Published online Jul 28, 2012. doi: 10.3748/wjg.v18.i28.3745
Revised: September 26, 2011
Accepted: April 12, 2012
Published online: July 28, 2012
AIM: To assess the significance of phosphatidylinositol 3-kinase (PI3K) in colorectal cancer (CRC) and toxicity of LY294002 in CRC cells with different metastatic abilities.
METHODS: Sixty formalin-fixed and paraffin-embedded CRC tumor specimens were investigated. Adjacent normal colonic mucosa specimens from 10 of these cases were selected as controls. PI3K protein was detected by immunohistochemistry and PIK3CA mutations were investigated by gene sequencing analysis. A flow-cytometry-based apoptosis detection kit was used to determine PI3K inhibitor-induced apoptosis in CRC cell lines SW480 and SW620. Expression of phosphorylated protein kinase B in CRC cell lines was detected by Western blotting.
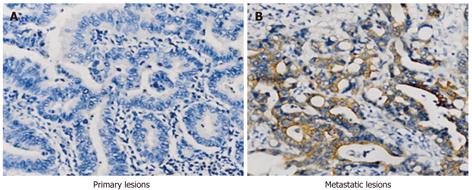
RESULTS: There was a significant difference in the proportion of primary lesions (30%, 18/60) vs metastatic lesions (46.7%, 28/60) that were positive for PI3K (P < 0.05). Mutations were detected in exon 9 (13.3%) and exon 20 (8.3%). Out of 60 cases, seven mutations were identified: two hotspot mutations, C.1633G>A resulting in E545A, and C.3140A>G resulting in H1047R; two novel missense mutations C.1624G>A and C.3079G>A; and three synonymous mutations (C.1641G>A, C.1581C>T and C.3027T>A). Exposure of SW480 cells to PI3K inhibitor for 48 h resulted in a significant increase of apoptotic cells in a dose-dependent manner [3.2% apoptotic cells in 0 μmol/L, 4.3% in 5 μmol/L, 6.3% in 10 μmol/L (P < 0.05), and 6.7% in 20 μmol/L (P < 0.05)]. Moreover, PI3K inhibitor induced a similar significant increase of apoptotic cells in the SW620 cell line for 48 h [3.3% apoptotic cells in 0 μmol/L, 13.3% in 5 μmol/L (P < 0.01), 19.2% in 10 μmol/L (P < 0.01), and 21.3% in 20 μmol/L (P < 0.01)].
CONCLUSION: High PI3K expression is associated with CRC metastasis. PI3K inhibitor induced apoptosis in CRC cells and displayed strong cytotoxicity for highly metastatic cells. PI3K inhibition may be an effective treatment for CRC.
- Citation: Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ, Xiao XY. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J Gastroenterol 2012; 18(28): 3745-3751
- URL: https://www.wjgnet.com/1007-9327/full/v18/i28/3745.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i28.3745
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States. The majority of these deaths are due to metastatic disease[1]. There is at least a 25-fold variation in occurrence of CRC worldwide, although rates are generally increasing. In high-risk areas, such as Europe, the rates are gradually increasing. The incidence tends to be low in Africa and Asia and intermediate in southern parts of South America[2]. However, in China, the incidence of CRC is increasing rapidly[3]. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway is activated in multiple cancers and it is an important regulator of cell growth, metabolism, proliferation, survival, motility, and invasion[4-7]. Highlighting the importance of the PI3K signaling pathway in human cancers, genetic alterations have been reported in various tumors including amplification of AKT1 and PIK3CA[8], somatic mutations of PIK3CA and AKT[9,10], and deletion of PTEN[11]. Somatic mutations in the catalytic subunit of PIK3CA have been found in a significant fraction of breast carcinomas, in which PIK3CA exon 20 mutation is associated with poor prognosis[12]. However, the different significance of PIK3CA mutations in primary CRC vs metastatic lesions has not been explored. Mutations in PIK3CA have been reported to occur in 13.6%-31.6% of CRC, making it one of the most frequently mutated genes[13,14]. A majority of mutations have been identified in the helical (exon 9) and kinase (exon 20) domains of PIK3CA. Expression of cancer-derived PIK3CA mutants in cultured human-derived CRC cells increases kinase activity, invasion, and resistance to apoptosis, suggesting that mutant PIK3CA plays a role in the initiating steps of CRC tumorigenesis[15,16]. In addition, the higher frequency of PIK3CA mutations in other advanced stage invasive tumors suggests a putative role in tumor progression[4,17].
Alongside genetic, molecular biological, and biochemical studies, chemical inhibitors have been valuable as tools in PI3K research[18-21]. They are helpful in understanding the role of PI3K enzymes in signal transduction and downstream physiological and pathological processes, as well as to assist validation of PI3Ks preclinically as therapeutic targets[18]. The earliest and still widely utilized inhibitors are wortmannin and LY294002. We utilized the PI3K inhibitor LY294002 in this study.
Based on PIK3CA data in other malignancies, we hypothesized that mutations resulting in the activation of the PIK3CA pathway may play a role in the progression of CRC. To test this hypothesis, we analyzed expression of PI3K protein in both primary and metastatic lesions; the mutational status of the two hotspot regions of PIK3CA (exons 9 and 20) in CRC; and the effect of PI3K inhibitor on cell apoptosis and AKT phosphorylation using two CRC cell lines.
Formalin-fixed and paraffin-embedded tumor specimens were obtained from surgical specimens from Shanghai Xuhui District Center Hospital. Specimens were from sporadic patients with colorectal adenocarcinoma who had not received preoperative radiotherapy, chemotherapy, or immunotherapy. All tumors were reviewed and confirmed by two senior pathologists using the World Health Organization classification of tumors of the digestive system[22]. Among the 60 patients with CRC, 33 were male and 27 were female. Their mean age was 52 years, ranging from 24 years to 78 years. There were 58 cases in stage III and two in stage IV according to the tumor, nodes, metastasis system. Lymph node metastasis was seen in 58 cases and liver metastasis in two. Twenty-six cases occurred in the colon and 34 in the rectum. Adjacent normal colonic mucosa specimens from 10 of these cases were selected as controls. Human colon cell lines SW480 and SW620 (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences); annexin V-flourescein iso-thiocyanate (FITC) Apoptosis Detection kit (BioVision, Palo Alto, CA, United States); and the detergent-compatible protein assay kit and ECL Plus Western blotting Detection System (Bio-Rad Laboratories, Hercules, CA, United States) were also used.
Paraffin-embedded blocks of CRC primary and metastatic tissue specimens were cut into 5 μm thick sections. One section from each block was stained with hematoxylin and eosin as a control. Known positive biopsies were used as positive controls, while specimens treated with phosphate buffered saline (PBS) instead of primary antibody were used as negative controls. All further steps were performed in strict accordance with the Envision two-step method instructions. PI3K (p110α) rabbit anti-human monoclonal antibody (Cell Signaling Technology, Beverly, MA, United States) was diluted 1:50. Envision Kit and DAB color kit (Zhongshan Biotechnology Company, Beijing, China) were used. PI3K presence was expressed in the cytoplasm as brown-yellow or brown granules. We observed the distribution of PI3K protein and scored the specimens[23]. The score was 0 if no positive tumor cells were found; 1 if positive tumor cells were < 10%; and 2 if positive tumor cells were > 10%. Tissues with scores of 0 or 1 were considered negative; those with scores of 2 were considered positive.
Genomic DNA was extracted from all samples using phenol-chloroform and ethanol precipitation. The DNA concentration of each sample was measured using NanoVue ultraviolet spectrophotometer. Exons 9 and 20 of PIK3CA were amplified using previously described polymerase chain reaction (PCR) primers[24]. The exon 9 forward and reverse primers were respectively 5’-GATTGGTTCTTTCCTGTCTCTG-3’ and 5’-CCACAAATATCAA TTTACAACCATTG-3’, and yielded a 487-bp expected product. The exon 20 forward and reverse primers were respectively 5’-TGGGGTAAAGGGAATCAAAAG-3’ and 5’-CCTATGCAATCGGTCTT TGC-3’, and yielded a 525-bp expected product. The PCR mixture contained 150 ng DNA, 5 pmol/L each primer, 2.5 nmol/L each dNTP, and 1.25 U Taq DNA polymerase in 20 μL buffer with 0.04 μmol/L Mg2+. After an initial denaturation step at 94 °C for 5 min, 35 PCR cycles were performed, which included 45 s for denaturation at 94 °C, 30 s for annealing at 60 °C, and 45 s for extension at 68 °C. PCR products were analyzed by 2% agarose gel electrophoresis. After observing clear and accurately sized bands, amplification products were then purified and directly sequenced on an ABI Prism 3730 sequence detection system (Applied Biosystems, Foster City, CA, United States).
SW480 and SW620 cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cell lines were incubated in 5% CO2 at 37 °C. PI3K-inhibitor-induced apoptosis in SW480 and SW620 cells was determined by flow cytometry using the annexin V-FITC Apoptosis Detection kit following the manufacturer’s instructions. Briefly, 3 × 105 cells were treated with PI3K inhibitor (0 μmol/L, 5 μmol/L, 10 μmol/L, and 20 μmol/L) for 24 h. The cells were then harvested, washed in PBS, and incubated with annexin V and propidium iodide for staining in binding buffer at room temperature for 10 min in the dark. The stained cells were analyzed using the fluorescence-activated cell sorting (FACS) Aria instrument (BD Biosciences, CA, United States).
Whole cell lysates were generated with cell lysis solution for Western blotting. After centrifugation, the supernatant fraction was collected for Western blotting. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. After blocking with 5% nonfat milk in blocking buffer [20 mmol/L tris-buffered saline (pH 7.5) containing 0.1% Tween 20], the membrane was incubated with the primary antibody at 4 °C overnight and then incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibody. The immunoreactive bands were visualized using the ECL Plus Western Blotting Detection System. The level of β-actin for each sample was used as a loading control. PI3K (p110α) rabbit anti-human monoclonal antibody was purchased from Cell Signaling Technology. β-actin (1-19) and the secondary antibodies (HRP-linked anti-mouse IgG) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States).
The statistical analysis software package SPSS 11.5 was used to conduct the Fisher’s exact test and χ2 test. P < 0.05 was considered to be statistically significant.
PI3K protein was localized in the cytoplasm of CRC tumor cells; brown granules were detected within the cytoplasm. The expression of PI3K in primary and metastatic lesions in the same patient is shown in Figure 1A and B. The positive PI3K rates were significantly lower in the primary tumors than in the associated metastases: 30% (18/60) vs 46.7% (28/60) (P < 0.05). Moreover, the positive rate of 10% (1/10) in normal mucosa was significantly lower than in cancer tissue (P < 0.05).
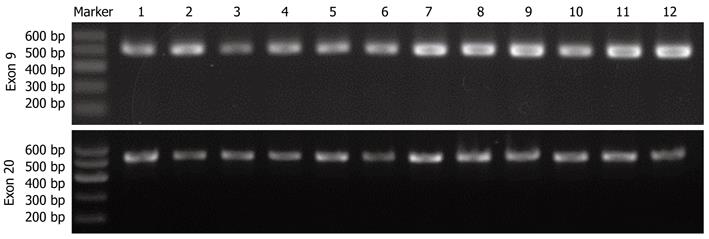
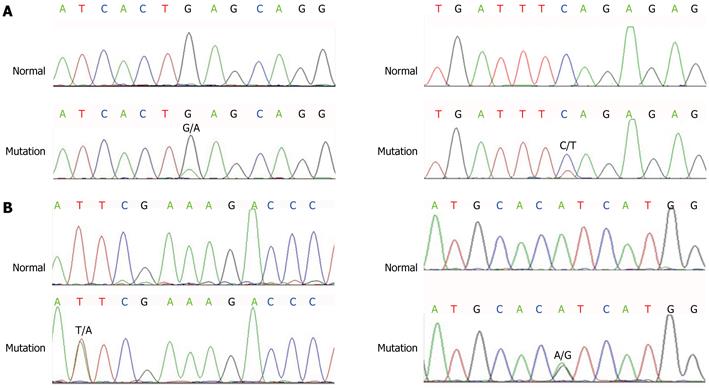
PCR products of PIK3CA exon 9 and exon 20 were analyzed by 2% agarose gel electrophoresis (Figure 2). After observing clear and accurately sized bands, amplification products were then purified and directly sequenced on an ABI Prism 3730 sequence detection system. Mutational analysis of PIK3CA was done in 60 primary CRC and 10 normal tissues (Figure 3). Mutation rates of PIK3CA exon 9 and exon 20 were 13.33% (8/60) and 8.33% (5/60) in CRC, respectively. A total of eight mutations were identified (Table 1). All the mutations were not detected in corresponding normal tissues, therefore, these mutations were confirmed as somatic mutations. Of these 13 mutations, eight were clustered in exon 9 and the remaining five in exon 20.
| Exon | Nucleotide | Codon | Domain | Cases |
| 9 | c.1624G>A | p.E541K | Helical | 3 |
| 9 | c.1633G>A | p.E545K | Helical | 3 |
| 9 | c.1581C>T | p.D527D | Helical | 1 |
| 9 | c.1641G>A | p.E547E | Helical | 1 |
| 20 | c.3027T>A | p.G1009G | Kinase | 3 |
| 20 | c.3079G>A | p.A1027H | Kinase | 1 |
| 20 | c.3140A>G | p.H1047R | Kinase | 1 |
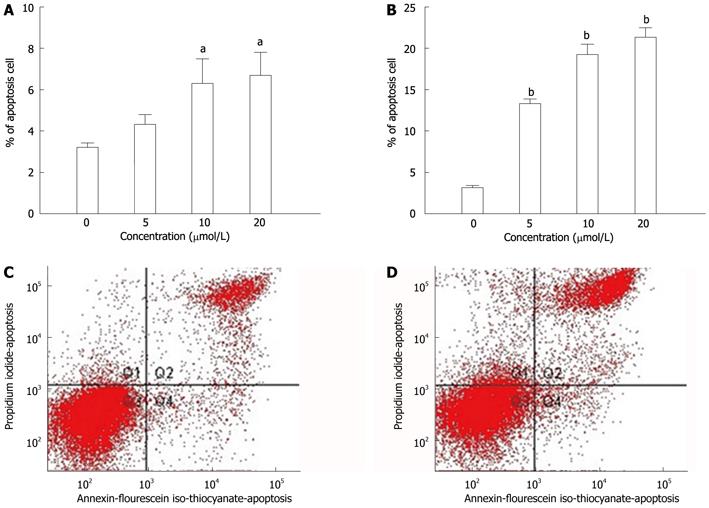
To determine whether the PI3K inhibitor (LY294002)-induced growth inhibition in human CRC cells was associated with metastasis of CRC, SW480 and SW620 cells were treated with PI3K inhibitor. The numbers of apoptotic cells were assessed using the annexin V-FITC Apoptosis Detection kit by the FACS Aria instrument. Exposure of SW480 cells to PI3K inhibitor for 48 h resulted in a significant increase of apoptotic cells in a dose-dependent manner [3.2% apoptotic cells in 0 μmol/L, 4.3% in 5 μmol/L, 6.3% in 10 μmol/L (P < 0.05), and 6.7% in 20 μmol/L (P < 0.05) ]. Inhibition was also obtained when the SW620 cell lines were exposed to the same PI3K inhibitor for 48 h [3.3% apoptotic cells in 0 μmol/L, 13.3% in 5 μmol/L (P < 0.01), 19.2% in 10 μmol/L (P < 0.01), and 21.3% in 20 μmol/L (P < 0.01)] (Figure 4). PI3K inhibitor, at a concentration of 20 μmol/L, induced apoptosis to a greater extent in SW620 cells, which have higher metastatic potential than SW480 cells.
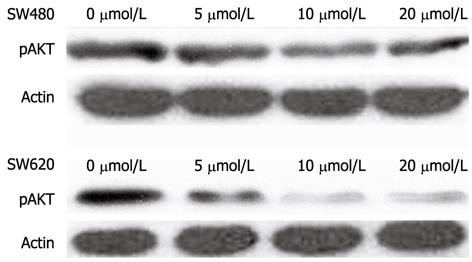
To determine the potential effect of PI3K inhibition on AKT phosphorylation, phosphorylated AKT (pAKT) was detected using different concentrations of PI3K inhibitors by Western blotting in two colon cancer cell lines with different metastatic activities. As shown in Figure 5, pAKT was detected in cells with 0 μmol/L inhibitor concentration, indicating the constitutive activation of AKT in two colon cancer cell lines. In SW620 and SW480 cells, the level of pAKT gradually decreased as inhibitor concentration gradually increased from 5 μmol/L to 10 μmol/L and 20 μmol/L. In SW620 and SW480 cells, pAKT at high inhibitory concentration was lower than that at low inhibitory concentration. In addition, pAKT was marginally lower in SW620 than SW480 cells at the same inhibitory concentration. These findings confirmed that this PI3K inhibitor was strongly cytotoxic for highly metastatic CRC cells.
It is well known that tumors are often genetically influenced and somatic mutations of oncogenes and tumor suppressor genes are the initiators of the carcinogenic process. The PI3K/AKT signaling pathway has previously been implicated in tumorigenesis; evidence over the past several years suggests a vital role for the PI3K catalytic subunit PIK3CA in human cancers[25,26]. In this study, we found that the expression of PI3K protein differed between primary and metastatic lesions in CRC. The positive rate of PI3K was higher in metastatic than in primary tumors, indicating that PI3K might be involved in CRC development, progression and metastases. The exact molecular mechanism deserves further study.
To probe the mechanism of PI3K high expression, we investigated the frequency of PIK3CA mutations in CRC and normal tissue adjacent to cancer. Due to the limited number of metastatic samples, PIK3CA mutations in metastases could not be evaluated. Instead, two previously characterized mutational hotspots, exon 9 and exon 20, were analyzed. PIK3CA mutations were identified in 21.67% of the tumor tissues. No mutations were detected in corresponding normal tissues, therefore, these mutations were confirmed as somatic mutations. We also found that the PI3K mutation rate was less than the expression rate of PI3K protein, indicating that PI3K mutations were not the sole cause of the high expression of PI3K protein in CRC samples.
Of these 13 mutations, eight were clustered in exon 9 and five in exon 20. In the two previously reported hotspots, we identified the point mutations c.1633G>A and c.3140A>G, which resulted in the amino acid substitutions E545A and H1047R, respectively[27,28]. Previous studies have also shown that mutations are more common in exon 9 in CRC[14]. We also explored associations between any PIK3CA mutation and other variables, as well as interactions between histological groups, and found no significant correlation with mutation frequency. This does not agree with previous studies[29], which might be related to the small number of cases in the present study. However, some similar reports have been found in breast cancer research. Dupont Jensen et al[30] have reported that PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer, and PIK3CA mutation was detected in 45% of the primary tumors; and there was a net gain in mutation in metastatic disease, to 53%. Nonetheless, there were instances where metastases were wild type in patients with PIK3CA mutant primary tumors. Thus, we believe that PIK3CA is altered between primary and metastatic disease, and it is necessary to assess the PIK3CA status in the metastatic lesions to select patients that would benefit from PIK3CA inhibitor treatment.
To remedy the problem that PIK3CA mutations in metastases were not evaluated due to the limited number of metastatic samples, we further investigated the effect of PI3K inhibitor on human CRC cells with different metastatic activities. We found that PI3K inhibitor (LY294002) induced growth inhibition in human CRC cells, which was associated with the induction of apoptosis. The number of apoptotic cells in SW620 cell line was obviously increased compared with that in in SW480 after LY294002 (20 μmol/L) treatment. Other research has confirmed that LY294002 has a strong sensitizing effect similar to to boswellic-acid-induced apoptosis in colon cancer cells, and inhibits bone-morphogenetic-protein-2-induced epithelial-mesenchymal transition and invasion[31,32]. Our results indicate that PI3K inhibitor induced different levels of apoptosis in cell lines with various metastatic capabilities, which suggests that PI3K is involved in the process of CRC metastasis. PIK3CA mutation and subsequent activation of the AKT pathway play an important role in colorectal carcinogenesis[29]. Therefore, we also investigated the influence of LY294002 on AKT activation in our study. We found that LY294002 (5 μmol/L, 10 μmol/L or 20 μmol/L) inhibited the phosphorylation of AKT significantly in two colon cancer cell lines, SW620 and SW480. PI3K inhibitor may have potential for the treatment of metastatic CRC. It is worth investigating further the relationship among the death receptors Fas, tumor necrosis factor-related apoptosis-inducing ligand receptor (TRAILR)1, TRAILR2 and PI3K. These experiments are planned in our future work.
In summary, our results suggest that activation of the PI3K pathway plays an important role in colorectal tumor metastasis, and PI3K mutation is likely to be involved in promoting tumor invasion. However, PI3K mutation rate was not in accordance with the expression rate of PI3K protein, indicating that PI3K mutations were not the unique cause leading to the high expression of PI3K protein in CRC samples. PI3K inhibitors might become a new therapeutic method for CRC metastasis. However, the association between exon 9 and exon 20 mutations and the risk of CRC progression needs further investigation of a larger number of samples. In addition, the upstream and downstream genes of the PI3K/AKT pathway including insulin-like growth factor 1 receptor and ligand, phosphatidyl inositol-3,4,5-triphosphate (PIP3), phosphatase and tensin homolog deleted on chromosome 10 (PTEN), mammalian target of rapamycin complex 2 and pyruvate dehydrogenase kinase warrant further study.
We gratefully thank American Journal Experts for their kind English editing.
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States. The majority of these deaths are due to metastatic disease. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B signaling pathway is an important regulator of cell growth, metabolism, proliferation, survival, motility, and invasion. This pathway is activated in multiple cancers. It is of important to identify expression of PI3K protein in both primary and metastatic lesions to understand better the molecular mechanism of CRC, and evaluate PI3K inhibitors as a new treatment method for metastatic CRC.
The expression of PI3K in primary CRC and metastatic tissues remains unclear. The differential effects of PI3K inhibition in CRC cell lines with various metastatic capabilities have been less well reported.
This is an important study that validates the hypothesis that activation of the PI3K pathway might play a role in CRC metastasis, and PI3K inhibitors should be evaluated as new candidates for the treatment of metastatic CRC.
These results suggest that the PI3K/AKT signaling pathway plays an important role in colorectal tumor metastasis. However, the PI3K mutation rate was not in accordance with the expression rate of PI3K protein, indicating that PI3K mutations were not the unique cause of the high expression of PI3K protein in CRC. PI3K inhibitor was strongly cytotoxic for cells with high metastatic ability, making PI3K targeting a new therapeutic candidate for the treatment of CRC metastasis. Further bioinformatics and functional studies using a large number of patient specimens will further determine the significance of these genes in CRC pathogenesis.
Flow cytometry (FCM) is a technique used to analyze cells rapidly and individually, and it allows for the quantitative analysis of distributions of a property or properties in a population. FCM has been a fundamental tool for biological discovery in recent years. It offers many advantages over conventional measurements for the analysis of biological cells, and it is a generally accepted tool to analyze apoptotic cells.
The intention of the authors is to focus on the PI3K/AKT pathway. This pathway is indeed very interesting concerning oncogenesis.
Peer reviewer: Alfred A Königsrainer, MD, Professor, Department General Surgery and Transplantation, University Hospital, Hoppe-Seyler-Str. 3, 72076 Tübingen, Germany
S- Editor Cheng JX L- Editor Kerr C E- Editor Zhang DN
| 1. | LeGolvan MP, Resnick M. Pathobiology of colorectal cancer hepatic metastases with an emphasis on prognostic factors. J Surg Oncol. 2010;102:898-908. [PubMed] [Cited in This Article: ] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] [Cited in This Article: ] |
| 3. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 4. | Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77-82. [PubMed] [Cited in This Article: ] |
| 5. | Richardson CJ, Schalm SS, Blenis J. PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol. 2004;15:147-159. [PubMed] [Cited in This Article: ] |
| 6. | Dunlap J, Le C, Shukla A, Patterson J, Presnell A, Heinrich MC, Corless CL, Troxell ML. Phosphatidylinositol-3-kinase and AKT1 mutations occur early in breast carcinoma. Breast Cancer Res Treat. 2010;120:409-418. [PubMed] [Cited in This Article: ] |
| 7. | Paradiso A, Mangia A, Azzariti A, Tommasi S. Phosphatidylinositol 3-kinase in breast cancer: where from here? Clin Cancer Res. 2007;13:5988-5990. [PubMed] [Cited in This Article: ] |
| 8. | de Araújo WM, Vidal FC, de Souza WF, de Freitas JC, de Souza W, Morgado-Diaz JA. PI3K/Akt and GSK-3β prevents in a differential fashion the malignant phenotype of colorectal cancer cells. J Cancer Res Clin Oncol. 2010;136:1773-1782. [PubMed] [Cited in This Article: ] |
| 9. | Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. [PubMed] [Cited in This Article: ] |
| 10. | Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439-444. [PubMed] [Cited in This Article: ] |
| 11. | Saal LH, Gruvberger-Saal SK, Persson C, Lövgren K, Jumppanen M, Staaf J, Jönsson G, Pires MM, Maurer M, Holm K. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102-107. [PubMed] [Cited in This Article: ] |
| 12. | Lai YL, Mau BL, Cheng WH, Chen HM, Chiu HH, Tzen CY. PIK3CA exon 20 mutation is independently associated with a poor prognosis in breast cancer patients. Ann Surg Oncol. 2008;15:1064-1069. [PubMed] [Cited in This Article: ] |
| 13. | Herreros-Villanueva M, Gomez-Manero N, Muñiz P, García-Girón C, Coma del Corral MJ. PIK3CA mutations in KRAS and BRAF wild type colorectal cancer patients. A study of Spanish population. Mol Biol Rep. 2011;38:1347-1351. [PubMed] [Cited in This Article: ] |
| 14. | Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455-459. [PubMed] [Cited in This Article: ] |
| 15. | Fumagalli D, Gavin PG, Taniyama Y, Kim SI, Choi HJ, Paik S, Pogue-Geile KL. A rapid, sensitive, reproducible and cost-effective method for mutation profiling of colon cancer and metastatic lymph nodes. BMC Cancer. 2010;10:101. [PubMed] [Cited in This Article: ] |
| 16. | Ollikainen M, Gylling A, Puputti M, Nupponen NN, Abdel-Rahman WM, Butzow R, Peltomäki P. Patterns of PIK3CA alterations in familial colorectal and endometrial carcinoma. Int J Cancer. 2007;121:915-920. [PubMed] [Cited in This Article: ] |
| 17. | Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184-192. [PubMed] [Cited in This Article: ] |
| 18. | Knight ZA, Shokat KM. Chemically targeting the PI3K family. Biochem Soc Trans. 2007;35:245-249. [PubMed] [Cited in This Article: ] |
| 19. | Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95-105. [PubMed] [Cited in This Article: ] |
| 20. | Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199-204. [PubMed] [Cited in This Article: ] |
| 21. | Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297-315. [PubMed] [Cited in This Article: ] |
| 22. | Hamilton SR, Aaltonen LA. Tumors of colon and rectum. World Health Organization classification of tumors: Pathology and genetics of tumors of digestive system. Lyon: IARC Press 2000; 103-105. [Cited in This Article: ] |
| 23. | Wang Y, Kristensen GB, Helland A, Nesland JM, Børresen-Dale AL, Holm R. Protein expression and prognostic value of genes in the erb-b signaling pathway in advanced ovarian carcinomas. Am J Clin Pathol. 2005;124:392-401. [PubMed] [Cited in This Article: ] |
| 24. | Baohua Y, Xiaoyan Z, Tiecheng Z, Tao Q, Daren S. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol. 2008;17:159-165. [PubMed] [Cited in This Article: ] |
| 25. | Phillips WA, Russell SE, Ciavarella ML, Choong DY, Montgomery KG, Smith K, Pearson RB, Thomas RJ, Campbell IG. Mutation analysis of PIK3CA and PIK3CB in esophageal cancer and Barrett's esophagus. Int J Cancer. 2006;118:2644-2646. [PubMed] [Cited in This Article: ] |
| 26. | Barbareschi M, Buttitta F, Felicioni L, Cotrupi S, Barassi F, Del Grammastro M, Ferro A, Dalla Palma P, Galligioni E, Marchetti A. Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res. 2007;13:6064-6069. [PubMed] [Cited in This Article: ] |
| 27. | Abubaker J, Bavi P, Al-Harbi S, Ibrahim M, Siraj AK, Al-Sanea N, Abduljabbar A, Ashari LH, Alhomoud S, Al-Dayel F. Clinicopathological analysis of colorectal cancers with PIK3CA mutations in Middle Eastern population. Oncogene. 2008;27:3539-3545. [PubMed] [Cited in This Article: ] |
| 28. | Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, Zepf D, Yan L, Longtine JA, Fuchs CS, Ogino S. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534-541. [PubMed] [Cited in This Article: ] |
| 29. | Ogino S, Nosho K, Kirkner GJ, Shima K, Irahara N, Kure S, Chan AT, Engelman JA, Kraft P, Cantley LC. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27:1477-1484. [PubMed] [Cited in This Article: ] |
| 30. | Dupont Jensen J, Laenkholm AV, Knoop A, Ewertz M, Bandaru R, Liu W, Hackl W, Barrett JC, Gardner H. PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer. Clin Cancer Res. 2011;17:667-677. [PubMed] [Cited in This Article: ] |
| 31. | Liu JJ, Duan RD. LY294002 enhances boswellic acid-induced apoptosis in colon cancer cells. Anticancer Res. 2009;29:2987-2991. [PubMed] [Cited in This Article: ] |