Published online Oct 14, 2013. doi: 10.3748/wjg.v19.i38.6479
Revised: August 28, 2013
Accepted: September 4, 2013
Published online: October 14, 2013
AIM: To develop a fuzzy classification method to score the texture features of pancreatic cancer in endoscopic ultrasonography (EUS) images and evaluate its utility in making prognosis judgments for patients with unresectable pancreatic cancer treated by EUS-guided interstitial brachytherapy.
METHODS: EUS images from our retrospective database were analyzed. The regions of interest were drawn, and texture features were extracted, selected, and scored with a fuzzy classification method using a C++ program. Then, patients with unresectable pancreatic cancer were enrolled to receive EUS-guided iodine 125 radioactive seed implantation. Their fuzzy classification scores, tumor volumes, and carbohydrate antigen 199 (CA199) levels before and after the brachytherapy were recorded. The association between the changes in these parameters and overall survival was analyzed statistically.
RESULTS: EUS images of 153 patients with pancreatic cancer and 63 non-cancer patients were analyzed. A total of 25 consecutive patients were enrolled, and they tolerated the brachytherapy well without any complications. There was a correlation between the change in the fuzzy classification score and overall survival (Spearman test, r = 0.616, P = 0.001), whereas no correlation was found to be significant between the change in tumor volume (P = 0.663), CA199 level (P = 0.659), and overall survival. There were 15 patients with a decrease in their fuzzy classification score after brachytherapy, whereas the fuzzy classification score increased in another 10 patients. There was a significant difference in overall survival between the two groups (67 d vs 151 d, P = 0.001), but not in the change of tumor volume and CA199 level.
CONCLUSION: Using the fuzzy classification method to analyze EUS images of pancreatic cancer is feasible, and the method can be used to make prognosis judgments for patients with unresectable pancreatic cancer treated by interstitial brachytherapy.
Core tip: Digital image processing (DIP) of endoscopic ultrasonography (EUS) images has been proven to be useful in diagnosis of malignant tumor. Currently commonly used method of DIP is only to concludes the differential diagnosis of solid tumors (“yes” or “no”), can not provide the numerical data describing the texture parameters in the EUS image. EUS-guided brachytherapy has been applied preliminarily in the study of advanced pancreatic cancer. However, prognosis judgment of these patients was still difficult. So we develop a fuzzy classification method to score texture features of pancreatic cancer in EUS images to supply more information and validated its utility in prognosis judgment of patients with unresectable pancreatic cancer treated by EUS-guided interstitial brachytherapy.
- Citation: Xu W, Liu Y, Lu Z, Jin ZD, Hu YH, Yu JG, Li ZS. A new endoscopic ultrasonography image processing method to evaluate the prognosis for pancreatic cancer treated with interstitial brachytherapy. World J Gastroenterol 2013; 19(38): 6479-6484
- URL: https://www.wjgnet.com/1007-9327/full/v19/i38/6479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i38.6479
The application of digital image processing (DIP) in endoscopic ultrasonography (EUS) images and other imaging scenarios has been proven to be a useful adjunct to endoscopic diagnoses and often comparable with specialists’ interpretation in different pathologic settings[1-3]. The texture parameters of EUS images are extracted and classified from the returned echoes to identify the tissue type present in the images. One effective approach is to use DIP based on a support vector machine (SVM), which is a computer algorithm that learns by example to assign labels to objects[4,5]. The SVM technique, as a subfield of digital signal processing, has been applied to a series of pathologically proven diseases[6-12].
The typical method of SVM, which is only able to provide a differential diagnosis for solid tumors (“yes” or “no”), cannot provide numerical data describing the texture parameters in the EUS image. In this study, a new DIP method based on fuzzy classification is applied to obtain the feature value of texture parameters in EUS images of pancreatic cancer and observe the change of texture parameters to evaluate its utility in making prognosis judgments for patients with unresectable pancreatic cancer after EUS-guided interstitial brachytherapy.
The whole study protocol was approved by the Institutional Review Board and Ethics Committees of the Second Military Medical University. All patients had provided their written informed consent before the study. DIP of EUS images using the fuzzy classification method was retrospective, whereas its application in the prognosis evaluation was prospective.
Given that the unidentified object u had p classes, which meant there were p cases that such an object could be classified to, a number of features were extracted from the object u, and the sum of these features had a membership degree A to every class. Therefore, the membership degree of the unidentified object u to each class was A1 (ui), A2 (ui),..., Ap (ui). It is generally assumed that the larger the membership degree’s value of a certain class is, the greater the feature value of the objects belonging to this class will be.
Given that the jth feature extracted from the unidentified object u was uj, its membership degree to the jth feature of class i was:
Aij (u) = (1 + (uj - aij)2/σij2)-1 (1)
where aij was the jth feature’s mean value for the training data belonging to class i, and σij was the variance.
Thus, every feature of the unidentified object u could obtain a membership degree to class i. In addition, a corresponding weight αj was also assigned to it. Therefore, the membership degree of belonging to class i should be:
(2)
The weights could be optimized by taking advantage of the training data.
In terms of the application object in this study, there were two classes: pancreatic cancer and non-pancreatic cancer. For an unidentified case, its membership degree to the two categories A1 and A2 was computed, and then the feature value was obtained according to the following normalized evaluation function: Eval = [A1/(A1 + A2)] × 100%. The object is more likely to be a cancer as the feature value gets closer to 100%, and vice versa. Thus, the fuzzy classification of pancreatic cancer was achieved.
The analysis database of EUS images was compiled from data collected between March 2005 and December 2007, which was described in a previous study of our group[14]. All EUS procedures were performed with an Olympus GF-UM2000 at 7.5 MHz. Regions of interest (ROIs) of all EUS images were manually outlined by endoscopic specialists who were blind to the final diagnosis. Texture features were extracted from every ROI and analyzed using a C++ program. Texture features generally referred to the spatial arrangement and interconnection of the basic elements of images[15]. The sequential forward search algorithm was applied to select the features after extracting the feature. Then, a few optimum feature combinations were obtained[16,17]. Finally, real time was taken into account, and 22 features falling into three categories were selected. First, the mean feature value of the image was extracted. It was a first-order statistical feature. Second, the gray level co-occurrence matrix (GLCM) features were selected, which were based on the second-order joint feature distribution matrix of the images proposed by Haralick et al[18]. GLCMs for four directions (0°, 45°, 90°, and 135°) were constructed. For each matrix, five features were extracted, which were energy, entropy, moment of inertia, correlation, and local stationary. Finally, the fractal feature was obtained. Recently, the fractal dimension feature has been widely used in pattern recognition and texture analysis. In this study, the second-order multi-fractal dimension feature was used, and the differential box-counting approach was applied to calculate the fractal dimension[19,20]. The previously described fuzzy classification method assessed all the features contained in the ROI of the EUS image and estimated a score between 0 and 100. Given that two states existed - cancer and a normal pancreas - 0 represented all the features of a “normal pancreas” that were contained in the ROI with no “cancer” features, whereas 100 represented all the features of “cancer” with no “normal pancreas” features.
Written informed consent for EUS-guided interstitial brachytherapy (EUS-guided iodine 125 radioactive seed implantation) was required from all included patients. Patient eligibility criteria included histologically confirmed unresectable pancreatic adenocarcinoma. To be included in the study, patients had to have a Karnofsky performance status score ≥ 60 and be expected to survive for more than 2 mo after diagnosis; in addition, they had to exhibit adequate bone-marrow function (absolute neutrophil count ≥ 1.5 × 109 cells/L, platelet count ≥ 100 × 109/L, and hemoglobin ≥ 100 g/L), kidney function (serum creatinine ≤ 132.6 μmol/L), and a prothrombin time within 3 s of the control. Exclusion criteria included the inability to give informed consent. Abdominal pain and other accompanying diseases had to be controlled in all patients before inclusion in the study. While receiving implantation treatment, the patients received other necessary treatments such as chemotherapy or biological therapy. The procedure used for radioactive seed implantation was the same one detailed in our previous description[21].
All patients received repeated EUS before and after brachytherapy. All images were reviewed by endoscopists who were blinded to the prognosis. A total of 10 EUS images, 5 images each before and after brachytherapy, were chosen for each patient. The boundary of the ROI was manually delineated, and all the feature values within the ROIs were averaged together. By setting the appropriate range for the estimated scores, the influences of necrotic tissue and radioactive seeds on the calculation results were avoided. The fuzzy classification method calculated two scores for every patient.
All patients were evaluated by weekly physical examinations, complete blood counts, and chemistry profiles. The serum level of carbohydrate antigen 199 (CA199) was measured every 3 wk after the therapy. Standard WHO response criteria were used to define the best anti-tumor effects, toxicities, complications, and adverse events[22]. Tumor assessment by the same endoscopic expert with an EUS scan was required every 3 mo. The largest and smallest diameters were recommended to be measured by EUS, and the tumor volume was estimated according the following formula: V = 1/2 ab2, where a and b are the largest and smallest tumor diameters, respectively, and V is the tumor volume. Overall survival (OS) was calculated from the day of treatment until the date of death.
The descriptive results of continuous variables were presented as the median (interquartile range, IQR). The relationships between the change of fuzzy classification score, tumor volume, CA199 level, and overall survival were assessed using the Spearman rank correlation test. The patients were divided into two groups according to an increase or decrease in the fuzzy classification score. The overall survival rates of the two groups were compared using the log-rank test. The inter-group comparison of the change of tumor volume and CA199 level was conducted by a Mann-Whitney U test. The results were considered statistically significant at P < 0.05. Statistical analyses were performed using the Statistical Package for Social Sciences software (SPSS version 18.0).
Between March 2005 and December 2007, 153 patients with pancreatic cancer and 63 non-cancer patients with a normal pancreas (20 patients) or chronic pancreatitis (43 patients) were included in the analysis database. All EUS images of these patients were analyzed. The ROIs were drawn, and texture features were extracted and selected.
From April 2007 to March 2009, a total of 25 consecutive patients were enrolled. There were fourteen men and eleven women, with a median age of 67 years (range 54-80 years) and a median KPS score of 80 (range 60-90). Five patients were in stage III, and twenty were in stage IV. The average number of seeds (0.5 mCi per seed) implanted was 14.6 per patient (range 5-30 per patient). All patients tolerated the brachytherapy well without any complications throughout the study.
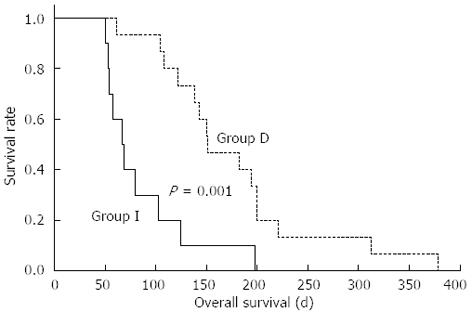
A total of 250 EUS images from the 25 patients were analyzed using the fuzzy classification method, and every patient was scored twice. There was a correlation between the change in the fuzzy classification score and overall survival (r = 0.616, P = 0.001), whereas no correlation was found to be significant between the change of tumor volume (P = 0.663), CA199 level (P = 0.659), and overall survival. There were 15 patients with a decrease in the fuzzy classification score after the brachytherapy, whereas the fuzzy classification score increased in other 10 patients (Table 1). There was a significant difference in the overall survival between the two groups (67 d vs 151 d, P = 0.001, Figure 1). There was no significant difference in the change of tumor volume (P = 0.345) and CA199 level (P = 0.371) between the two groups (Table 1).
| No. | FCS before brachytherapy | FCS after brachytherapy | FCS1 | Tumor volume1 | CA19912 | Survival time (d) |
| 1 | 22.30 | 80.40 | -260.50% | -93% | -53% | 58 |
| 2 | 16.30 | 33.52 | -105.60% | 14% | 69% | 69 |
| 3 | 70.60 | 88.61 | -25.50% | 42% | 75% | 50 |
| 4 | 50.30 | 60.24 | -19.80% | 53% | 0% | 80 |
| 5 | 52.60 | 56.80 | -8.00% | 2% | 0% | 53 |
| 6 | 60.30 | 63.50 | -5.30% | 0% | 8% | 67 |
| 7 | 87.52 | 90.31 | -3.20% | 0% | 66% | 198 |
| 8 | 88.50 | 90.20 | -1.90% | -320% | 17% | 54 |
| 9 | 83.10 | 83.68 | -0.70% | 0% | NA | 103 |
| 10 | 90.80 | 91.20 | -0.40% | 12% | 0% | 125 |
| 11 | 88.20 | 87.20 | 1.10% | -15% | NA | 200 |
| 12 | 90.20 | 88.93 | 1.40% | -171% | 0% | 143 |
| 13 | 91.30 | 88.70 | 2.80% | 0% | 0% | 138 |
| 14 | 92.43 | 89.20 | 3.50% | 48% | 0% | 221 |
| 15 | 95.10 | 88.20 | 7.30% | -1% | 6% | 312 |
| 16 | 89.21 | 80.12 | 10.20% | -104% | -30% | 108 |
| 17 | 93.70 | 81.10 | 13.40% | 71% | 85% | 61 |
| 18 | 87.52 | 75.21 | 14.10% | 44% | -265% | 182 |
| 19 | 92.20 | 61.23 | 33.60% | 96% | 0% | 122 |
| 20 | 82.30 | 47.60 | 42.20% | 78% | -3% | 200 |
| 21 | 78.56 | 44.00 | 44.00% | -66% | -1% | 150 |
| 22 | 75.90 | 35.62 | 53.10% | 42% | 13% | 104 |
| 23 | 51.20 | 18.30 | 64.30% | 40% | -358% | 151 |
| 24 | 90.10 | 22.70 | 74.80% | 61% | 96% | 194 |
| 25 | 89.30 | 18.64 | 79.10% | 4% | 95% | 378 |
| Groups | ||||||
| Increase (n = 10) (interquartile range) | -6.7% (43.9%) | 1% (44%) | 8% (68%) | 67 (49)3 | ||
| Decrease (n = 15) (interquartile range) | 14.1% (49.6%) | 40% (76%) | 0% (41%) | 151 (78)3 | ||
The analysis of texture features is the core of DIP of digital images. Texture features are helpful for classifying lesions on sonography, and the potential of sonographic texture analysis to improve tumor diagnosis has already been demonstrated[23-27]. However, only a few reports exist about the application of DIP techniques to EUS. For the diagnosis of pancreatic cancer, research using DIP and pattern recognition remains rare. Two recent studies successfully used neural network analysis of EUS images to differentiate pancreatic cancer from chronic pancreatitis[1,3]. Das et al[3] reported high sensitivity (93%) and specificity (92%), with excellent positive predictive values (87%) and negative predictive values (96%). An SVM model was evaluated as a potential method to differentiate between malignant and benign lesions with excellent accuracy rates[28]. Its performance characteristics in differentiating pancreatic cancer from benign lesions or normal tissue of the pancreas are closely rivaled by those of EUS-FNA.
In our study, the feature extraction and selection based on fuzzy classification was applied to EUS images of pancreatic cancer patients. All the work was carried out by the developed C++ program. According to the fuzzy algorithm, the classification result was not just “yes” or “no”, but a score from 0 to 100[2,13,29]. Compared with the SVM method[13], the fuzzy classification method proposed in our study could additionally give the precise numerical difference between a cancer case and a non-cancer case.
EUS-guided brachytherapy has been applied preliminarily in the study of advanced pancreatic cancer[30]. Two clinical series showed that pancreatic cancer could be treated safely with EUS-guided brachytherapy with pain control[31,32]. The number of patients enrolled in these two series was 22 and 100, respectively, with stage III or IV pancreatic cancer in a majority of cases. The estimated median overall survival in the two studies was 9.0 and 7.0 mo. The brachytherapy’s effect on overall survival was uncertain because of the lack of a control. Meanwhile, making prognosis judgments for these patients is still difficult. Given that brachytherapy aims to destroy the tumor closely, if it were effective, the EUS images of pancreatic cancer ought to change to be more similar to those from a normal pancreas, and the fuzzy classification score of EUS images after the brachytherapy ought to show a decrease. Thus, the change of the fuzzy classification score most likely reflected the treatment effect to some extent and the prognosis after brachytherapy. Our study results validated this hypothesis. First, the change of the fuzzy classification score was significantly correlated with overall survival, which meant the more the score decreased, the longer the patient survived. Second, 15 of 25 patients (60%) had a decreased fuzzy classification score after the brachytherapy. The median overall survival was nearly 5 mo. As a control, the fuzzy classification results increased in 10 patients after treatment, and the median overall survival was only approximately 2 mo. The log-rank test indicated a significant difference between these two groups.
The tumor volume is an important candidate for making prognosis evaluations for pancreatic cancer. In our study setting, the metal package of radioactive seeds made it difficult to measure the tumor volume by computed tomography. Thus, the EUS scan was a more suitable and convenient way to measure the volume. Meanwhile, as a diagnostic marker, CA199 is also another candidate for prognosis evaluation[33]. However, our results found no association between the change of tumor or CA199 and the overall survival, which meant they were not a suitable prognosis marker in the patient population.
There were some limitations in our study. The new method can distinguish pancreatic cancer from chronic pancreatitis or a normal pancreas, but it cannot differentiate different cancer types. The probable approach to overcome this problem is to train multiple, 1-vs-all classifiers[19]. Furthermore, enlarging the sample size and selecting new effective features are future possibilities for further study to improve the practicability of the technique.
In conclusion, the fuzzy classification method to score texture features of pancreatic cancer in EUS images is feasible and can be used as an effective tool to judge the prognosis of patients with unresectable pancreatic cancer treated by interstitial brachytherapy.
The application of digital image processing (DIP) in endoscopic ultrasonography (EUS) images and other imaging scenarios has been proven to be a useful adjunct to endoscopic diagnoses and is often comparable with specialists’ interpretations in different pathologic settings. The typical method of support vector machine, which is only able to provide a differential diagnosis for solid tumors (“yes” or “no”), cannot provide numerical data describing the texture parameters in the EUS image. Thus, authors applied a new DIP method based on fuzzy classification to quantify the images and supply more information about the status of pancreatic cancer.
The prognosis for pancreatic cancer is poor, and the effects of all currently available therapies are poor. EUS-guided brachytherapy has been applied preliminarily in the study of advanced pancreatic cancer as a potential new therapy. However, making prognosis judgments for these patients after brachytherapy is still difficult. EUS has become a useful tool to monitor cancer lesions. DIP of the change in EUS images after brachytherapy may be useful for making prognosis judgements.
Making prognosis judgments for patients with unresectable pancreatic cancer after EUS-guided interstitial brachytherapy is difficult. Authors developed a new DIP method based on fuzzy classification to analyze EUS images of pancreatic cancer, and they validated its utility in making prognosis judgements.
The new DIP method based on using fuzzy classification to analyze EUS images supplies more information than other DIP methods and has a significant potential to assist in clinical decision making in terms of diagnosis, prognosis, and diseases monitoring, especially for solid tumors.
Fuzzy classification is the process of grouping elements into a fuzzy set whose membership function is defined by the truth value of a fuzzy propositional function.
This study provided a new method to evaluate the effect of EUS-guided interstitial brachytherapy on unresectable pancreatic cancer. Through digital image processing of EUS images, the current study indicates that using the fuzzy classification method to score the texture features of pancreatic cancer in EUS images is useful for making prognosis judgments for patients with unresectable pancreatic cancer treated by interstitial brachytherapy.
P- Reviewers Du YQ, Reddy DN, Roy PK S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Norton ID, Zheng Y, Wiersema MS, Greenleaf J, Clain JE, Dimagno EP. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointest Endosc. 2001;54:625-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Levy MJ, Clain JE, Clayton A, Halling KC, Kipp BR, Rajan E, Roberts LR, Root RM, Sebo TJ, Topazian MD. Preliminary experience comparing routine cytology results with the composite results of digital image analysis and fluorescence in situ hybridization in patients undergoing EUS-guided FNA. Gastrointest Endosc. 2007;66:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Das A, Nguyen CC, Li F, Li B. Digital image analysis of EUS images accurately differentiates pancreatic cancer from chronic pancreatitis and normal tissue. Gastrointest Endosc. 2008;67:861-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Noble WS. What is a support vector machine? Nat Biotechnol. 2006;24:1565-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1395] [Cited by in F6Publishing: 1219] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 5. | Vapnik V. The nature of statistical learning theory. 2nd ed. New York: Wiley 1998; . [Cited in This Article: ] |
| 6. | Zhu ZH, Sun BY, Ma Y, Shao JY, Long H, Zhang X, Fu JH, Zhang LJ, Su XD, Wu QL. Three immunomarker support vector machines-based prognostic classifiers for stage IB non-small-cell lung cancer. J Clin Oncol. 2009;27:1091-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Levman J, Leung T, Causer P, Plewes D, Martel AL. Classification of dynamic contrast-enhanced magnetic resonance breast lesions by support vector machines. IEEE Trans Med Imaging. 2008;27:688-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Mohamed SS, Salama MM, Kamel M, El-Saadany EF, Rizkalla K, Chin J. Prostate cancer multi-feature analysis using trans-rectal ultrasound images. Phys Med Biol. 2005;50:N175-N185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Mohamed SS, Salama MA. Prostate cancer spectral multifeature analysis using TRUS images. IEEE Trans Med Imaging. 2008;27:548-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Chang RF, Wu WJ, Moon WK, Chou YH, Chen DR. Support vector machines for diagnosis of breast tumors on US images. Acad Radiol. 2003;10:189-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Vomweg TW, Buscema M, Kauczor HU, Teifke A, Intraligi M, Terzi S, Heussel CP, Achenbach T, Rieker O, Mayer D. Improved artificial neural networks in prediction of malignancy of lesions in contrast-enhanced MR-mammography. Med Phys. 2003;30:2350-2359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Nattkemper TW, Arnrich B, Lichte O, Timm W, Degenhard A, Pointon L, Hayes C, Leach MO. Evaluation of radiological features for breast tumour classification in clinical screening with machine learning methods. Artif Intell Med. 2005;34:129-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Chen SL, Li JG, Wang XG. Fuzzy Set Theory and Applications. 1st ed. Beijing: Science Press 2005; 170-175. [Cited in This Article: ] |
| 14. | Zhang MM, Yang H, Jin ZD, Yu JG, Cai ZY, Li ZS. Differential diagnosis of pancreatic cancer from normal tissue with digital imaging processing and pattern recognition based on a support vector machine of EUS images. Gastrointest Endosc. 2010;72:978-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Amadasun M, King R. Textural features corresponding to textural properties. IEEE Trans Syst Man Cybern. 1989;19:269-285. [DOI] [Cited in This Article: ] [Cited by in Crossref: 737] [Cited by in F6Publishing: 732] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 16. | Cai ZY, Yu JG, Zhang MM, Jin ZD. Texture Feature Extraction and Classification of Pancreatic Endoscopic Ultrasonography Images. Shengwu Yixue Gongchengxue Jinzhan. 2008;29:141-145. [DOI] [Cited in This Article: ] |
| 17. | Cai ZY, Yu JG, Li XP, Jin ZD. Feature Selection Algorithm Based on Kernel Distance Measure. Moshi Shibie Yu Rengong Zhineng. 2010;23:235-240. [DOI] [Cited in This Article: ] |
| 18. | Haralick RM, Shanmugam K, Dinstein I. Texture features for image classification. IEEE Trans Syst Man Cybern. 1973;3:610-621. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13956] [Cited by in F6Publishing: 13816] [Article Influence: 270.9] [Reference Citation Analysis (1)] |
| 19. | Xia Y, Feng D, Zhao R. Morphology-based multifractal estimation for texture segmentation. IEEE Trans Image Process. 2006;15:614-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Sarkar N, Chaudhuri BB. An efficient differential box-counting approach to compute fractal dimension of image. IEEE Trans Syst Man Cybern. 1994;24:115-120. [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 559] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [PubMed] [Cited in This Article: ] |
| 23. | Kolios MC, Czarnota GJ, Lee M, Hunt JW, Sherar MD. Ultrasonic spectral parameter characterization of apoptosis. Ultrasound Med Biol. 2002;28:589-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Van Holsbeke C, Van Calster B, Valentin L, Testa AC, Ferrazzi E, Dimou I, Lu C, Moerman P, Van Huffel S, Vergote I. External validation of mathematical models to distinguish between benign and malignant adnexal tumors: a multicenter study by the International Ovarian Tumor Analysis Group. Clin Cancer Res. 2007;13:4440-4447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Van Holsbeke C, Van Calster B, Testa AC, Domali E, Lu C, Van Huffel S, Valentin L, Timmerman D. Prospective internal validation of mathematical models to predict malignancy in adnexal masses: results from the international ovarian tumor analysis study. Clin Cancer Res. 2009;15:684-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Garra BS, Krasner BH, Horii SC, Ascher S, Mun SK, Zeman RK. Improving the distinction between benign and malignant breast lesions: the value of sonographic texture analysis. Ultrason Imaging. 1993;15:267-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 75] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Goldberg V, Manduca A, Ewert DL, Gisvold JJ, Greenleaf JF. Improvement in specificity of ultrasonography for diagnosis of breast tumors by means of artificial intelligence. Med Phys. 1992;19:1475-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Mavroforakis ME, Georgiou HV, Dimitropoulos N, Cavouras D, Theodoridis S. Mammographic masses characterization based on localized texture and dataset fractal analysis using linear, neural and support vector machine classifiers. Artif Intell Med. 2006;37:145-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Kumon RE, Olowe K, Faulx AL, Farooq FT, Chen VK, Zhou Y, Wong RC, Isenberg GA, Sivak MV, Chak A. EUS spectrum analysis for in vivo characterization of pancreatic and lymph node tissue: a pilot study. Gastrointest Endosc. 2007;66:1096-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Du YQ, Li ZS, Jin ZD. Endoscope-assisted brachytherapy for pancreatic cancer: From tumor killing to pain relief and drainage. J Interv Gastroenterol. 2011;1:23-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Du YQ, Jin ZD, Meng H, Zou DW, Chen J, Liu Y, Zhan XB, Wang D, LiaoZ , Li ZS. Long-term effect of gemcitabine-combined endoscopic ultrasonography- guided brachytherapy in pancreatic cancer. J Interv Gastroenterol. 2013;3:18-24. [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Nishida K, Kaneko T, Yoneda M, Nakagawa S, Ishikawa T, Yamane E, Nishioka B, Miyamoto Y, Takano H, Yoshikawa T. Doubling time of serum CA 19-9 in the clinical course of patients with pancreatic cancer and its significant association with prognosis. J Surg Oncol. 1999;71:140-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |