Published online Jul 28, 2010. doi: 10.3748/wjg.v16.i28.3521
Revised: April 6, 2010
Accepted: April 13, 2010
Published online: July 28, 2010
AIM: To clarify the association of glypican-3 (GPC3) expression with Wnt and other growth signaling molecules in hepatocellular carcinoma (HCC).
METHODS: Expression of GPC3, Wnt, matrix metalloproteinases (MMPs), sulfatase (SULF)1, SULF2, and other growth signaling molecules was analyzed in HCC cell lines and tissue samples by real-time reverse transcription-polymerase chain reaction, immunoblotting, and/or immunostaining. Expression of various genes in GPC3 siRNA-transfected HCC cells was analyzed.
RESULTS: GPC3 was overexpressed in most HCCs at mRNA and protein levels and its serum levels were significantly higher in patients with HCC than in non-HCC subjects (P < 0.05). Altered expressions of various MMPs and growth signaling molecules, some of which were correlated with GPC3 expression, were observed in HCCs. Down-regulation of GPC3 expression by siRNA in GPC3-overexpressing HCC cell lines resulted in a significant decrease in expressions of MMP2, MMP14, fibroblast growth factor receptor 1, insulin-like growth factor 1 receptor. GPC3 expression was significantly correlated with nuclear/cytoplasmic localization of β-catenin.
CONCLUSION: These results suggest that GPC3, in conjunction with MMPs and growth signaling molecules, might play an important role in the progression of HCC.
- Citation: Akutsu N, Yamamoto H, Sasaki S, Taniguchi H, Arimura Y, Imai K, Shinomura Y. Association of glypican-3 expression with growth signaling molecules in hepatocellular carcinoma. World J Gastroenterol 2010; 16(28): 3521-3528
- URL: https://www.wjgnet.com/1007-9327/full/v16/i28/3521.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i28.3521
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world[1]. HCC is associated with well-defined viral and non-viral etiological factors. Chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), chemical carcinogens (i.e. aflatoxins), and other environmental and host factors causing liver injury have etiologically been linked to HCC[2,3]. Various genetic and epigenetic abnormalities have been identified in HCC, suggesting a multi-step nature of hepatocarcinogenesis[3,4].
The pathogenesis of HCC has been known to involve p53[5], β-catenin[6], TGFβ[7] and the retinoblastoma gene[8]. p53 gene mutation occurs in one-third of HCC[5,9]. Activating mutations in β-catenin have been reported in 18% of HCC patients and axin mutations in 6%[6,10]. HCCs are also known to express various Wnt family members[11] and the activation of the canonical Wnt signaling pathway occurs in 18% of HCC[10].
Glypican-3 (GPC3) is a member of the glypican family of glycosylphosphatidylinositol-anchored cell-surface heparan sulfate proteoglycans. GPC3 is highly expressed in HCC cells and tissues[12-19]. It is thought that GPC3 stimulates the growth of HCC cells by upregulating autocrine/paracrine canonical Wnt signaling[20]. GPCs have been reported to stimulate both the canonical and non-canonical pathways[20]. GPC3 reportedly regulates migration, adhesion, and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation[21].
Matrix metalloproteinases (MMPs) also play an important role in HCC[22]. It has been reported that GPC3 may regulate MMP activity in breast cancer[23]. It has also been demonstrated that secreted MMP-9 associates with glypican-like proteoglycans through their heparan sulphate chains, and plays a crucial role in cell motility of murine colon cancer cell line LuM1 cells[24].
GPC3 has been shown to bind to fibroblast growth factor (FGF)2 and may function as a coreceptor for FGF2[25]. Two recently identified human heparin-degrading endosulfatases, named sulfatase 1 (SULF1) and SULF2, have been found to be involved in liver carcinogenesis[26,27]. Interestingly, SULF2 reportedly up-regulates GPC3, promotes FGF signaling, and decreases survival in HCC[27].
Moreover, GPC3 reportedly confers oncogenicity through the interaction between insulin-like growth factor (IGF)-II and its receptor, and the subsequent activation of the IGF signaling pathway[28]. Specific interactions both between GPC3 and IGF-II and between GPC3 and IGF 1 receptor (IGF1R) have been reported[28].
These results suggest that GPC3 joins a multiprotein complex, which is composed of the ligand, receptor, GPC3, and probably other proteins[28]. However, the association of GPC3 with Wnt, MMPs, SULF1, SULF2, and other growth signaling molecules has not been systematically analyzed in HCC. In this study, we analyzed expression of these molecules in HCC cell lines and tissue samples by real-time reverse transcription-polymerase chain reaction (RT-PCR), immunoblotting, and/or immunostaining. Expression of various genes in GPC3 siRNA-transfected HCC cells was analyzed.
HCC cell lines, HepG2, Hep3B, JHH-4, HuH-7, HLE, HLF, PLC/PRF/5, Li-7, huH-1, HT17, CHC4, and CHC32, were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan), Riken Cell Bank (Tokyo), or the American Type Culture Collection (Rockville, MD, USA), and cultured as recommended. Cells were maintained at 37°C in an atmosphere of humidified air with 5% CO2.
All tissue and serum samples were obtained from Japanese patients. Informed consent was obtained from each patient. Tissue microarray of HCC tissues was purchased from SuperBioChips Laboratories (Seoul, Korea). Each tissue specimen was divided into two pieces. One sample was used for total RNA extraction. The other sample was processed for pathological examination using hematoxylin and eosin staining for the evaluation of the tumor cell content. Only specimens containing more than 80% tumor cells were used for analysis. The tumor-node-metastasis (TNM) system of the American Joint Committee on Cancer and the International Union against Cancer was used for the pathologic diagnosis and classification of variables.
Semiquantitative RT-PCR was performed as described previously[29]. The primer sequences used were 5'-AGGTAGCTGCGAGGAAAC-3' and 5'-AGGTCACGTCTTGCTCCTC-3' for GPC3 and 5'-TGGACATCAATGAGTGCCTC-3' and 5'-CACATTCT GGTGAGCATTCG-3' for GAPDH. Real-time RT-PCR was performed by using TaqMan real-time PCR system as described previously[30]. The following genes were analyzed: GPC3, WNT (WNT1, WNT2, WNT2b, WNT3a, WNT4, WNT5a, and WNT7b), MMP (MMP2, MMP3, MMP7, MMP9, and MMP14), SULF1, SULF2, FGF2, FGF receptor (FGFR) 1, FGFR2, FGFR3, FGFR4, epidermal growth factor receptor (EGFR), erb-b2 (ERBB2), IGF2 and IGF1R. A comparative threshold cycle was used to determine gene expression relative to the no-tissue control (calibrator).
Immunoblotting using total cell lysates was performed as previously described[29]. The antibodies used were MMP2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), MMP14 (Abcam, Cambridge, UK), FGFR1 (Abcam), and IGF1R (Santa Cruz Biotechnology). The protein was visualized using the enhanced chemiluminescence plus detection system (Amersham Biosciences, Piscataway, NJ, USA), and the membranes were stripped and reprobed with mouse anti-actin monoclonal antibody (Chemicon, Temecula, CA, USA).
Gelatinase activity of conditioned media of siRNA-transfected HepG2 was measured using SDS-polyacrylamide gels containing 0.1% gelatin as described previously[22]. Gels were washed in 2.5% Triton X-100 for 30 min prior to incubation in 20 mmol/L glycine, 10 mmol/L CaCl2, 1 μmol/L ZnCl2 (pH 8.3) for 48 h at 37°C, before staining with Commassie Brilliant Blue.
siRNAs for the GPC3 gene were purchased from Ambion (Silencer® Pre-designed siRNA, Austin, TX, USA). Unrelated nonspecific siRNAs (Silencer® Negative Control siRNA) were used as a control. siRNA transfection was performed following the manufacturer’s instructions[29]. After incubation for 72 h, siRNA-transfected HepG2 cells were analyzed by real-time RT-PCR and immunoblotting to validate knockdown effect on GPC3. These cells were used for real-time RT-PCR.
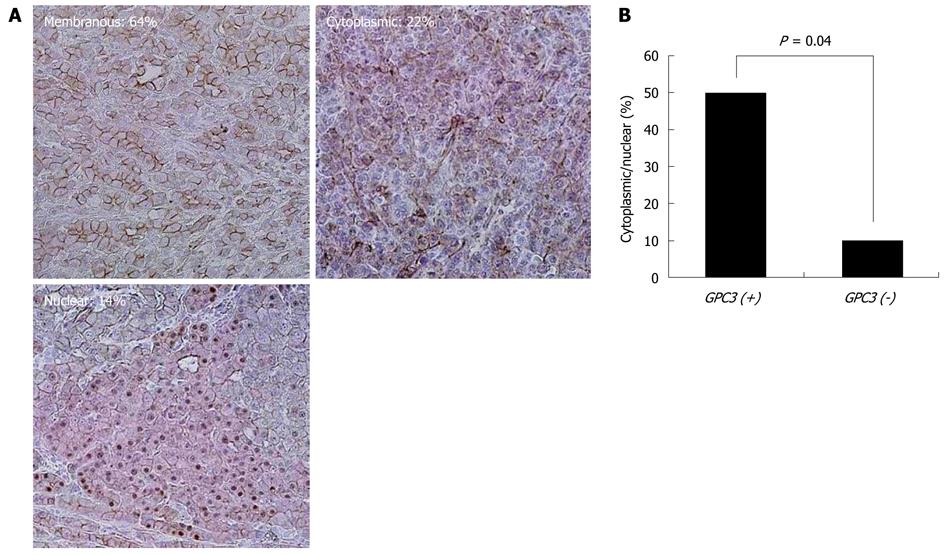
Immunohistochemistry with an anti-human GPC3 mouse monoclonal antibody (Clone 1G12 used at 5 mg/mL, BioMosaics, Burlington, VT, USA) and an anti-human β-catenin monoclonal antibody (Santa Cruz Biotechnology) was performed as described previously[29]. A case was considered negative for GPC3 if < 5% of the carcinoma cells exhibited immunoreactivity[31]. Cancer cases were categorized into the following 4 groups corresponding to immunostaining patterns of β-catenin as previously described: membranous, membranous staining pattern similar to that in normal; weak, no staining or weaker staining than normal; cytoplasmic, staining in the cytoplasm or cytoplasm/cell membrane; accumulated, staining in the nucleus or nucleus/cytoplasm.
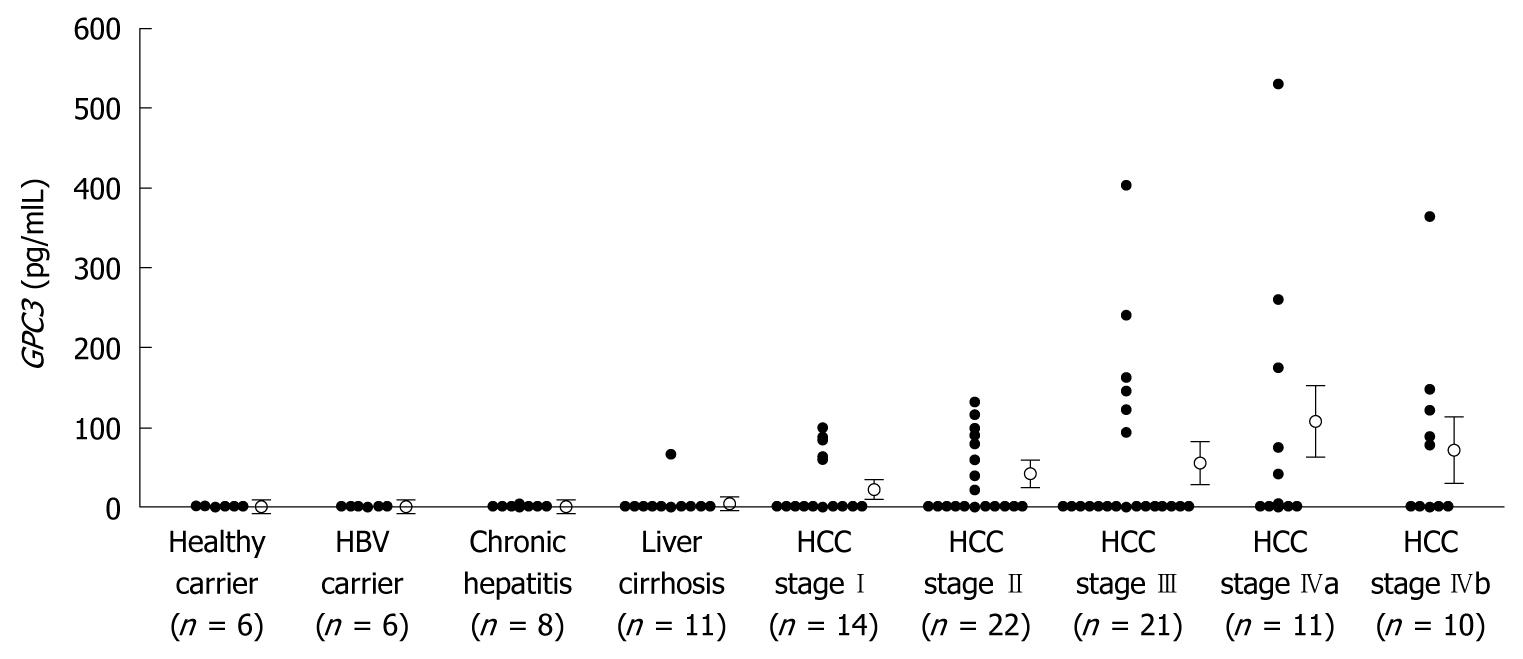
Serum GPC3 levels were measured using a commercially available sandwich ELISA kit (Biomosaics) following the manufacturer’s protocol[16]. The subjects were 6 healthy volunteers, 6 healthy HBV carriers, 8 patients with chronic hepatitis, 11 patients with cirrhosis, and 64 patients with HCC.
The results were assessed for associations with clinicopathological parameters using the following statistical tests: Student’s t test for age, the Mann-Whitney test for lymph node metastasis and pTNM stage, and the χ2 test or Fisher’s exact test for the remaining parameters. To explore relationships between the variables selected for the models, Spearman’s correlation coefficients were calculated. A P value of less than 0.05 was considered significant. A P value between 0.05 and 0.10 was considered as trend toward an association.
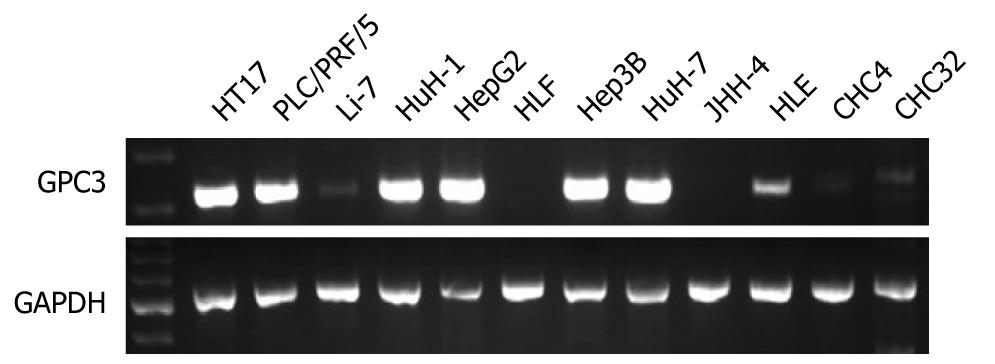
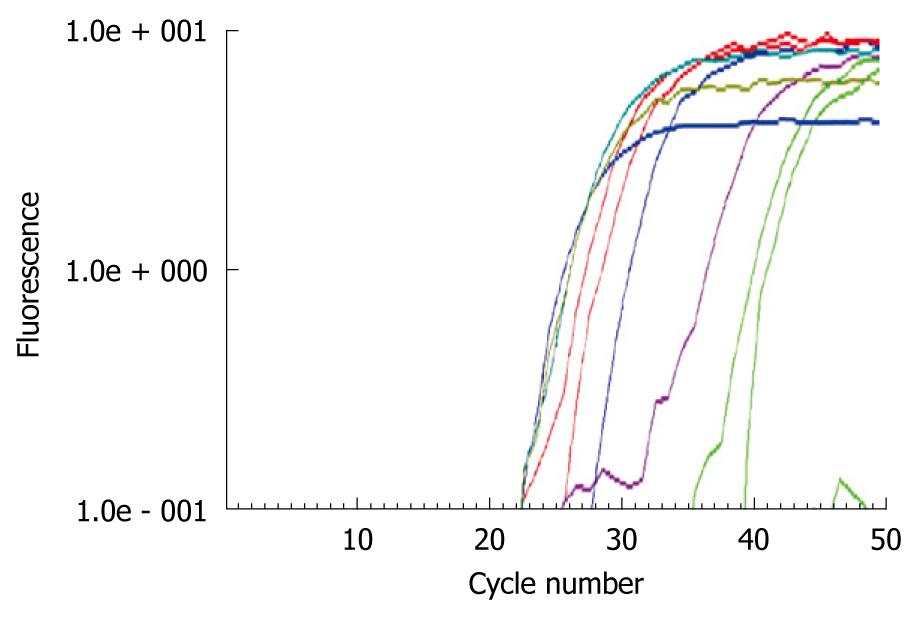
GPC3 expression was detected in 7 (58%) of 12 HCC cell lines (Figure 1). GPC3 mRNA was detected in HT17, PLC/PRF/5, HuH-1, HepG2, Hep3B, and HuH-7 at a high level and HLE at a low level. GPC3 mRNA was positive in well differentiated HuH7 and poorly differentiated HT17 cell lines. There was no correlation between GPC3 positivity and histopathology. Concordant results were obtained by real-time RT-PCR (Figure 2). There was no correlation between quantitative GPC3 mRNA levels and histopathology.
WNT1, WNT2, WNT2b, WNT3a, WNT4, WNT5a, WNT7b expression was detected in 0 (0%), 7 (58%), 0 (0%), 0 (0%), 5 (41%), 9 (75%), 4 (33%) of 12 HCC cell lines. Thus, WNT2 and WNT5a were frequently detected compared with other WNTs. MMP2, MMP3, MMP7, MMP9, MMP14 expression was detected in 7 (58%), 2 (16%), 8 (67%), 2 (16%), 11 (92%) of 12 HCC cell lines. Thus, the positivity was considerably different among MMPs. SULF1 and SULF2 expression was detected in all of the 12 HCC cell lines. EGFR, ERBB2, FGF2, FGFR1, FGFR2, FGFR3, FGFR4 expression was detected in 12 (100%), 12 (100%), 12 (100%), 12 (100%), 6 (50%), 2 (16%), 12 (100%) of 12 HCC cell lines. Thus, growth factor receptors were frequently detected in HCC cell lines. GPC3 expression was significantly correlated with expression of MMP14, ERBB2, FGFR3, and FGFR4.
In 18 (78%) of 23 cases, mRNA expression of GPC3 was upregulated in cancer tissue compared with non-tumor tissue samples. There was no significant difference in GPC3 mRNA levels among non-tumor liver tissue, including from chronic hepatitis and liver cirrhosis cases. Overexpression of GPC3 was not related to HBV or to HCV infection. Overexpression of GPC3 was not significantly correlated with other clinicopathological characteristics (data not shown).
mRNA expression of SULF1 was downregulated in 17 (74%) and SULF2 was upregulated in 7 (30%) of 23 HCC tissue samples compared with non-tumor tissue. Overexpression of GPC3 was significantly correlated with MMP2 (P = 0.0460), FGF2 (P = 0.0001), FGFR1 (P = 0.0417), FGFR2 (P = 0.0023), SULF1 (P = 0.0202), and SULF2 (P = 0.0081).
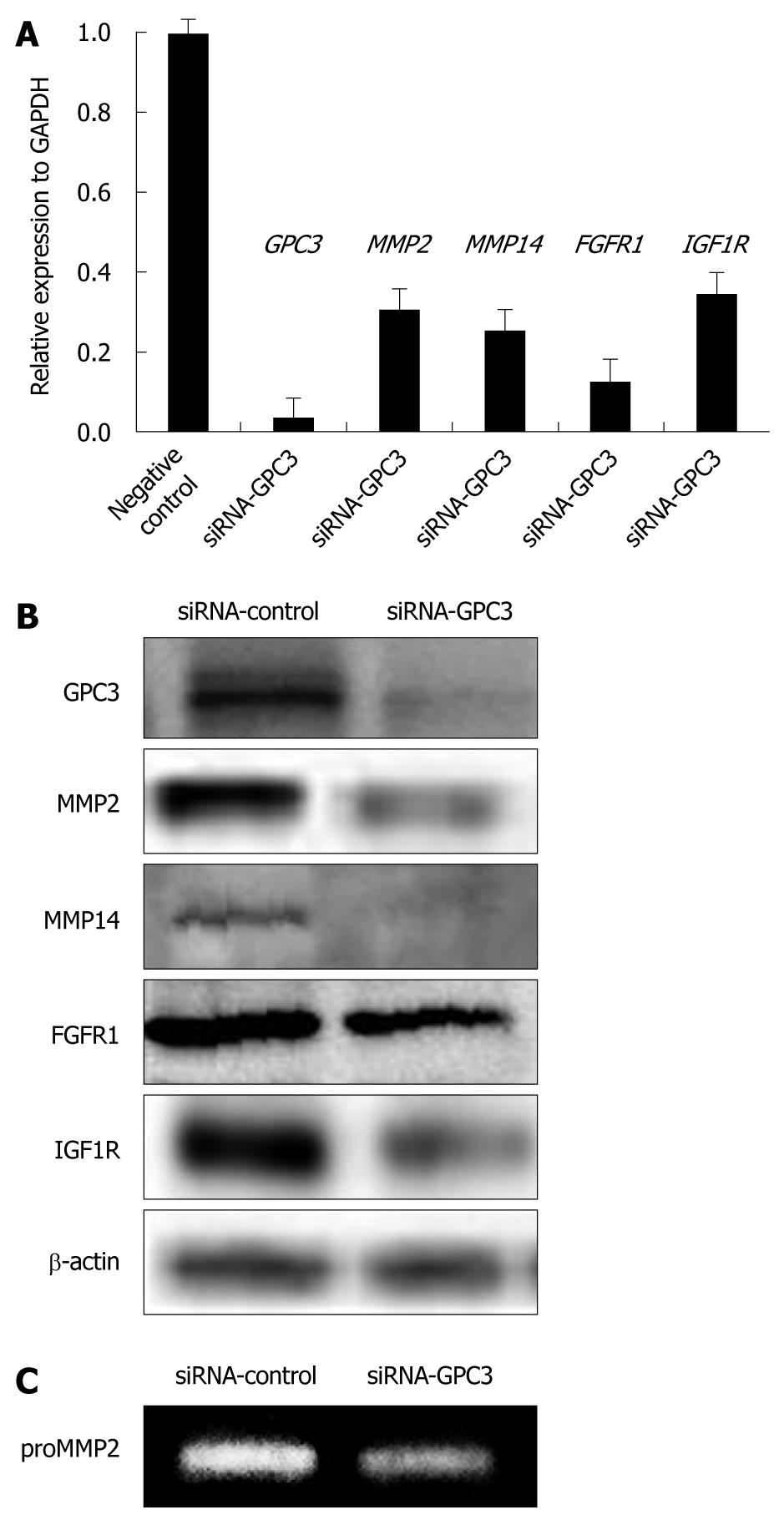
To assess the effect of GPC3 expression on gene expression in HCC, mRNA expression of WNT, MMPs, SULF1, SULF2, and other growth signaling molecules was analyzed by real-time PCR after treatment with specific siRNA for the GPC3 gene. Transfection with siRNA resulted in over 80% inhibition of mRNA and protein expression of GPC3 (Figure 3). Among the genes analyzed, mRNA expression of MMP2, MMP14, FGFR1, and IGF1R was downregulated in GPC3 siRNA-transfected cells compared with control siRNA-transfected counterparts (Figure 3A). Down-expression of MMP2, MMP14, FGFR1, and IGF1R was confirmed at protein levels analyzed by immunoblotting (Figure 3B). Down-regulation of MMP2 activity was further confirmed by zymography (Figure 3C).
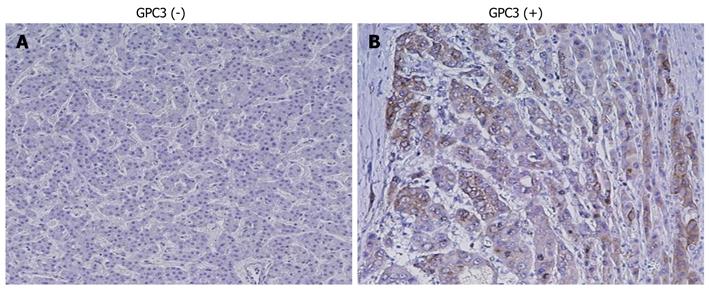
Figure 4 shows representative results of immunohistochemistry for GPC3 in HCC tissue samples. GPC3 protein was strongly expressed in the cytoplasm and/or membrane of carcinoma cells, when compared with adjacent non-tumor cells. GPC3 expression was positive in 54 (75%) of the 72 cases. GPC3 positivity was not significantly correlated with clinicopathological characteristics. Figure 5 shows representative results of immunohistochemistry for β-catenin in HCC tissues. Membranous, weak, cytoplasmic, and accumulated pattern was observed in 18 (64%), 0 (0%), 6 (22%), and 4 (14%), respectively. Nuclear/cytoplasmic localization of β-catenin was observed in a significantly higher percentage of carcinomas with GPC3 expression (9 of 18, 50%) than in those without (1 of 10, 10%, P = 0.040).
Serum GPC3 levels were under cut-off levels in healthy volunteers, HBV carriers and chronic hepatitis patients (Figure 6). One patient with liver cirrhosis and 32 (50%) of the 64 patients with HCC showed elevated serum GPC3 levels. Serum GPC3 levels in patients with HCC were significantly higher than in non-HCC subjects (P < 0.05). There was no correlation in positivity between GPC3, α-fetoprotein (AFP) levels and vitamin K absence or antagonist-II (PIVKA-II) levels (data not shown).
In this study, we found overexpression of GPC3 mRNA in HCC cell lines and tissue samples. The overexpression of GPC3 in HCC was also observed at protein level analyzed by immunohistochemistry. These results further support the notion that GPC3 plays an important role in hepatocarcinogenesis.
We analyzed the association of GPC3 with WNT, MMPs, SULF1, SULF2, and other growth signaling molecules, in HCC cell lines and tissue samples. GPC3 expression was correlated with expression of MMP14, ERBB2, FGFR3, and FGFR4 in HCC cell lines. Overexpression of GPC3 was significantly correlated with MMP2, FGF2, FGFR1, FGFR2, SULF1, and SULF2 in HCC tissue. To assess the effect of GPC3 expression on gene expression in HCC cells, expression of WNT, MMPs, SULF1, SULF2, and other growth signaling molecules was analyzed in HCC cell lines after treatment with specific siRNA for the GPC3 gene. Down-expression of MMP2, MMP14, FGFR1, and IGF1R was observed at mRNA and protein levels, and that of MMP2 was also observed at gelatinolytic activity levels.
MMP2 and its activator MMP14 play an important role in HCC progression[22]. Therefore, down-regulation of both MMP2 and MMP14 by GPC3 suppression is interesting. It has been reported that GPC3 may regulate MMP activity[23]. FGF-FGFR signaling plays an important role in hepatocarcinogenesis[27,32]. GPC3 has been shown to bind to FGF2 and may function as a coreceptor for FGF2[25]. SULF2 reportedly up-regulates GPC3, and promotes FGF signaling in HCC[27]. Moreover, GPC3 reportedly confers oncogenicity through the interaction between IGF-II and IGF1R, and the subsequent activation of the IGF signaling pathway[28]. Specific interactions both between GPC3 and IGF-II and between GPC3 and IGF-IR have been reported[28]. Therefore, the association of GPC3 expression with expressions of these molecules found in this study is interesting (Figure 7).
Nuclear/cytoplasmic localization of β-catenin was observed in a significantly higher percentage of HCCs with GPC3 expression than in those without. GPC3-induced accumulation of cytoplasmic β-catenin has been reported in HCC cells[20]. Therefore, in addition to β-catenin mutation or deletion, axin mutations, and other alterations of molecules in the canonical WNT signaling pathway, GPC3 may play a role in nuclear/cytoplasmic localization of β-catenin in HCC. These results may explain, in part, the association between GPC3 and growth signaling molecules in HCC. Further studies are necessary to clarify the direct and/or indirect interactions between GPC3 and growth signaling molecules in HCC.
The implication of a part played by GPC3 in HCC was further substantiated by the fact that serum GPC3 levels were significantly higher in patients with HCC than non-HCC subjects. However, there was a discrepancy between GPC3 overexpression in HCC tissues (75%) and GPC secretion (50%). The discrepancy was also reported in previous studies[14,16]. The sandwich ELISA kit used in this study used a polyclonal antibody and a monoclonal antibody, both raised against the last 70 amino acids of the COOH-terminal portion of GPC3, to detect glycanated GPC3[16,19]. This may be one of the reasons why serum GPC3 positivity was lower than GPC3 positivity in HCC tissue[17]. Comparison of our results with those analyzed by a kit using antibody against the NH2-terminal portion of GPC3 will further strengthen the notion that GPC3 is a useful serum marker for HCC[17]. There was no correlation between GPC and AFP levels. Therefore, detection of both glycanated and NH2-terminal truncated GPC3, as well as AFP, may provide additional useful markers for HCC.
Taken together, our results suggest that GPC3 overexpression plays an important role in HCC. As a target gene for molecular therapy, its expression in normal adult tissues is important. Considering the expression pattern of GPC3 together with its oncogenic function, GPC3 could be an attractive target for molecular therapy. Antitumor effects of the anti-GPC3 antibody have been reported[33]. Interestingly, we have recently reported the tumor suppressive effect of tyrosine kinase inhibitor of IGF1R, NVP-AEW541, on GPC-3-expressing HCC cell line PLC/PRF/5[34]. Combination of the anti-GPC3 antibody and molecular therapy targeting GPC3-related molecules, such as FGFR, found in this study will be a promising new cancer therapy in the future.
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world. Understanding the molecular biological features of HCC is necessary for early diagnosis and better prognosis. The potential role of glypican-3 (GPC3) in human HCC is receiving increasing attention.
GPC3 is highly expressed in HCC cells and tissues. However, little is known about the association of GPC3 expression with Wnt and other growth signaling molecules. In this study, the authors demonstrate that GPC3, in conjunction with matrix metalloproteinases (MMPs) and growth signaling molecules, might play an important role in the progression of HCC.
GPC3 was overexpressed in most HCCs at mRNA and protein levels and its serum levels were significantly higher in patients with HCC than in non-HCC subjects. Altered expressions of various MMPs and growth signaling molecules, some of which were correlated with GPC3 expression, were observed in HCCs. This is the first study to report an association of GPC3 expression with MMPs and growth signaling molecules in HCC.
Considering the tumor specific expression pattern of GPC3 together with its oncogenic function, GPC3 could be an attractive target for molecular diagnosis and/or therapy in clinical settings. Understanding of the direct and/or indirect interactions between GPC3 and growth signaling molecules may represent a future strategy for therapeutic intervention in the treatment of patients with HCC.
GPC3: GPC3 is a member of the glypican family of glycosylphosphatidylinositol-anchored cell-surface heparan sulfate proteoglycans. Sulfatase (SULF): Two recently identified human heparin-degrading endosulfatases, named SULF1 and SULF2, are extracellular neutral-pH SULFs. SULFs modulate several signaling pathways, including the promotion of Wnt signaling.
This paper reports an association of GPC3 expression with MMPs and growth signaling molecules in HCC. The authors showed that GPC3, in conjunction with MMPs and growth signaling molecules, might play a key role in the progression of HCC. The study sounds interesting and significant in a potentially important area involving HCC.
Peer reviewers: Ned Snyder, MD, FACP, AGAF, Professor, Chief of Clinical Gastroenterology and Hepatology, Department of Internal Medicine, The University of Texas Medical Branch, 301 University Blvd., Galveston, TX 77555-0764, United States; Fanyin Meng, MD, PhD, Assistant Professor, Department of Internal Medicine, Ohio State University, Room 514A Medical Research Facility, 420 West 12th Avenue, Columbus, OH 43210, United States
S- Editor Wang JL L- Editor Logan S E- Editor Zheng XM
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [Cited in This Article: ] |
| 2. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [Cited in This Article: ] |
| 3. | Kaneto H, Sasaki S, Yamamoto H, Itoh F, Toyota M, Suzuki H, Ozeki I, Iwata N, Ohmura T, Satoh T. Detection of hypermethylation of the p16(INK4A) gene promoter in chronic hepatitis and cirrhosis associated with hepatitis B or C virus. Gut. 2001;48:372-377. [Cited in This Article: ] |
| 4. | Takagi H, Sasaki S, Suzuki H, Toyota M, Maruyama R, Nojima M, Yamamoto H, Omata M, Tokino T, Imai K. Frequent epigenetic inactivation of SFRP genes in hepatocellular carcinoma. J Gastroenterol. 2008;43:378-389. [Cited in This Article: ] |
| 5. | Hsu HC, Tseng HJ, Lai PL, Lee PH, Peng SY. Expression of p53 gene in 184 unifocal hepatocellular carcinomas: association with tumor growth and invasiveness. Cancer Res. 1993;53:4691-4694. [Cited in This Article: ] |
| 6. | Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157:763-770. [Cited in This Article: ] |
| 7. | Kitisin K, Ganesan N, Tang Y, Jogunoori W, Volpe EA, Kim SS, Katuri V, Kallakury B, Pishvaian M, Albanese C. Disruption of transforming growth factor-beta signaling through beta-spectrin ELF leads to hepatocellular cancer through cyclin D1 activation. Oncogene. 2007;26:7103-7110. [Cited in This Article: ] |
| 8. | Zhang X, Xu HJ, Murakami Y, Sachse R, Yashima K, Hirohashi S, Hu SX, Benedict WF, Sekiya T. Deletions of chromosome 13q, mutations in Retinoblastoma 1, and retinoblastoma protein state in human hepatocellular carcinoma. Cancer Res. 1994;54:4177-4182. [Cited in This Article: ] |
| 9. | Hsu HC, Tseng HJ, Lai PL, Lee PH, Peng SY. Expression of p53 gene in 184 unifocal hepatocellular carcinomas: association with tumor growth and invasiveness. Cancer Res. 1993;53:4691-4694. [Cited in This Article: ] |
| 10. | Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1-24. [Cited in This Article: ] |
| 11. | Xu XR, Huang J, Xu ZG, Qian BZ, Zhu ZD, Yan Q, Cai T, Zhang X, Xiao HS, Qu J. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci USA. 2001;98:15089-15094. [Cited in This Article: ] |
| 12. | Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, Büchler MW. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48:558-564. [Cited in This Article: ] |
| 13. | Sung YK, Hwang SY, Park MK, Farooq M, Han IS, Bae HI, Kim JC, Kim M. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94:259-262. [Cited in This Article: ] |
| 14. | Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16-25. [Cited in This Article: ] |
| 15. | Midorikawa Y, Ishikawa S, Iwanari H, Imamura T, Sakamoto H, Miyazono K, Kodama T, Makuuchi M, Aburatani H. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int J Cancer. 2003;103:455-465. [Cited in This Article: ] |
| 16. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [Cited in This Article: ] |
| 17. | Hippo Y, Watanabe K, Watanabe A, Midorikawa Y, Yamamoto S, Ihara S, Tokita S, Iwanari H, Ito Y, Nakano K. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res. 2004;64:2418-2423. [Cited in This Article: ] |
| 18. | Yamauchi N, Watanabe A, Hishinuma M, Ohashi K, Midorikawa Y, Morishita Y, Niki T, Shibahara J, Mori M, Makuuchi M. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591-1598. [Cited in This Article: ] |
| 19. | Capurro M, Filmus J. Glypican-3 as a serum marker for hepatocellular carcinoma. Cancer Res. 2005;65:372; author reply 372-372; author reply 373. [Cited in This Article: ] |
| 20. | Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245-6254. [Cited in This Article: ] |
| 21. | Stigliano I, Puricelli L, Filmus J, Sogayar MC, Bal de Kier Joffé E, Peters MG. Glypican-3 regulates migration, adhesion and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation. Breast Cancer Res Treat. 2009;114:251-262. [Cited in This Article: ] |
| 22. | Yamamoto H, Itoh F, Adachi Y, Sakamoto H, Adachi M, Hinoda Y, Imai K. Relation of enhanced secretion of active matrix metalloproteinases with tumor spread in human hepatocellular carcinoma. Gastroenterology. 1997;112:1290-1296. [Cited in This Article: ] |
| 23. | Peters MG, Farías E, Colombo L, Filmus J, Puricelli L, Bal de Kier Joffé E. Inhibition of invasion and metastasis by glypican-3 in a syngeneic breast cancer model. Breast Cancer Res Treat. 2003;80:221-232. [Cited in This Article: ] |
| 24. | Koyama Y, Naruo H, Yoshitomi Y, Munesue S, Kiyono S, Kusano Y, Hashimoto K, Yokoi T, Nakanishi H, Shimizu S. Matrix metalloproteinase-9 associated with heparan sulphate chains of GPI-anchored cell surface proteoglycans mediates motility of murine colon adenocarcinoma cells. J Biochem. 2008;143:581-592. [Cited in This Article: ] |
| 25. | Song HH, Shi W, Filmus J. OCI-5/rat glypican-3 binds to fibroblast growth factor-2 but not to insulin-like growth factor-2. J Biol Chem. 1997;272:7574-7577. [Cited in This Article: ] |
| 26. | Lai JP, Chien JR, Moser DR, Staub JK, Aderca I, Montoya DP, Matthews TA, Nagorney DM, Cunningham JM, Smith DI. hSulf1 Sulfatase promotes apoptosis of hepatocellular cancer cells by decreasing heparin-binding growth factor signaling. Gastroenterology. 2004;126:231-248. [Cited in This Article: ] |
| 27. | Lai JP, Sandhu DS, Yu C, Han T, Moser CD, Jackson KK, Guerrero RB, Aderca I, Isomoto H, Garrity-Park MM. Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology. 2008;47:1211-1222. [Cited in This Article: ] |
| 28. | Cheng W, Tseng CJ, Lin TT, Cheng I, Pan HW, Hsu HC, Lee YM. Glypican-3-mediated oncogenesis involves the Insulin-like growth factor-signaling pathway. Carcinogenesis. 2008;29:1319-1326. [Cited in This Article: ] |
| 29. | Taniguchi H, Yamamoto H, Akutsu N, Nosho K, Adachi Y, Imai K, Shinomura Y. Transcriptional silencing of hedgehog-interacting protein by CpG hypermethylation and chromatic structure in human gastrointestinal cancer. J Pathol. 2007;213:131-139. [Cited in This Article: ] |
| 30. | Oki M, Yamamoto H, Taniguchi H, Adachi Y, Imai K, Shinomura Y. Overexpression of the receptor tyrosine kinase EphA4 in human gastric cancers. World J Gastroenterol. 2008;14:5650-5656. [Cited in This Article: ] |
| 31. | Wang HL, Anatelli F, Zhai QJ, Adley B, Chuang ST, Yang XJ. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723-1728. [Cited in This Article: ] |
| 32. | Huang X, Yu C, Jin C, Kobayashi M, Bowles CA, Wang F, McKeehan WL. Ectopic activity of fibroblast growth factor receptor 1 in hepatocytes accelerates hepatocarcinogenesis by driving proliferation and vascular endothelial growth factor-induced angiogenesis. Cancer Res. 2006;66:1481-1490. [Cited in This Article: ] |
| 33. | Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68:9832-9838. [Cited in This Article: ] |
| 34. | Piao W, Wang Y, Adachi Y, Yamamoto H, Li R, Imsumran A, Li H, Maehata T, Ii M, Arimura Y. Insulin-like growth factor-I receptor blockade by a specific tyrosine kinase inhibitor for human gastrointestinal carcinomas. Mol Cancer Ther. 2008;7:1483-1493. [Cited in This Article: ] |