Published online Jul 14, 2010. doi: 10.3748/wjg.v16.i26.3310
Revised: January 27, 2010
Accepted: February 3, 2010
Published online: July 14, 2010
AIM: To evaluate the prevalence of preoperative and postoperative malnutrition and the relationships between objective and subjective nutritional assessment of gastric cancer patients.
METHODS: From October 2005 to July 2006, we studied 80 patients with no evidence of recurrent disease and no loss to follow-up after curative surgery for gastric cancer. In this group, 9 patients underwent total gastrectomy and 71 patients subtotal gastrectomy. At admission, 6 and 12 mo after surgery, the patients were assessed on the subjective global assessment (SGA), nutritional risk screening (NRS-2002), nutritional risk index (NRI) and by anthropometric measurements and laboratory data. Differences between the independent groups were assessed with the Student’s t test and one-way analysis of variance. Spearman’s rank correlation coefficients were calculated to evaluate the association between the scores and variables.
RESULTS: The prevalence of malnutrition at admission was 31% by SGA and 43% by NRS-2002. At admission, the anthropometric data were lower in the malnourished groups defined by the SGA and NRS-2002 assessments, but did not differ between the groups using the NRI assessment. Body weight (BW), body mass index (BMI), triceps skin fold and midarm circumference were significantly reduced, but the total lymphocyte count, albumin, protein, cholesterol and serum iron levels did not decrease during the postoperative period. Six months after surgery, there was a good correlation between the nutritional assessment tools (SGA and NRS-2002) and the other nutritional measurement tools (BW, BMI, and anthropometric measurements). However, 12 mo after surgery, most patients who were assessed as malnourished by SGA and NRS-2002 had returned to their preoperative status, although their BW, BMI, and anthropometric measurements still indicated a malnourished status.
CONCLUSION: A combination of objective and subjective assessments is needed for the early detection of the nutritional status in case of gastric cancer patients after gastrectomy.
- Citation: Ryu SW, Kim IH. Comparison of different nutritional assessments in detecting malnutrition among gastric cancer patients. World J Gastroenterol 2010; 16(26): 3310-3317
- URL: https://www.wjgnet.com/1007-9327/full/v16/i26/3310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i26.3310
We should pay special attention to the alarming report that 30%-50% of patients in general hospitals have some degree of malnutrition[1-5]. Malnutrition is defined as a state of deficiency in energy, protein or other specific nutrients, producing an appreciable change in body function[6]. Patients who have had gastrointestinal problems and who have undergone gastrointestinal surgery constitute an important risk group for malnutrition[7,8]. Malnutrition is an important factor, influencing both their morbidity and recovery after gastrectomy surgery including radical lymphadenectomy[9,10]. The early detection of nutritional risk would allow early intervention, which may prevent later complications.
The assessment of a patient’s initial nutritional status and its evaluation during the disease and/or treatment plays an important role in tailoring nutritional support[11]. The goals of a formal nutrition assessment are: to identify patients who are malnourished or are at risk of malnutrition; to collect the information necessary to create a nutrition care plan; and to monitor the adequacy of nutritional therapy[12]. Studies have consistently revealed the inadequacy of any single method or tool in assessing a patient’s nutritional status. The absence of a single gold-standard objective measure has led investigators to develop various nutritional indices that can be used to stratify patients at increased risk of poor outcomes[13]. As a result, combinations of diverse measurements have been developed into subjective scoring systems designed to increase the sensitivity and specificity of nutritional status determinations[14]. Traditionally, scoring systems have been based on objective measurements of nutritional status, such as oral energy intake, body weight, weight loss over time, loss of subcutaneous fat, muscle wasting, serum protein levels, and immune competence. These prognostic indices include the nutritional risk index (NRI)[15], which is based on mathematical equations, and the subjective global assessment (SGA)[16] and nutritional risk screening (NRS-2002), which are based on clinical and subjective assessments[17].
This study was performed to evaluate the prevalence of preoperative and postoperative malnutrition in patients with gastric cancer who underwent radical gastrectomy, and the relationships between the objective variables (anthropometric and laboratory measurements) and the subjective scoring systems in the assessment of nutritional status during the postoperative follow-up period.
Between October 2005 and July 2006, 80 patients were studied following curative surgery for gastric cancer. Among this group, 9 patients underwent total gastrectomy and 71 patients underwent subtotal gastrectomy. We assessed the nutritional status and laboratory parameters of the patients on admission and at 6 and 12 mo after surgery. Patients with evidence of recurrent disease or who were lost to follow-up were excluded.
This study was approved by the research ethics committee of the institution, and informed consent was obtained from all patients.
The patients were assessed on the following items: SGA, NRS-2002, NRI, anthropometric measurements and laboratory data. The nutritional assessments were performed by a trained nurse specializing in nutrition and a dietitian.
The SGA is a screening tool to determine the nutritional status of patients and was developed by Detsky et al[16]. The SGA is a clinical technique with subjective elements and assesses nutritional status based on features of the patient’s history and physical examination. During the SGA, two trained investigators used a standardized questionnaire concerning the patients’ height and weight (current, before illness, and weight range during the previous 6 mo) and took a nutritional history (appetite, intake, gastrointestinal symptoms). In addition, the dietitian evaluated their physical appearances (subjective assessment of fat loss, muscle wasting, edema and ascites) and noted any existing medical conditions (e.g. encephalopathy, infection, renal insufficiency). Based on this evaluation, the patients were classified as being well nourished (SGA A), moderately malnourished (SGA B), or severely malnourished (SGA C). The SGA examiner was not aware of the laboratory test results at the time of the assessment.
The NRS-2002 was introduced by the European Society of Parenteral and Enteral Nutrition as the preferred method for screening and assessing hospital patients[17]. Its stated purpose was “Identification of those hospitalized patients, who are malnourished or at risk for malnourishment and who would gain benefit from the improvement of their nutritional situation.” The NRS-2002 consists of a nutritional score and a severity of disease score and an age adjustment for patients aged > 70 years (+1). Nutritional score: weight loss > 5% in 3 mo or food intake below 50%-75% in the preceding week = 1; weight loss > 5% in 2 mo or BMI 18.5-20.5 kg/m2 and impaired general condition or food intake 25%-60% in the preceding week = 2; weight loss > 5% in 1 mo or > 15% in 3 mo or BMI < 18.5 kg/m2 and impaired general condition or food intake 0%-25% in the preceding week = 3. Severity of disease score: hip fracture, chronic patients with acute complications = 1; major abdominal surgery, stroke, severe pneumonia, hematological malignancies = 2; head injury, bone marrow transplantation, intensive care patients with APACHE > 10 = 3. The NRS-2002 score is the total of the nutritional score, severity of disease score and age adjustment. Patients are classified as no risk = 0, low risk = 0-1, medium risk = 3-4 and high risk = > 5.
The NRI was developed by the Veteran’s Affairs Total Parenteral Nutrition group[15] in 1991 for use in the evaluation of the efficacy of perioperative total parenteral nutrition in patients undergoing thoracic or abdominal surgery. The NRI is a simple equation that uses serum albumin and recent weight loss: NRI = [1.519 × serum albumin (g/L)] + 0.417 × (present weight/usual weight × 100). An NRI score higher than 100 indicates that the patient is not malnourished, a score of 97.5 to 100 indicates mild malnourishment, a score of 83.5 to 97.5 indicates moderate malnourishment, and a score lower than 83.5 indicates severe malnourishment.
Body weight (BW; nearest 0.1 kg) and height (nearest cm) were measured while the patient was standing without shoes and in light clothes. Body mass index (BMI) was derived as weight (kg) divided by height (m) squared (kg/m2). The triceps skinfold thickness (TSF), to the nearest mm, was measured at the midpoint between the acromion and olecranon processes on the nondominant side with a Holtain caliper (Holtain Ltd., Crymych, UK). The midarm circumference (MAC) was measured to the nearest 0.1 cm with a tape at the same point as the TSF. All anthropometric measurements were made at least three times by the same investigator, and the reported values are the means of the repeated measurements.
Blood samples were taken from the cubital vein and tests included the measurement of serum protein, albumin, and cholesterol, and total lymphocyte counts (TLC). Laboratory data were collected using standard laboratory methods.
The data were analyzed with the statistical software “Statistical Package for Social Science (SPSS)” version 12.0 for Windows (SPSS, Inc., Chicago, IL, USA). Differences between the independent groups were assessed with Student’s t test and one-way analysis of variance. Spearman’s rank correlation coefficients were calculated to evaluate the association between the scores and variables. Data are presented as mean ± SD. Differences were considered to be statistically significant at P < 0.05. Agreement between two assessment methods was analyzed with the κ statistic. The value of κ varies from 0 to 1; a value of 0.4 or less indicates that chance alone can account for the observed agreement, and a value of 1 indicates perfect concordance.
Eighty patients who were treated with gastrectomy for gastric carcinoma were enrolled. The patients’ characteristics are summarized in Table 1.
| Subtotal gastrectomy (n = 71) | Total gastrectomy (n = 9) | P value | |
| Age (yr) | 58.5 ± 11.9 | 56.5 ± 13.2 | 0.641 |
| Sex | |||
| Male | 37 (52.1) | 6 (66.7) | 0.409 |
| Female | 34 (47.9) | 3 (33.3) | |
| Cancer Stage | 0.003 | ||
| I | 57 (81.4) | 5 (55.6) | |
| II | 8 (11.4) | 0 (0) | |
| III | 2 (2.9) | 3 (33.3) | |
| IV | 3 (4.3) | 1 (11.1) | |
| Complications | 0.219 | ||
| Major | 2 (2.8) | 1 (11.1) | |
| Minor | 24 (33.8) | 1 (11.1) | |
| Hospital stay (d) | 12.8 ± 8.4 | 16.4 ± 6.8 | 0.223 |
We assessed the nutritional status and laboratory parameters of patients within 24 h of their hospital admission. The prevalence of malnutrition at admission was 31% when determined with the SGA (moderately and severely malnourished) or 43% when determined with the NRS-2002 (medium and high risk). The frequency of any degree of malnutrition at admission was 31% according to the NRI (mild, moderate, and severe malnutrition). There was no difference in age or TLC between malnourished and well-nourished groups defined according to the three assessments, but the percentage weight loss differed between the groups (Table 2). The anthropometric data were lower in the malnourished groups based on SGA and NRS-2002 assessments, but did not differ between the groups defined with the NRI assessment. Albumin, protein, and total cholesterol levels differed between the malnourished and well-nourished groups based on the NRI assessment, but there was no significant difference between the groups defined with the SGA and NRS-2002 techniques (Table 2).
| SGA | NRS-2002 | NRI | |||||||
| Well-nourished (n = 55) | Malnourished (n = 25) | P value | Well-nourished (n = 45) | Malnourished (n = 35) | P value | Well-nourished (n = 55) | Malnourished (n = 25) | P value | |
| Age (yr) | 57.58 ± 11.5 | 60.00 ± 13.2 | 0.410 | 56.53 ± 9.9 | 60.65 ± 14.1 | 0.130 | 58.07 ± 12.3 | 58.92 ± 11.6 | 0.769 |
| Weight (kg) | 62.04 ± 9.1 | 59.30 ± 8.3 | 0.207 | 63.64 ± 8.4 | 58.03 ± 8.6 | 0.005 | 61.80 ± 9.0 | 59.83 ± 8.7 | 0.360 |
| Weight loss (%) | 1.03 ± 1.5 | 7.31 ± 4.5 | 0.000 | 0.89 ± 1.4 | 6.42 ± 4.6 | 0.000 | 2.71 ± 3.1 | 5.32 ± 5.9 | 0.047 |
| TSF (mm) | 17.88 ± 7.5 | 13.72 ± 6.5 | 0.020 | 18.25 ± 7.8 | 14.44 ± 6.4 | 0.020 | 16.99 ± 8.0 | 15.69 ± 6.2 | 0.435 |
| MAC (cm) | 27.95 ± 2.5 | 26.70 ± 2.3 | 0.040 | 28.37 ± 2.4 | 26.51 ± 2.2 | 0.001 | 27.81 ± 2.6 | 26.99 ± 2.1 | 0.155 |
| BMI (kg/m2) | 24.26 ± 2.7 | 22.47 ± 2.6 | 0.008 | 24.69 ± 2.4 | 22.42 ± 2.7 | 0.000 | 23.88 ± 2.9 | 23.29 ± 2.6 | 0.366 |
| Albumin (g/dL) | 3.86 ± 0.3 | 3.85 ± 0.3 | 0.924 | 3.87 ± 0.3 | 3.84 ± 0.3 | 0.618 | 4.02 ± 0.2 | 3.50 ± 0.1 | 0.000 |
| Total protein (g/dL) | 6.89 ± 0.6 | 6.75 ± 0.6 | 0.391 | 6.88 ± 0.6 | 6.80 ± 0.6 | 0.568 | 7.13 ± 0.5 | 6.23 ± 0.4 | 0.000 |
| Total cholesterol (mg/dL) | 169.65 ± 42.3 | 167.8 ± 42.5 | 0.863 | 166.04 ± 42.2 | 173.02 ± 42.2 | 0.466 | 176.4 ± 39.6 | 152.8 ± 43.6 | 0.026 |
| TLC (× 103/mm3) | 1856.6 ± 503 | 1905.9 ± 569 | 0.698 | 1874.7 ± 536 | 1868.6 ± 511 | 0.959 | 1903.9 ± 511 | 1801.8 ± 549 | 0.698 |
Malnutrition scores correlated significantly with the percentage weight loss according to the SGA and NRS-2002 groupings. BMI and anthropometric data correlated inversely in the SGA and NRS-2002 groupings, but did not correlate in the NRI grouping, which correlated inversely with the nutrition factors albumin, protein, and total cholesterol (Table 3). Concordance between the SGA and NRS-2002 assessments was observed in 68 of the 80 (85%) patients, but was not observed between the SGA and NRI assessments in 50 of the 80 (63%) patients (Table 4). Sensitivity was 80% with the NRS-2002 and 73% with the NRI. Specificity was 96% and 40% with the NRS-2002 and NRI, respectively. Agreement was higher between the SGA and NRS-2002 (κ = 0.685, P = 0.000) than between the SGA and NRI (κ = 0.127, P = 0.255) (Table 4).
| SGA1 | NRS-20022 | NRI3 | ||||
| r | P value | r | P value | r | P value | |
| Age (yr) | 0.118 | 0.297 | 0.246 | 0.028 | 0.035 | 0.758 |
| Weight (kg) | -0.132 | 0.243 | -0.314 | 0.005 | -0.091 | 0.425 |
| Weight loss (%) | 0.754 | 0.000 | 0.690 | 0.000 | 0.199 | 0.166 |
| TSF (mm) | -0.272 | 0.015 | -0.234 | 0.037 | -0.048 | 0.669 |
| MAC (cm) | -0.228 | 0.042 | -0.378 | 0.001 | -0.170 | 0.132 |
| BMI (kg/m2) | -0.279 | 0.012 | -0.393 | 0.000 | -0.109 | 0.335 |
| Albumin (g/dL) | 0.004 | 0.971 | -0.043 | 0.703 | -0.783 | 0.000 |
| Total protein (g/dL) | -0.086 | 0.448 | -0.062 | 0.583 | -0.636 | 0.000 |
| Total cholesterol (mg/dL) | -0.006 | 0.955 | 0.088 | 0.436 | -0.285 | 0.010 |
| TLC (× 103/mm3) | 0.038 | 0.738 | -0.008 | 0.943 | -0.116 | 0.305 |
| NRS-2002 | NRI | |||||
| Low | Medium/high | Total | Low | Medium/high | Total | |
| SGA well-nourished | 44 | 11 | 55 | 40 | 15 | 55 |
| SGA malnourished | 1 | 24 | 25 | 15 | 10 | 25 |
| Total | 45 | 35 | 80 | 55 | 25 | 80 |
| Sensitivity | 80.0% (44/55) | 72.7% (40/55) | ||||
| Specificity | 96.0% (24/25) | 40.0% (10/25) | ||||
| κ = 0.685, P = 0.000 | κ = 0.127, P = 0.255 | |||||
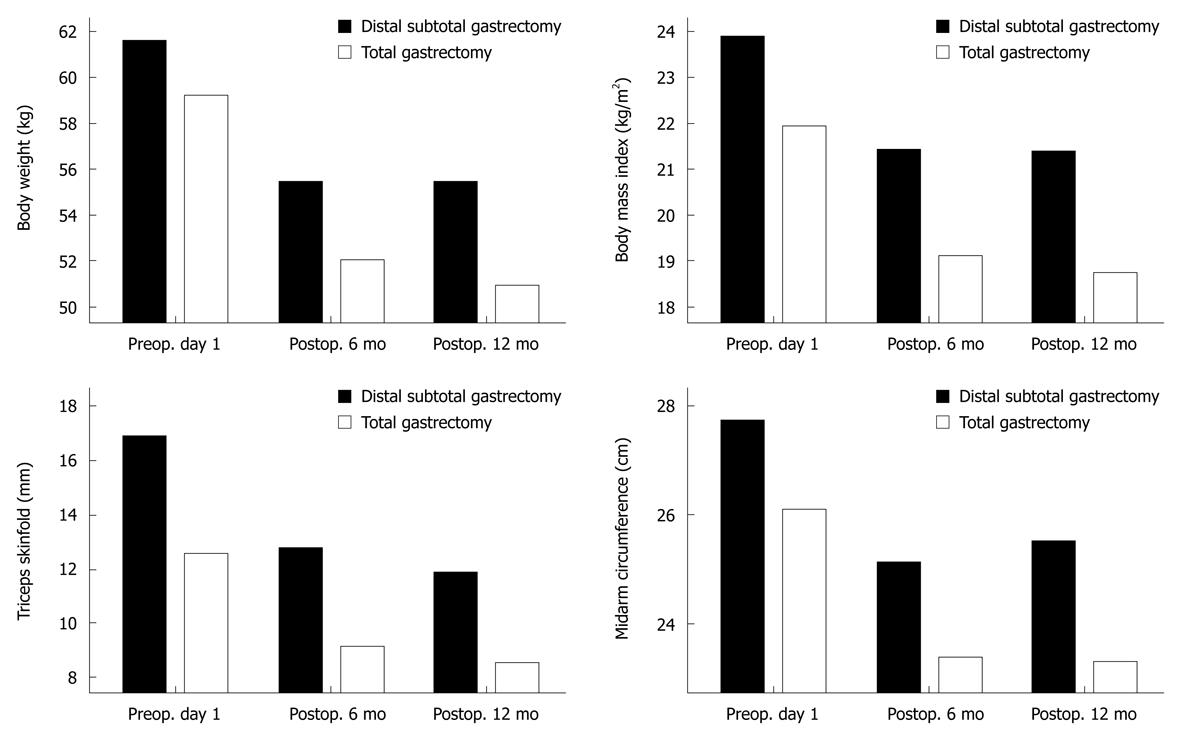
At 6 and 12 mo after surgery, BW, BMI, TSF and MAC were significantly reduced, whereas the TLC, and albumin, protein, cholesterol and serum iron levels did not decrease during the postoperative period (Table 5). The nutritional status of the patients who had undergone subtotal gastrectomy stabilized 6 mo after surgery, but the total gastrectomy patients showed a significantly reduced nutritional status in terms of BW, BMI and anthropometric measurements 12 mo after surgery (Figure 1).
| Preoperative | Postoperative | Postoperative | P value | |
| day 1 | 6 mo | 12 mo | ||
| Weight (kg) | 61.4 ± 8.8 | 55.4 ± 8.2 | 55.2 ± 8.6 | 0.000 |
| TSF (mm) | 17.0 ± 7.5 | 12.7 ± 6.5 | 12.1 ± 6.2 | 0.000 |
| MAC (cm) | 27.7 ± 2.5 | 25.1 ± 4.9 | 25.5 ± 2.4 | 0.000 |
| BMI (kg/m2) | 23.9 ± 2.7 | 21.4 ± 2.4 | 21.5 ± 2.4 | 0.000 |
| Albumin (g/dL) | 3.88 ± 0.3 | 4.28 ± 0.2 | 4.30 ± 0.2 | 0.000 |
| Total protein (g/dL) | 6.87 ± 0.6 | 7.18 ± 0.5 | 7.25 ± 0.5 | 0.000 |
| Total cholesterol (mg/dL) | 170.9 ± 37.4 | 168.2 ± 38.1 | 173.3 ± 31.4 | 0.679 |
| TLC (× 103/mm3) | 1863 ± 493 | 1799 ± 633 | 1752 ± 578 | 0.328 |
| Serum iron (μg/dL) | 72.9 ± 37.6 | 110.4 ± 52.8 | 118.2 ± 57.8 | 0.000 |
| Serum ferritin (µg/L) | 77.7 ± 75.3 | 74.9 ± 81.3 | 60.2 ± 64.4 | 0.358 |
| Vitamin B12 (pg/mL) | 686.5 ± 249 | 666.8 ± 281 | 709.1 ± 330 | 0.731 |
At 6 mo after surgery, a good correlation was observed between the results of the nutritional assessment tools (SGA, and NRS-2002) and those of the other nutritional measurement tools (BW, BMI, and anthropometric measurements).
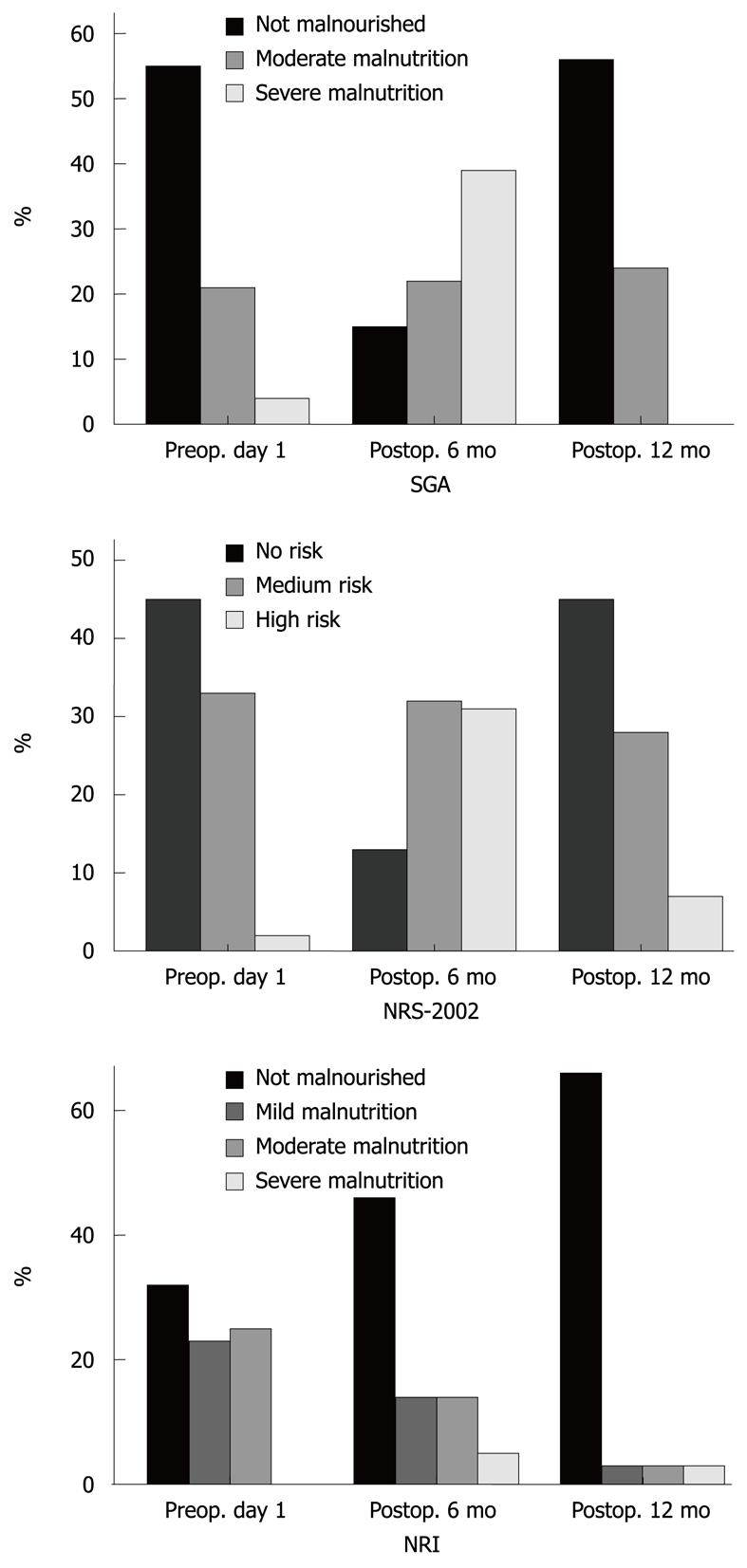
According to the SGA and NRS-2002, the proportion of malnourished patients was 80% and 83%, respectively, 6 mo after surgery. At 12 mo after surgery, most patients who had been assessed as malnourished by SGA and NRS-2002 had returned to their preoperative status (Figure 2), although the other nutritional measurement tools (BW, BMI, and anthropometric measurements) still showed a malnourished status.
Nutritional depletion is a common problem in critically ill patients with cancer and is associated with a poor outcome. It is specifically reversible with nutritional support. Several studies have shown that patients with malignant gastrointestinal disease have a higher prevalence of weight loss before surgery, and during the first postoperative months, an additional weight loss of approximately 10% has been reported[7,18-21]. Plausible reasons for the development of malnutrition are a reduced food intake because of poor appetite, postprandial symptoms, and malabsorption[22,23]. Hospitalization, surgery, and chemo/radiotherapy can also cause malnutrition. In this study, the overall prevalence of malnutrition in patients with gastric cancer at admission was 31% according to the SGA and 43% according to the NRS-2002. Based on the objective assessment techniques, BW loss, BMI and anthropometric data were lower in the malnourished groups.
The purpose of nutritional screening is to identify those patients who are at nutritional risk and therefore at higher risk of complications. Malnutrition in hospitalized patients is a critical issue and has been associated with a significant increase in morbidity and mortality[9,10,24]. The detection of malnourished patients is possible if the importance of the issue is understood and the patient’s nutritional status is evaluated on admission to hospital. Multiple clinical parameters are available to assess the nutritional status of critically ill patients, but no standard recommendation can be made at this time. Each method has its own advantages and disadvantages[25].
A traditional nutritional assessment often includes dietary and medical evaluations to identify significant weight loss over time, significantly low or high BW or BMI, reduction in MAC, SFT, serum protein levels, or immune competence, and functional measurements of muscle strength may be incorporated into the overall final assessment[26]. Individually, these measurements often have limited value in accurately determining a patient’s nutritional risk. Studies have consistently revealed the inadequacy of any single assessment method or tool in evaluating a patient’s nutritional status. An effective nutritional screening tool will generally combine both objective and subjective factors.
In this analysis of the preoperative and postoperative anthropometric p arameters of patients with gastric cancer, an interesting observation was that, although the mean BMI was within the normal range, malnutrition scores correlated significantly with the percentage weight loss according to the SGA and NRS-2002. This means that BMI alone is not sufficient to determine the real malnutrition rate. Aydin et al[27] reported that a patient can be malnourished even when the BMI is normal and that the SGA can detect malnutrition before the BMI drops below 20 kg/m2. For this reason, it is very important to use several methods in combination to evaluate a patient’s nutritional status.
Albumin is commonly considered a good marker of nutritional status and visceral protein stores. The NRI is derived with an equation from the serum albumin concentration and the ratio of the actual to the usual weight. In this study, serum protein and albumin levels correlated statistically with the malnourished and well-nourished groups based on NRI assessment, but there was no statistical correlation with the groups defined by the SGA or NRS-2002. From this result, it can be inferred that albumin and serum protein parameters are not as sensitive as anthropometric measurements in the evaluation of nutritional status. Some studies have demonstrated that low serum albumin concentrations correlate with longer hospital stays, medical complications, and increased mortality[28,29], whereas other studies have reported that low serum protein levels do not always indicate malnutrition and malnutrition does not always accompany low serum protein levels[27,30,31]. Usually, many serum proteins and albumin are affected by the inflammatory response, liver disease, cancer, or idiopathically[32]. For these reasons, hypoalbuminemia has been reported to be a predictor of risk in a broad sense, rather than a parameter that indicates malnutrition[33]. Therefore, there are arguments for discounting hypoalbuminemia as a marker of malnutrition because patient populations differ. In this study, we evaluated only preoperative patients with gastric cancer who had no other serious medical problems. There was no good correlation between the NRI and objective assessments. The concordance and agreement were higher between the SGA and NRS-2002 than between the SGA and NRI. In this respect, the NRI may not be specific for the diagnosis of malnutrition in preoperative cancer patients.
Weight loss is a common problem after gastrectomy. The main mechanisms implicated include impaired food intake and malabsorption[34]. Patients who undergo gastrectomy consume fewer calories during the first 3-6 mo after surgery, after which their intake improves[20]. In this study, mean BW, BMI, TSF, and MAC were significantly reduced from the time of hospital discharge until 6 mo after surgery. Conversely, serum albumin levels, total protein, cholesterol, and TLC were similar between the groups before and 6 mo after surgery.
According to the subjective assessment of nutritional status 6 mo after surgery, 80% and 83% of the patients were malnourished according to the SGA and NRS-2002, respectively, compared with 31% and 43% of patients who were malnourished on preoperative day 1, respectively. In the group of patients who underwent subtotal gastrectomy, the patients’ anthropometric parameters did not change between 6 and 12 mo after surgery. At 12 mo after surgery, their nutritional status was assessed as similar to its preoperative value according to the SGA, NRS-2002 and NRI, but their objective nutritional parameters were still low, especially mean BW, BMI, TSF, and MAC. Subjective assessment is a validated method of nutritional assessment when based on a medical history (weight change, dietary intake change, gastrointestinal symptoms, changes in functional capacity) and physical examination (loss of subcutaneous fat, muscle wasting). Therefore, according to the SGA and NRS-2002, the proportion of malnourished patients was high at 6 mo after surgery, but weight loss was not significant between 6 and 12 mo after surgery. Most of the patients who were assessed as malnourished had returned to their preoperative status, although the other nutritional measurement tools (BW, BMI, and anthropometric measurements) still indicated a malnourished status.
Patients with malignant gastrointestinal disease have a high prevalence of malnutrition. In cancer, reduced food intake and an increased energy gap result in the deterioration of nutritional status. It is very important to detect malnourished patients during the preoperative period and postoperative follow-up. Not only objective nutritional parameters but also subjective assessments have some limitations in the accurate measurement of nutritional status. Therefore, measuring the nutritional status of patients who have undergone gastrectomy requires a combination of objective variables (anthropometric and laboratory measurements) and a subjective scoring system during the postoperative follow-up period.
Nutritional depletion is a common problem in critically ill patients with cancer and is associated with a poor outcome. The assessment of nutritional status and its evaluation plays an important role in tailoring nutritional support. Multiple clinical parameters are available to assess the nutritional status of gastric cancer patients, but no standard recommendation can be made at this time. This study would suggest that a specific tailored nutritional assessment is needed for the accurate measurement of nutritional status in patients.
A traditional nutritional assessment often includes dietary and medical evaluations to identify significant weight loss over time, significantly low or high body weight, skinfold thickness, serum nutritional factor levels and functional measurements of muscle strength. Individually, these measurements often have limited value in accurately determining a patient’s nutritional risk. As a result, combinations of diverse measurements have been developed into subjective scoring systems [subjective global assessment (SGA) and nutritional risk screening (NRS-2002)] designed to increase the sensitivity and specificity of nutritional status determinations. Scoring systems have been based on objective measurements of nutritional status, such as oral energy intake, body weight, weight loss over time, loss of subcutaneous fat, muscle wasting, serum protein levels, and immune competence.
When the authors analyzed the nutritional status in gastric cancer patients after gastrectomy surgery, body weight (BW), body mass index (BMI) and fat thickness were significantly reduced, but the total lymphocyte count, albumin, protein, cholesterol and serum iron levels did not decrease during the postoperative period. From this result, it can be inferred that albumin and serum protein parameters are not as sensitive as anthropometric measurements in the evaluation of nutritional status. Six months after surgery, there was a good correlation between the scoring nutritional assessment tools and the other general nutritional measurement tools (BW, BMI, and anthropometric measurements). However, 12 mo after surgery, most patients who were assessed as malnourished by the scoring nutritional assessment tool had returned to their preoperative normal nutritional status, although their BW, BMI, and anthropometric measurements still indicated a malnourished status.
The authors studied the prevalence of preoperative and postoperative malnutrition in patients with gastric cancer who underwent radical gastrectomy. This is the first study to report on the relationship between nutritional assessment tools and the nutritional status of gastric cancer patients after gastrectomy. From this study, not only objective nutritional parameters but also subjective scoring assessments have some limitations in the accurate measurement of nutritional status. Therefore, measuring the nutritional status of patients who have undergone gastrectomy requires a combination of objective variables (anthropometric and laboratory measurements) and a subjective scoring system during the postoperative follow-up period.
This is a nicely written paper and well executed small study. There is enough presented to alert clinicians to both the problem of malnutrition in the sample studies and the problem of nutritional assessment tools used. The combination of assessment tools should allow for improvement in the identification of at risk patients.
Peer reviewer: Wallace F Berman, MD, Professor, Division of Pediatric GI/Nutrition, Department of Pediatrics, Duke University Medical Center, Duke University School of Medicine, Durham, Box 3009, NC 27710, United States
S- Editor Wang YR L- Editor Webster JR E- Editor Ma WH
| 1. | Walesby RK, Goode AW, Spinks TJ, Herring A, Ranicar AS, Bentall HH. Nutritional status of patients requiring cardiac surgery. J Thorac Cardiovasc Surg. 1979;77:570-576. [Cited in This Article: ] |
| 2. | Bistrian BR, Blackburn GL, Vitale J, Cochran D, Naylor J. Prevalence of malnutrition in general medical patients. JAMA. 1976;235:1567-1570. [Cited in This Article: ] |
| 3. | Agradi E, Messina V, Campanella G, Venturini M, Caruso M, Moresco A, Giacchero A, Ferrari N, Ravera E. Hospital malnutrition: incidence and prospective evaluation of general medical patients during hospitalization. Acta Vitaminol Enzymol. 1984;6:235-242. [Cited in This Article: ] |
| 4. | Waitzberg DL, Cordeiro AC, Faintuch J, Gama-Rodrigues J, Habr-Gama A. [Nutritional status in pre and immediate postoperative periods in patients with digestive diseases]. Rev Paul Med. 1983;101:7-13. [Cited in This Article: ] |
| 5. | Doerr TD, Marks SC, Shamsa FH, Mathog RH, Prasad AS. Effects of zinc and nutritional status on clinical outcomes in head and neck cancer. Nutrition. 1998;14:489-495. [Cited in This Article: ] |
| 6. | Allison SP. Malnutrition, disease, and outcome. Nutrition. 2000;16:590-593. [Cited in This Article: ] |
| 7. | Copland L, Liedman B, Rothenberg E, Bosaeus I. Effects of nutritional support long time after total gastrectomy. Clin Nutr. 2007;26:605-613. [Cited in This Article: ] |
| 8. | Tian J, Chen JS. Nutritional status and quality of life of the gastric cancer patients in Changle County of China. World J Gastroenterol. 2005;11:1582-1586. [Cited in This Article: ] |
| 9. | Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition. 1996;12:23-29. [Cited in This Article: ] |
| 10. | Hill GL, Blackett RL, Pickford I, Burkinshaw L, Young GA, Warren JV, Schorah CJ, Morgan DB. Malnutrition in surgical patients. An unrecognised problem. Lancet. 1977;1:689-692. [Cited in This Article: ] |
| 11. | Wong PW, Enriquez A, Barrera R. Nutritional support in critically ill patients with cancer. Crit Care Clin. 2001;17:743-767. [Cited in This Article: ] |
| 12. | Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26:1SA-138SA. [Cited in This Article: ] |
| 13. | Jones HJ, de Cossart L. Risk scoring in surgical patients. Br J Surg. 1999;86:149-157. [Cited in This Article: ] |
| 14. | Schneider SM, Hebuterne X. Use of nutritional scores to predict clinical outcomes in chronic diseases. Nutr Rev. 2000;58:31-38. [Cited in This Article: ] |
| 15. | Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325:525-532. [Cited in This Article: ] |
| 16. | Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8-13. [Cited in This Article: ] |
| 17. | Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415-421. [Cited in This Article: ] |
| 18. | Braga M, Molinari M, Zuliani W, Foppa L, Gianotti L, Radaelli G, Cristallo M, Di Carlo V. Surgical treatment of gastric adenocarcinoma: impact on survival and quality of life. A prospective ten year study. Hepatogastroenterology. 1996;43:187-193. [Cited in This Article: ] |
| 19. | Hyltander A, Bosaeus I, Svedlund J, Liedman B, Hugosson I, Wallengren O, Olsson U, Johnsson E, Kostic S, Henningsson A. Supportive nutrition on recovery of metabolism, nutritional state, health-related quality of life, and exercise capacity after major surgery: a randomized study. Clin Gastroenterol Hepatol. 2005;3:466-474. [Cited in This Article: ] |
| 20. | Liedman B, Andersson H, Berglund B, Bosaeus I, Hugosson I, Olbe L, Lundell L. Food intake after gastrectomy for gastric carcinoma: the role of a gastric reservoir. Br J Surg. 1996;83:1138-1143. [Cited in This Article: ] |
| 21. | Takahashi S, Maeta M, Mizusawa K, Kaneko T, Naka T, Ashida K, Tsujitani S, Kaibara N. Long-term postoperative analysis of nutritional status after limited gastrectomy for early gastric cancer. Hepatogastroenterology. 1998;45:889-894. [Cited in This Article: ] |
| 22. | Bae JM, Park JW, Yang HK, Kim JP. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg. 1998;22:254-260; discussion 260-261. [Cited in This Article: ] |
| 23. | Iivonen MK, Ahola TO, Matikainen MJ. Bacterial overgrowth, intestinal transit, and nutrition after total gastrectomy. Comparison of a jejunal pouch with Roux-en-Y reconstruction in a prospective random study. Scand J Gastroenterol. 1998;33:63-70. [Cited in This Article: ] |
| 24. | Naber TH, Schermer T, de Bree A, Nusteling K, Eggink L, Kruimel JW, Bakkeren J, van Heereveld H, Katan MB. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66:1232-1239. [Cited in This Article: ] |
| 25. | Waitzberg DL, Correia MI. Nutritional assessment in the hospitalized patient. Curr Opin Clin Nutr Metab Care. 2003;6:531-538. [Cited in This Article: ] |
| 26. | DeLegge MH, Drake LM. Nutritional assessment. Gastroenterol Clin North Am. 2007;36:1-22, v. [Cited in This Article: ] |
| 27. | Aydin N, Karaöz S. Nutritional assessment of patients before gastrointestinal surgery and nurses’ approach to this issue. J Clin Nurs. 2008;17:608-617. [Cited in This Article: ] |
| 28. | Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36-42. [Cited in This Article: ] |
| 29. | Delgado-Rodríguez M, Medina-Cuadros M, Gómez-Ortega A, Martínez-Gallego G, Mariscal-Ortiz M, Martinez-Gonzalez MA, Sillero-Arenas M. Cholesterol and serum albumin levels as predictors of cross infection, death, and length of hospital stay. Arch Surg. 2002;137:805-812. [Cited in This Article: ] |
| 30. | Shenkin A. Impact of disease on markers of macronutrient status. Proc Nutr Soc. 1997;56:433-441. [Cited in This Article: ] |
| 31. | Young VR, Marchini JS, Cortiella J. Assessment of protein nutritional status. J Nutr. 1990;120 Suppl 11:1496-1502. [Cited in This Article: ] |
| 32. | Hill GL. Surgical nutrition: time for some clinical common sense. Br J Surg. 1988;75:729-730. [Cited in This Article: ] |
| 33. | Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20:265-269. [Cited in This Article: ] |
| 34. | Armbrecht U, Lundell L, Lindstedt G, Stockbruegger RW. Causes of malabsorption after total gastrectomy with Roux-en-Y reconstruction. Acta Chir Scand. 1988;154:37-41. [Cited in This Article: ] |