcome of D2 lymph node dissection in LAG and open surgery for gastric cancer[11-13]. Du et al[11] evaluated 82 patients with AGC who underwent LATG with D2 dissection compared with 94 patients who received open surgery; a similar number of harvested lymph nodes (HLNs) was obtained in both groups. Cui et al[13] retrospectively analyzed 131 cases including a single LATG group, and found that laparoscopic D2 dissection is equivalent to open gastrectomy in the number of HLNs, regardless of tumor location.
The mean operation time for LATG with OrVilTM was 198 min, which was significantly shortened compared with the traditional mini-laparotomy group (240 min), and the mean operation time for LATG was also significantly shorter than for mini-laparotomy (180 min vs 406 min) in the previous studies[9,10,13]. It takes a longer time to perform esophagojejunal anastomosis through a narrow mini-laparotomy in LATG, which can be avoided by the use of OrVilTM. The same conclusion is confirmed by other operative team and with OrVilTM their mean operation time was 152-243 min which mainly affected by tumor stage[3,5,9,14].
The incidence of postoperative complications in patients who underwent LATG has been reported to be 9.4%-39.4%, and common complications include anastomotic leakage, anastomotic stenosis, and pancreatic fistula[9,2,14,15]. Some studies revealed that the incidence of complications in the LATG group was similar to that in the open total gastrectomy group; however, other studies showed a lower or higher rate of complications in the LATG group[15-17]. In this study, 1 case developed anastomotic leakage and 1 case had anastomotic stenosis. The complication rate was 27%, being slightly lower compared with those from previous studies[15,18,19]. The high frequency of anastomotic complications in patients who underwent LATG might result from the excessive traction of the distal esophagus and the extensive mobilization of the jejunal limb. In our series, the rates of complications associated with anastomosis were not statitistically different between the LATG with OrVilTM and traditional mini-laparoscopy groups. However, it should be mentioned that the number of the mini-laparotomy group was small which may produce statistics bias. The same procedure was performed postoperatively in these two groups, so the comparison of mean time to first oral intake and postoperative hospital stays was meaningless.
There are some reconstructive methods used after LATG, such as Roux-en-Y esophagojejunostomy, and extracorporeal or intracorporeal anastomosis using a hand-sewn, circular stapler, or side-to-side linear stapler[1,9,20,21]. Roux-en-Y esophagojejunostomy by extracorporeal anastomosis through a small skin incision is the most common approach. However, it is difficult to perform through a mini-laparotomy, particularly on obese patients, and too larger laparotomy makes it similar to conventional open surgery[22]. OrVilTM as an intracorporeal circular stapling esophagojejunostomy can simplify the reconstruction procedure after total gastrectomy[23]. This device requires no purse-string sutures and offers wide intracorporeal operating views[24,25]. In this study, compared with control group, the smaller body incision and less operation time were observed. Moreover, two respective studies concluded that this technique was simple, safe, and efficient for performing gastrojejunostomy, and additionally less expensive and accelerated the surgical learning curve[23,26,27]. However, the earlier studies reported postoperative infection and recommended oral gargling with hexamidine solution and abdominal irrigation after anvil insertion[9,28]. No postoperative abdominal infection occurred in our series.
There were some limitations in this study. First, this retrospective analysis might have selection bias as a result of comparison of these nonrandomized groups with a retrospective profile. Second, there was no survival data. Thus, long-term oncological outcomes of LATG with OrVil TM need to be evaluated by future studies. Third, the sample size of the mini-laparotomy group is small and the operation was performed in relatively early period which cause the learning curve effect.
In conclusion, LATG using OrVilTM for gastric cancer may be a technically feasible surgical procedure with advantages of sufficient lymph node dissection, less operation time and acceptable morbidity. However, the number of patients is small in this study. It will be necessary to confirm these results by a large cohort study in the validity of LATG with OrVilTM.
Laparoscopic gastrectomy (LG) including laparoscopy-assisted gastrectomy (LAG) and totally LG with regional lymph node dissection as an alternative surgical treatment for gastric cancer has become increasingly common worldwide, especially in Asia.
Since the first report of LG in 1992, laparoscopy-assisted gastrectomy has been carried out not only in distal and proximal gastrectomy, but also in total gastrectomy which was more often used in advanced gastric cancer. Although performance of laparoscopy-assisted total gastrectomy for gastric cancer has been increasing worldwide, especially in Asia, it remains controversial if laparoscopic D2 dissection is equivalent to open surgery for advanced gastric cancer.
LATG using orally inserted anvil (OrVilTM) for gastric cancer may be a technically feasible surgical procedure with advantages of sufficient lymph node dissection, less operation time and acceptable morbidity. However, the number of patients is small in this study. It will be necessary to confirm these results by a large cohort study in the validity of LATG with OrVilTM.
This is an interesting manuscript on LG with trans-orally anastomosis. Since little is known about this technique, many readers would be interested to learn this experience.
P- Reviewers Du JJ, Pogorelic Z, Milone M S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 2. | Kinoshita T, Shibasaki H, Oshiro T, Ooshiro M, Okazumi S, Katoh R. Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc. 2011;25:1395-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Ahn SH, Lee JH, Park DJ, Kim HH. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Cho HJ, Kim W, Hur H, Jeon HM. Laparoscopy-assisted completion total gastrectomy for gastric cancer in remnant stomach: report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2009;19:e57-e60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Kawamura H, Yokota R, Homma S, Kondo Y. Comparison of invasiveness between laparoscopy-assisted total gastrectomy and open total gastrectomy. World J Surg. 2009;33:2389-2395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Eom BW, Kim YW, Lee SE, Ryu KW, Lee JH, Yoon HM, Cho SJ, Kook MC, Kim SJ. Survival and surgical outcomes after laparoscopy-assisted total gastrectomy for gastric cancer: case-control study. Surg Endosc. 2012;26:3273-3281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Kodera Y. Introduction of laparoscopy-assisted distal gastrectomy: a tale of two cities. Gastric Cancer. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Goh P, Tekant Y, Isaac J, Kum CK, Ngoi SS. The technique of laparoscopic Billroth II gastrectomy. Surg Laparosc Endosc. 1992;2:258-260. [PubMed] [Cited in This Article: ] |
| 9. | Jeong GA, Cho GS, Kim HH, Lee HJ, Ryu SW, Song KY. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009;146:469-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Du J, Zheng J, Li Y, Li J, Ji G, Dong G, Yang Z, Wang W, Gao Z. Laparoscopy-assisted total gastrectomy with extended lymph node resection for advanced gastric cancer--reports of 82 cases. Hepatogastroenterology. 2010;57:1589-1594. [PubMed] [Cited in This Article: ] |
| 12. | Huang JL, Wei HB, Zheng ZH, Wei B, Chen TF, Huang Y, Guo WP, Hu B. Laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer. Dig Surg. 2010;27:291-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Cui M, Xing JD, Yang W, Ma YY, Yao ZD, Zhang N, Su XQ. D2 dissection in laparoscopic and open gastrectomy for gastric cancer. World J Gastroenterol. 2012;18:833-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bracale U, Marzano E, Nastro P, Barone M, Cuccurullo D, Cutini G, Corcione F, Pignata G. Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc. 2010;24:2475-2479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, Yamashita K, Watanabe M. Laparoscopy-assisted pancreas- and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc. 2009;23:2416-2423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Hamabe A, Omori T, Tanaka K, Nishida T. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2012;26:1702-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Gordon AC, Kojima K, Inokuchi M, Kato K, Sugihara K. Long-term comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy in advanced gastric cancer. Surg Endosc. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010;211:e25-e29. [PubMed] [Cited in This Article: ] |
| 19. | Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, Kim CG, Choi IJ. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392-395. [PubMed] [Cited in This Article: ] |
| 20. | Gould JC, Garren M, Boll V, Starling J. The impact of circular stapler diameter on the incidence of gastrojejunostomy stenosis and weight loss following laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2006;20:1017-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Usui S, Yoshida T, Ito K, Hiranuma S, Kudo SE, Iwai T. Laparoscopy-assisted total gastrectomy for early gastric cancer: comparison with conventional open total gastrectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:309-314. [PubMed] [Cited in This Article: ] |
| 22. | Omori T, Oyama T, Mizutani S, Tori M, Nakajima K, Akamatsu H, Nakahara M, Nishida T. A simple and safe technique for esophagojejunostomy using the hemidouble stapling technique in laparoscopy-assisted total gastrectomy. Am J Surg. 2009;197:e13-e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc. 2009;23:2624-2630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Chavarriaga LF, Cook MW, White B, Jeansonne L, Gletsu N, Parker CB, Sweeney J, Davis SS, Lin E. Transoral technique for gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass (LRYGBP) can accelerate learning curve and reduce cost. Obes Surg. 2010;20:846-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Hirahara N, Monma H, Shimojo Y, Matsubara T, Hyakudomi R, Yano S, Tanaka T. Reconstruction of the esophagojejunostomy by double stapling method using EEA™ OrVil™ in laparoscopic total gastrectomy and proximal gastrectomy. World J Surg Oncol. 2011;9:55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Kunisaki C, Makino H, Oshima T, Fujii S, Kimura J, Takagawa R, Kosaka T, Akiyama H, Morita S, Endo I. Application of the transorally inserted anvil (OrVil) after laparoscopy-assisted total gastrectomy. Surg Endosc. 2011;25:1300-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Park do J, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, Ryu SW, Song KY, Lee HJ, Cho GS. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548-1553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Kim KH, Kim MC, Jung GJ, Choi HJ, Jang JS, Kwon HC. Comparative analysis of five-year survival results of laparoscopy-assisted gastrectomy versus open gastrectomy for advanced gastric cancer: a case-control study using a propensity score method. Dig Surg. 2012;29:165-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |