Published online Aug 7, 2010. doi: 10.3748/wjg.v16.i29.3716
Revised: April 16, 2010
Accepted: April 23, 2010
Published online: August 7, 2010
AIM: To present a series of cases with life-threatening hemorrhage from ruptured hepatic artery pseudoaneurysm after pancreaticoduodenectomy (PD) treated with placement of stent-grafts.
METHODS: Massive hemorrhage from ruptured hepatic artery pseudoaneurysm after PD in 9 patients (6 men, 3 women) at the age of 23-75 years (mean 48 years), were treated with placement of percutaneous endovascular balloon-expandable coronary stent-grafts. All patients were not suitable for embolization because of a non-patent portal vein. One or more stent-grafts, ranging 3-6 mm in diameter and 16-55 mm in length, were placed to exclude ruptured pseudoaneurysm. Follow-up data, including clinical condition, liver function tests, and Doppler ultrasound examination, were recorded at the outpatient clinic.
RESULTS: Immediate technical success was achieved in all the 9 patients. All stent-grafts were deployed in the intended position for immediate cessation of bleeding and preservation of satisfactory hepatic arterial blood flow. No significant procedure-related complications occurred. Recurrent bleeding occurred in 2 patients at 16 and 24 h, respectively, after placement of stent-grafts and treated with surgical revision. One patient died of sepsis 12 d after the interventional procedure. The remaining 6 patients were survived when they were discharged. The mean follow-up time was 10.5 mo (range 4-16 mo). No patient had recurrent bleeding after discharge. Doppler ultrasound examination verified the patency of hepatic artery and stent-grafts during the follow-up.
CONCLUSION: Placement of stent-grafts is an effective and safe procedure for acute life-threatening hemorrhage from ruptured hepatic artery pseudoaneurysm.
- Citation: Wang MQ, Liu FY, Duan F, Wang ZJ, Song P, Fan QS. Stent-grafts placement for treatment of massive hemorrhage from ruptured hepatic artery after pancreaticoduodenectomy. World J Gastroenterol 2010; 16(29): 3716-3722
- URL: https://www.wjgnet.com/1007-9327/full/v16/i29/3716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i29.3716
Delayed hepatic arterial hemorrhage after pancreaticoduodenectomy (PD) is not a common, but a potentially fatal complication[1-4]. Massive hepatic arterial bleeding occurs as a result of inflammatory vascular erosion related to pancreatic juice or bile leaking from an insufficient anastomosis and/or due to local infection. Treatment options include re-operation or endovascular catheter techniques such as coil embolization of the bleeding vessels. Surgical exploration and identification of the bleeding vessel may be difficult in acute situations and hazardous because of adhesions and surrounding postsurgical tissue friability[5,6]. Embolization of the bleeding artery has an immediate impact on patient survival but often excludes the distal circulation, which may be a risk factor for hepatic ischemia and even fatal hepatic necrosis, particularly in patients with portal vein stenosis or thrombosis[3,7-9].
Recently the use of stent-grafts to exclude aneurysms in splenic and other visceral arteries has been presented in single case reports[10-15]. We report our experience in 9 patients with delayed massive hemorrhage from ruptured hepatic artery pseudoaneurysm after PD, demonstrating the potential of interventional radiology in initial treatment of hemorrhage, instead of re-operation and coil embolization, using transluminal stent graft placement for emergency vessel repair retaining patency of hepatic artery.
From March 2003 to December 2009, 9 patients (6 men, 3 women) at the age of 23-75 years (mean 48 years), referred to our department for delayed massive hemorrhage from ruptured hepatic artery pseudoaneurysm occurring 6 or more days after PD, were treated with percutaneous endovascular placement of balloon-expandable stent-grafts. All medical records, radiological reports, and images of the patients were retrospectively reviewed. Delayed massive hemorrhage occurring 5 or more days after PD in patients with stable hemodynamics was defined as a potentially life-threatening bleeding leading to hemorrhagic shock needing blood transfusions as previously described[2,3].
The indications for PD are listed in Table 1. Classic resection procedures were performed in all the 9 patients, including Whipple procedure (n = 6) and pylorus preserving pancreatoduodenectomy (n = 3). Pancreatic fistula (PF) after PD, diagnosed by routine assay of drainage fluid amylase levels, was present in 7 patients with local infection. PF was defined as drain output of any measurable volume of fluid on or after postoperative day 3 with amylase content 3 times greater than that of serum amylase activity[4]. Embolization of splenic artery was performed in 2 patients (Patients No. 1 and No. 2) due to bleeding from the splenic artery.
| Patient No. | Age (yr)/sex | Indication for surgery | PF | Bleeding at POD | Initial presentation of bleeding | Transfusion of units of blood | Stent-graft |
| 1 | 75/F | Carcinoma of pancreatic head | Yes | 14 | Abdominal drain | 9, 9 FFP | Jostent |
| 4 mm × 19 mm × 2 pieces | |||||||
| 2 | 23/F | Pancreatic trauma | Yes | 9 | Abdominal drain | 12 | Jostent |
| 4 mm × 19 mm × 2 pieces | |||||||
| 3 | 42/M | Distal common bile cholangiocarcinoma | Yes | 15 | Abdominal drain | 11, 6 FFP | Jostent |
| 4 mm × 19 mm × 3 pieces | |||||||
| 4 | 56/M | Carcinoma of pancreatic head | Yes | 7 | Abdominal drain and hematemesis | 11, 7 FFP | Jostent |
| 3.5 mm × 19 mm × 1 piece | |||||||
| 4 mm × 19 mm × 1 piece | |||||||
| 5 | 62/M | Carcinoma of pancreatic head | No | 35 | Hematemesis and melena | 9 | Jostent |
| 4 mm × 19 mm × 1 piece | |||||||
| 4 mm × 16 mm × 1 piece | |||||||
| 6 | 67/M | Pancreatic carcinoma | Yes | 6 | Surgical drainage, nasogastric tube | 11 | Jostent |
| 6 mm × 55 mm × 1 piece | |||||||
| 7 | 53/M | Periampullary cancer | No | 38 | Hematemesis and melena | 9 | Jostent |
| 4 mm × 19 mm × 3 pieces | |||||||
| 8 | 68/M | Pancreatic carcinoma | Yes | 8 | Abdominal drain | 11, 9 FFP | Jostent |
| 3.5 mm × 19 mm × 1 piece | |||||||
| 4 mm × 16 mm × 1 piece | |||||||
| 9 | 50/F | Carcinoma of pancreatic head | Yes | 6 | Surgical drainage, nasogastric tube | 12, 7 FFP | Jostent |
| 4 mm × 19 mm × 2 pieces |
All the 9 patients presented with an unstable clinical condition with their heart rate higher than 90 beats per min, blood pressure lower than 80/40 mmHg, hemoglobin lower than 6.5 g/dL (reference range 13-18 g/dL), hematocrit lower than 40% (reference range 40%-54%), and blood transfusion greater than 5 U. Angiography revealed active hemorrhage from the hepatic artery pseudoaneurysm, including bleeding from the abdominal drain (n = 4), gastrointestinal tract (n = 2), or both (n = 3). Bleeding occurred in 7 patients (78%) before they were discharged and in 2 patients (Patients No. 5 and No. 7) after they were discharged following an uneventful postoperative course. The mean time between PD and onset of massive bleeding was 15.3 d (range 6-38 d).
All patients received an average blood transfusion of 10.6 U (9-12 U) before the interventional procedure. Of the 9 patients, 5 needed an average of 1520 mL (1200-1800 mL) fresh frozen plasma and 4 required intubation.
Emergency endoscopy performed in 3 patients showed mixed old and fresh blood in gastric lumen but no bleeding site. Computed tomography (CT) and ultrasonography were not performed because of the emergency situation.
Informed consent was obtained from the patients or their guardians before the interventional procedure.
Instead of coil embolization, stent-graft was placed to exclude hepatic pseudoaneurysm because the patients had an obstructed portal vein prior to massive pseudoaneurysm bleeding, including thrombosis of the portal vein in 5 patients, tumor infiltration of the portal vein in 3 patients, and ligation of the portal vein during the surgical procedure in 1 patient. In patients with a non-patent portal vein, embolization of the common or proper hepatic artery was a high risk factor for hepatic ischemia and even fatal hepatic necrosis as previously described[9,16].
After restoration of hemodynamic stability by aggressive resuscitation with intravenous fluids and administration of blood products, the patients underwent emergency abdominal angiography with standard Seldinger technique. All procedures were performed under local anesthesia (2% lidocaine). The patients were monitored by electrocardiogram and blood pressure measurements.
A pigtail catheter (4 Fr, Cordis, the Netherlands) was inserted into the abdominal aorta at level of the 12th thoracic vertebra, and an abdominal aortography was performed with digital subtraction angiography technique. The celiac trunk, hepatic artery, splenic artery, and superior mesenteric artery were selectively catheterized using a 4 Fr Cobra catheter (Cordis). Both arterial and portal venous phases were assessed, in order to detect the bleeding site and exclude the portal vein thrombosis.
Diameter of the affected artery at the located bleeding site was measured. A 0.35-inch guide-wire (Terumo Co., Tokyo, Japan) was passed into a distal branch of the right or left hepatic artery through the 4 Fr Cobra catheter under fluoroscopic guidance. The femoral 4 Fr introducer was replaced with an 8 Fr introducer with a 60 cm long vascular sheath (Arrow, Arrow International, USA).
A stent-graft (Jostent, Graftmaster, Coronary stent graft, Germany) was advanced into the distal extravasation site through the 8 Fr Arrow sheath over a 0.014 inch guide-wire (Table 2). The sheath was then pulled back to the proximal extravasation site to allow initial exposure of the stent-graft. The stent-graft was then deployed by inflating the balloon to a pressure of 8 atmospheres. A 6/20-mm balloon catheter (Abbott Lab, IL) was then used to post-dilate the stent-graft. If placement of one stent-graft failed to completely exclude the pseudoaneurysm, a second or even a third stent-graft was placed in a coaxial overlapping manner. Control angiography was repeated to confirm the exclusion of pseudoaneurysm, no sign of contrast medium extravasation, and patency of the hepatic artery.
| Patient No. | Immediate technical success | Intensive care unit/length of stay | Clinical outcome | Length of hospital stay (d) | Follow-up |
| 1 | Yes | Yes/19 d | Bleeding stopped, no further hemorrhage | 48 | 4 mo exitus, AMI |
| 2 | Yes | Yes/7 d | Bleeding stopped, no further bleeding | 28 | 16 mo, clinical and laboratory findings normal |
| 3 | Yes | Yes/10 d | Bleeding stopped, no further bleeding | 38 | 10 mo exitus, underlying malignancy |
| 4 | Yes | Yes/15 d | Recurrent bleeding 16 h later, underwent surgical revision | 32 | 2 d exitus, uncontrolled bleeding |
| 5 | Yes | No | Cessation of bleeding, no further hemorrhage | 11 | 14 mo exitus, underlying malignancy |
| 6 | Yes | Yes/8 d | Bleeding stopped, no further hemorrhage | 36 | 8 mo exitus, underlying malignancy |
| 7 | Yes | No | Bleeding stopped, no further hemorrhage | 12 | 11 mo, clinical and laboratory findings normal |
| 8 | Yes | Yes/21 d | Recurrent bleeding 24 h later, underwent surgical revision | 40 | 3 d exitus, multiorgan failure |
| 9 | Yes | Yes/24 d | Bleeding stopped, no further hemorrhage | 43 | 12 d exitus, abdominal sepsis |
All individuals were given antibiotics to prevent infection with aerobic and non-aerobic bacteria but no anticoagulation or anti-platelet drugs immediately after the procedure due to the emergent hemorrhagic conditions.
Six patients were survived when they were discharged and aspirin (100 mg/d) was given lifelong after discharge. Follow-up data, including clinical condition and laboratory (liver function test) findings, were recorded at the outpatient clinic. Doppler ultrasound studies were performed every day for 1 wk after placement of stent-grafts, then every 2-3 d, followed by every month on an outpatient basis.
Technical success was defined as the successful deployment of stent-graft within the intended artery, exclusion of pseudoaneurysm without evidence for contrast extravasation, cessation of immediate hemorrhage, and preservation of hepatic arterial flow. Clinical success was defined as the disappearance of signs or symptoms and improvement in laboratory findings. Re-bleeding from the same arterial focus after treatment was defined as clinical failure.
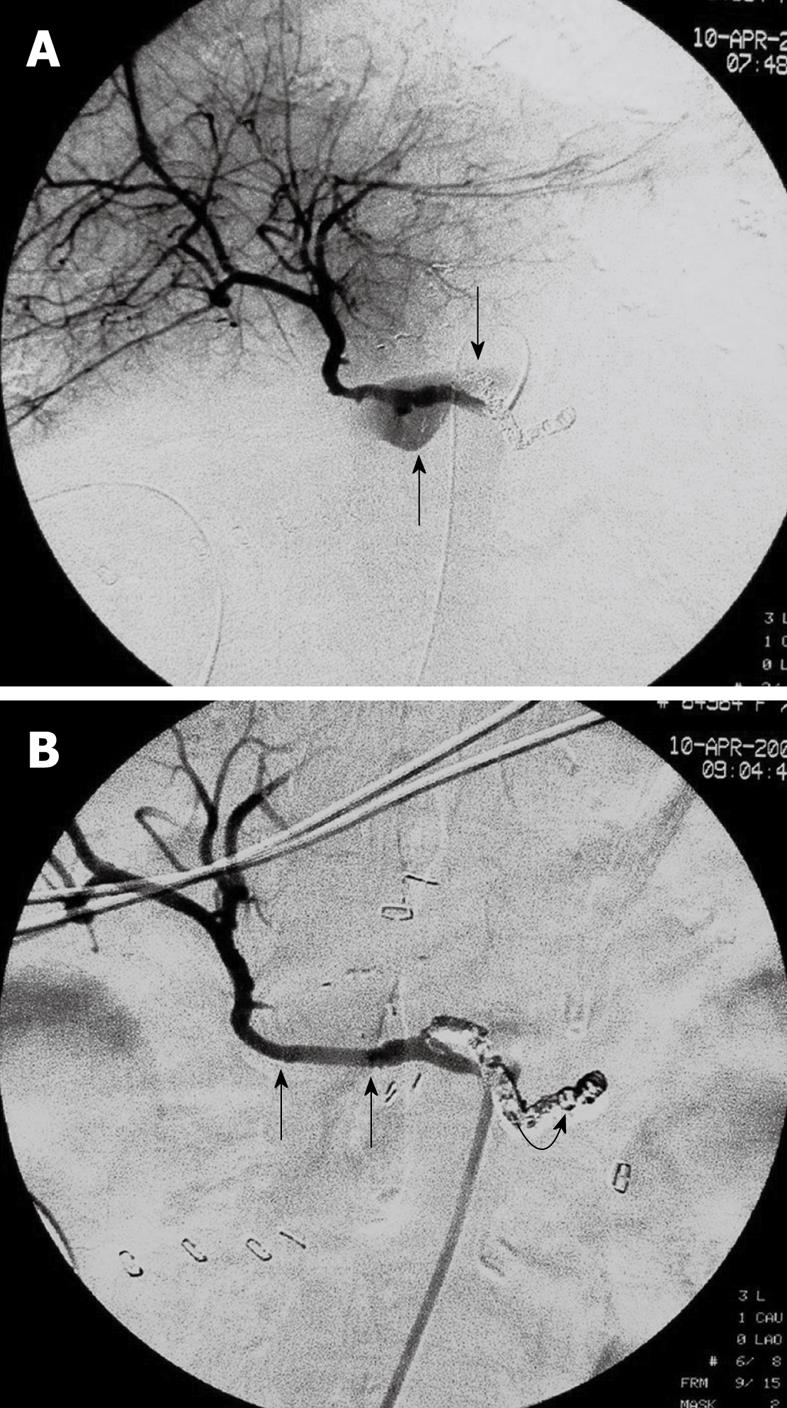
The selective angiography of celiac axis demonstrated active bleeding (extravasation of contrast agent) from the ruptured hepatic artery pseudoaneurysm in all patients (Figure 1A). A non-patent main portal vein was observed at the delayed phase in all patients.
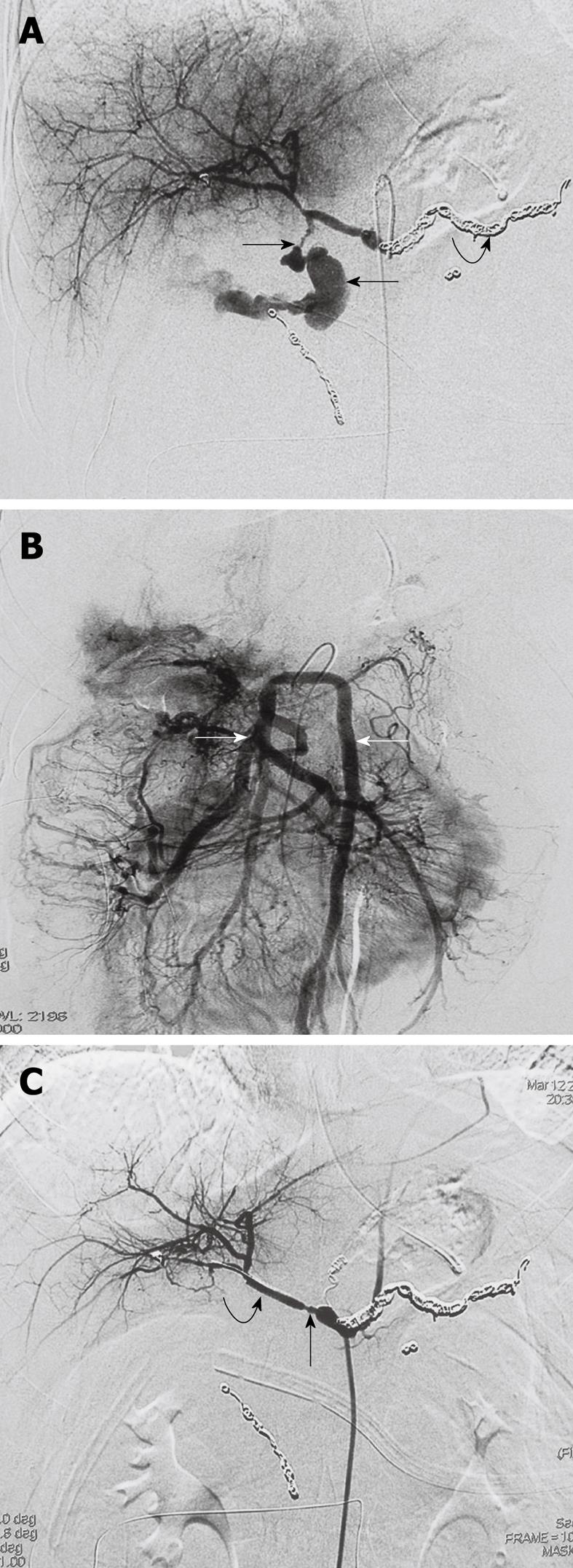
Stent-graft placement was technically successful in all patients with the bleeding pseudoaneurysm completely excluded and stable hemodynamically achieved immediately (Figure 1B). The control angiography demonstrated patency of the hepatic artery in all individuals (Figure 2A and B). Each patient needed one or more stent-grafts to exclude his or her ruptured hepatic pseudoaneurysm. A total of 19 stent-grafts were implanted in 9 patients (Table 1).
The median time of interventional procedure, including diagnostic angiography, was 65 min (range 35-100 min). No vascular adverse event occurred during the procedure. Control angiography showed vasospasm of the proximal and distal hepatic artery during and immediately after the procedure as expected because of the intra-arterial guide wire manipulation (Figure 2C), which did not affect the outcome of intervention. No major complication of the procedure was observed.
Seven patients were transferred to intensive care unit after placement of stent-grafts with a mean stay time of 13.7 d (7-24 d). Four patients required intubation.
Hemodynamically stabilization was achieved in 7 of the 9 patients after the procedure without evidence of further bleeding (Table 2). Laboratory findings (liver function tests) were within the normal range 1, 3 and 7 d after the procedures and at the time when the patients were discharged.
Two patients (Patients No. 4 and No. 8) with clinical failure underwent surgical revision. In patient No. 4, the stent-graft was successfully placed, but his hemoglobin level dropped again 16 h after the procedure due to bleeding from the same artery probably due to the dislodgment of stent-grafts. This patient underwent surgical revision and died of uncontrolled bleeding 2 d after the procedure. In patient No. 8, recurrent bleeding occurred 24 h after the placement of stent-grafts due to bleeding from the same artery. He underwent surgical revision with haemostasis achieved. The patient died of multiorgan failure 3 d after the procedure.
Of the 7 patients with their bleeding completely controlled, one (Patient No. 9) died of intra-abdominal sepsis 12 d after the interventional procedure with no active bleeding until her death. Four patients were subjected to CT-guided interventional placement of additional drains close to the pancreaticojejunostomy due to considerable fluid collections. Two patients underwent re-operation for persistent abscess and PF.
Six patients were survived when they were discharged (Table 2). Their mean hospital stay time was 24.5 d (range 11-48 d). The mean follow-up time was 10.5 mo (range 4-16 mo). No further bleeding was seen during the follow-up. Clinical and laboratory follow-up findings were unremarkable. Doppler ultrasound examination verified the patency of hepatic artery and stent-grafts during the follow-up.
It was reported that approximately two thirds of delayed arterial hemorrhage cases after PD have an underlying collection or anastomotic leak, with pseudoaneurysm formation[1,2,4,5,9]. In our study, delayed hepatic arterial hemorrhage occurred in 7 of the 9 patients due to the local complication (PF).
Transcatheter arterial embolization (TAE) has been advocated as the first-line treatment modality for late-onset bleeding after PD[17-20], with a success rate of 83%-100% and a mortality of 0%-20%. In fact, the liver can tolerate embolization of the main hepatic artery without major consequences, since it has a dual blood supply from the portal and arterial circulations. Collateral arterial blood flow to the liver can also be expected. However, the number of collaterals is less than that of normal blood vessels after PD because of lymphadenectomy and skeletonization of the vasculature[3]. Embolization of the hepatic artery may result in liver failure and necrosis as well as intrahepatic abscesses[5,21,22].
In this study, all patients had an obstructed portal vein. Embolization of the hepatic artery itself, proximal and distal to the bleeding pseudoaneurysm, may influence the liver perfusion with an undesirable effect[9,16]. Thus, for preserving hepatic arterial blood flow, implantation of stent-grafts (covered stent) may be better than TAE[10-12].
In recent years, stent-grafts have been increasingly used in endovascular repair of thoracic and abdominal aneurysm, repair of traumatic subclavian artery and iatrogenic vascular injuries, and in exclusion of peripheral arterial aneurysms[23,24]. However, conventional peripheral vascular stent-grafts are 6-8 mm in diameter and 6 mm in length, making implantation difficult through the tortuous celiac arterial system. Moreover, stent-graft itself is less flexible and seldom used in the celiac system[25,26]. The Jostent, used in our patients for sealing perforation of coronary artery, is a covered stent, ranging 3.5-4.0 mm in diameter and 16-19 mm in length[27]. In the present study, placement of coronary stent-grafts was a useful procedure for the repair of ruptured hepatic artery pseudoaneurysm. However, angiography follow-up was not performed, but hepatic arterial flow was confirmed by Doppler ultrasound examination.
In this study, a high immediate technical success rate was archived using stent-grafts for bleeding hepatic artery pseudoaneurysm, which is consistent with the reported findings[10-15,22]. Bleeding was immediately controlled after placement of stent-grafts and all patients remained stable after the procedure. However, recurrent bleeding occurred in 2 patients, at 16 and 24 h, respectively, after the interventional procedure, possibly due to the dislodgment of stent-grafts.
Of the 7 patients with their bleeding successfully controlled, one died of intra-abdominal sepsis 12 d after the interventional procedure. Consequently, the patency of hepatic artery and stent-grafts without recurrent bleeding was achieved in 6 patients during a mean follow-up time of 10.5 mo (range 4-16 mo).
Placement of stent-grafts with preservation of organ arterial flow, if technically possible, may represent the best treatment option with the following advantages[10-15]. First, it permits immediate and effective control of hemorrhage, thus avoiding emergency surgery, and a second operation can be performed if hemodynamics is stable. Second, placement of stent-grafts is a minimally invasive technique with a low morbidity. Third, placement of stent-grafts preserves end-organ perfusion in the acute stage, thus reducing the risk of organ failure or infarction.
Placement of stent-grafts for hemorrhage from ruptured hepatic artery pseudoaneurysm has several limitations. First, although technical success has been achieved in most published case reports, stent-graft implantation in branches of the celiac trunk is not always possible[10,12,28]. Second, the procedure may lead to rupture of artery because of its eroded and fragile vascular wall, thus requiring emergency surgery. Third, placement of stent-grafts may lead to in-stent-graft stenosis and occlusion[23,24,27]. It has been recommended that antiplatelet medication should be given after stent-graft deployment in order to prevent in-stent-graft stenosis.
In addition, placement of stent-grafts can control hemorrhage but cannot treat other potential complications[5,16]. To reduce the risk of recurrent hemorrhage, antibiotic therapy for peripancreatic infection and surgical revision of pancreatic or biliary anastomotic leakage may be necessary. Alternatively, pancreatic or biliary leakage can be treated by percutaneous drainage in some patients. In the present study, 6 patients recovered after placement of stent-grafts. Of the 6 patients, 4 underwent CT-guided placement of additional drains, and 2 underwent re-operation due to persistent abscess and PF.
This study has the following limitations: lack of a control group, randomization, and uniformity of evaluation and treatment. In fact, it is almost impossible to perform a prospective randomized study and no statistically significant conclusion could be drawn because of the limited number of patients.
In summary, placement of stent-grafts for acute life-threatening bleeding from hepatic artery pseudoaneurysm is a valuable alternative to embolization and surgical intervention. If technically possible, this technique should be considered the first-line treatment for bleeding from the common and proper hepatic artery, particularly in patients with a non-portal vein. Further data are required to evaluate its technical success rate, complications, and long-term outcome in a larger number of patients.
Delayed hepatic arterial hemorrhage after pancreaticoduodenectomy (PD) is not a common but a fatal complication, occurring in 7% of all patients. Its ideal management remains unclear and controversial.
There are many diagnostic and therapeutic options for massive hepatic arterial hemorrhage after PD but no established guidelines are available. Traditional treatment modalities for massive bleeding include re-operation or endovascular catheter techniques such as coil embolization. Surgical exploration and identification of the bleeding vessel can be difficult in acute situations and hazardous because of adhesions and surrounding postsurgical tissue friability. Embolization of the bleeding artery has an immediate impact on patient survival but often excludes the distal circulation, which may have a risk of hepatic ischemia and even fatal hepatic necrosis, particularly in patients with portal vein stenosis or thrombosis. Placement of stent-grafts is a new procedure for control of bleeding without interruption of the distal circulation.
The authors reported the clinical outcome of 9 patients with life-threatening hemorrhage from a ruptured hepatic artery pseudoaneurysm after PD after treatment with a new interventional technique, namely placement of stent-grafts. This technique provides a good alternative option for the control of hemorrhage from ruptured hepatic artery pseudoaneurysm after PD, especially in those who cannot undergo embolization. Although the number of patients was small, the procedure demonstrated a lower mortality than conventional surgical intervention.
Instead of coil embolization, the authors used stent-graft placement to exclude ruptured hepatic pseudoaneurysm because the patients had an obstructed portal vein prior to massive bleeding. In patients with a non-patent portal vein, embolization of the common or proper hepatic artery may have a high risk of hepatic ischemia and even fatal hepatic necrosis. Placement of stent-grafts in bleeding hepatic artery can immediately and effectively stop the hemorrhage, thus avoiding emergency surgery, and a second operation can be performed if the hemodynamics is stable. Placement of stent-grafts is a minimally invasive technique with a low morbidity. Placement of stent-grafts preserves end-organ perfusion in the acute stage, thus reducing the risk of liver ischemia or failure.
The authors describe a small number of patients with life-threatening hemorrhage from ruptured hepatic artery pseudoaneurysm after PD, who were treated with implantation of endovascular stent-grafts. The study is quite interesting and innovative. Placement of stent-grafts is a good procedure for the control of massive bleeding from hepatic artery, especially in those who cannot undergo embolization. Although the number of patients is small, the results can be considered satisfactory and encouraging.
Peer reviewers: Luis Grande, Professor, Department of Surgery, Hospital del Mar, Passeig Marítim 25-29, Barcelona 08003, Spain; Laura Lladó, PhD, Department of Surgery, Liver Transplant Unit, Hospital Universitari de Bellvitge, IDIBELL, 08907 Barcelona, Spain
S- Editor Wang JL L- Editor Wang XL E- Editor Zheng XM
| 1. | Limongelli P, Khorsandi SE, Pai M, Jackson JE, Tait P, Tierris J, Habib NA, Williamson RC, Jiao LR. Management of delayed postoperative hemorrhage after pancreaticoduodenectomy: a meta-analysis. Arch Surg. 2008;143:1001-1007; discussion 1007. [Cited in This Article: ] |
| 2. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [Cited in This Article: ] |
| 3. | Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, Kaifi J, Schurr PG, Bubenheim M, Nolte-Ernsting C. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269-280. [Cited in This Article: ] |
| 4. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [Cited in This Article: ] |
| 5. | de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Laméris JS, van Gulik TM, Obertop H, Gouma DJ. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241:85-91. [Cited in This Article: ] |
| 6. | Miura F, Asano T, Amano H, Yoshida M, Toyota N, Wada K, Kato K, Yamazaki E, Kadowaki S, Shibuya M. Management of postoperative arterial hemorrhage after pancreato-biliary surgery according to the site of bleeding: re-laparotomy or interventional radiology. J Hepatobiliary Pancreat Surg. 2009;16:56-63. [Cited in This Article: ] |
| 7. | Fujii Y, Shimada H, Endo I, Yoshida K, Matsuo K, Takeda K, Ueda M, Morioka D, Tanaka K, Togo S. Management of massive arterial hemorrhage after pancreatobiliary surgery: does embolotherapy contribute to successful outcome? J Gastrointest Surg. 2007;11:432-438. [Cited in This Article: ] |
| 8. | Sato N, Yamaguchi K, Shimizu S, Morisaki T, Yokohata K, Chijiiwa K, Tanaka M. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg. 1998;133:1099-1102. [Cited in This Article: ] |
| 9. | Baker TA, Aaron JM, Borge M, Pierce K, Shoup M, Aranha GV. Role of interventional radiology in the management of complications after pancreaticoduodenectomy. Am J Surg. 2008;195:386-390; discussion 390. [Cited in This Article: ] |
| 10. | Won YD, Ku YM, Kim KT, Kim KH, Kim JI. Successful management of a ruptured hepatic artery pseudoaneurysm with a stent-graft. Emerg Radiol. 2009;16:247-249. [Cited in This Article: ] |
| 11. | Sasaki K, Ueda K, Nishiyama A, Yoshida K, Sako A, Sato M, Okumura M. Successful utilization of coronary covered stents to treat a common hepatic artery pseudoaneurysm secondary to pancreatic fistula after Whipple's procedure: report of a case. Surg Today. 2009;39:68-71. [Cited in This Article: ] |
| 12. | Rossi M, Rebonato A, Greco L, Citone M, David V. Endovascular exclusion of visceral artery aneurysms with stent-grafts: technique and long-term follow-up. Cardiovasc Intervent Radiol. 2008;31:36-42. [Cited in This Article: ] |
| 13. | Pasklinsky G, Gasparis AP, Labropoulos N, Pagan J, Tassiopoulos AK, Ferretti J, Ricotta JJ. Endovascular covered stenting for visceral artery pseudoaneurysm rupture: report of 2 cases and a summary of the disease process and treatment options. Vasc Endovascular Surg. 2008;42:601-606. [Cited in This Article: ] |
| 14. | Ichihara T, Sato T, Miyazawa H, Shibata S, Hashimoto M, Ishiyama K, Yamamoto Y. Stent placement is effective on both postoperative hepatic arterial pseudoaneurysm and subsequent portal vein stricture: a case report. World J Gastroenterol. 2007;13:970-972. [Cited in This Article: ] |
| 15. | Stoupis C, Ludwig K, Inderbitzin D, Do DD, Triller J. Stent grafting of acute hepatic artery bleeding following pancreatic head resection. Eur Radiol. 2007;17:401-408. [Cited in This Article: ] |
| 16. | Gebauer T, Schulz HU, Tautenhahn J, Halloul Z, Effenberger O, Lippert H, Bürger T. [Interventional and vascular surgical management for inflammatory arrosion hemorrhage from visceral arteries following pancreatic surgery]. Chirurg. 2004;75:1021-1028. [Cited in This Article: ] |
| 17. | Tessier DJ, Fowl RJ, Stone WM, McKusick MA, Abbas MA, Sarr MG, Nagorney DM, Cherry KJ, Gloviczki P. Iatrogenic hepatic artery pseudoaneurysms: an uncommon complication after hepatic, biliary, and pancreatic procedures. Ann Vasc Surg. 2003;17:663-669. [Cited in This Article: ] |
| 18. | Bassi C, Falconi M, Salvia R, Mascetta G, Molinari E, Pederzoli P. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. 2001;18:453-457; discussion 458. [Cited in This Article: ] |
| 19. | Tsirlis T, Vasiliades G, Koliopanos A, Kopanakis N, Katseli A, Tsipras H, Margaris H. Pancreatic leak related hemorrhage following pancreaticoduodenectomy. A case series. JOP. 2009;10:492-495. [Cited in This Article: ] |
| 20. | Khorsandi SE, Limongelli P, Jackson JE, Tait P, Williamson RC, Habib NA, Jiao LR. Management of delayed arterial hemorrhage after pancreaticoduodenectomy. A case series. JOP. 2008;9:172-178. [Cited in This Article: ] |
| 21. | Teramoto K, Kawamura T, Takamatsu S, Noguchi N, Arii S. A case of hepatic artery embolization and partial arterialization of the portal vein for intraperitoneal, hemorrhage after a pancreaticoduodenectomy. Hepatogastroenterology. 2003;50:1217-1219. [Cited in This Article: ] |
| 22. | Makowiec F, Riediger H, Euringer W, Uhl M, Hopt UT, Adam U. Management of delayed visceral arterial bleeding after pancreatic head resection. J Gastrointest Surg. 2005;9:1293-1299. [Cited in This Article: ] |
| 23. | Xenos ES, Freeman M, Stevens S, Cassada D, Pacanowski J, Goldman M. Covered stents for injuries of subclavian and axillary arteries. J Vasc Surg. 2003;38:451-454. [Cited in This Article: ] |
| 24. | Baltacioğlu F, Cimşit NC, Cil B, Cekirge S, Ispir S. Endovascular stent-graft applications in latrogenic vascular injuries. Cardiovasc Intervent Radiol. 2003;26:434-439. [Cited in This Article: ] |
| 25. | Tielliu IF, Verhoeven EL, Zeebregts CJ, Prins TR, Oranen BI, van den Dungen JJ. Endovascular treatment of iliac artery aneurysms with a tubular stent-graft: mid-term results. J Vasc Surg. 2006;43:440-445. [Cited in This Article: ] |
| 26. | White R, Krajcer Z, Johnson M, Williams D, Bacharach M, O'Malley E. Results of a multicenter trial for the treatment of traumatic vascular injury with a covered stent. J Trauma. 2006;60:1189-1195; discussion 1195-1196. [Cited in This Article: ] |
| 27. | Lansky AJ, Yang YM, Khan Y, Costa RA, Pietras C, Tsuchiya Y, Cristea E, Collins M, Mehran R, Dangas GD. Treatment of coronary artery perforations complicating percutaneous coronary intervention with a polytetrafluoroethylene-covered stent graft. Am J Cardiol. 2006;98:370-374. [Cited in This Article: ] |
| 28. | Larson RA, Solomon J, Carpenter JP. Stent graft repair of visceral artery aneurysms. J Vasc Surg. 2002;36:1260-1263. [Cited in This Article: ] |