Published online Jan 14, 2010. doi: 10.3748/wjg.v16.i2.167
Revised: October 26, 2009
Accepted: November 2, 2009
Published online: January 14, 2010
AIM: To investigate whether probiotic bacteria, given perioperatively, might adhere to the colonic mucosa, reduce concentration of pathogens in stools, and modulate the local immune function.
METHODS: A randomized, double-blind clinical trial was carried out in 31 subjects undergoing elective colorectal resection for cancer. Patients were allocated to receive either a placebo (group A, n = 10), or a dose of 107 of a mixture of Bifidobacterium longum (BB536) and Lactobacillus johnsonii (La1) (group B, n = 11), or the same mixture at a concentration of 109 (group C, n = 10). Probiotics, or a placebo, were given orally 2 doses/d for 3 d before operation. The same treatment continued postoperatively from day two to day four. Stools were collected before treatment, during surgery (day 0) and 5 d after operation. During the operation, colonic mucosa samples were harvested to evaluate bacterial adherence and to assess the phenotype of dendritic cells (DCs) and lymphocyte subsets by surface antigen expression (flow cytometry). The presence of BB536 and La1 was evaluated by the random amplified polymorphism DNA method with specific polymerase chain reaction probes.
RESULTS: The three groups were balanced for baseline and surgical parameters. BB536 was never found at any time-points studied. At day 0, La1 was present in 6/10 (60%) patients in either stools or by biopsy in group C, in 3/11 (27.2%) in group B, and none in the placebo group (P = 0.02, C vs A). There was a linear correlation between dose given and number of adherent La1 (P = 0.01). The rate of mucosal colonization by enterobacteriacae was 30% (3/10) in C, 81.8% (9/11) in B and 70% (7/10) in A (P = 0.03, C vs B). The Enterobacteriacae count in stools was 2.4 (log10 scale) in C, 4.6 in B, and 4.5 in A (P = 0.07, C vs A and B). The same trend was observed for colonizing enterococci. La1 was not found at day +5. We observed greater expression of CD3, CD4, CD8, and naive and memory lymphocyte subsets in group C than in group A with a dose response trend (C > B > A). Treatment didnot affect DC phenotype or activation, but after ex vivo stimulation with lipopolysaccharides, groups C and B had a lower proliferation rate compared to group A (P = 0.04). Moreover, dendritic phenotypes CD83-123, CD83-HLADR, and CD83-11c (markers of activation) were significantly less expressed in patients colonized with La1 (P = 0.03 vs not colonized).
CONCLUSION: La1, but not BB536, adheres to the colonic mucosa, and affects intestinal microbiota by reducing the concentration of pathogens and modulates local immunity.
- Citation: Gianotti L, Morelli L, Galbiati F, Rocchetti S, Coppola S, Beneduce A, Gilardini C, Zonenschain D, Nespoli A, Braga M. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol 2010; 16(2): 167-175
- URL: https://www.wjgnet.com/1007-9327/full/v16/i2/167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i2.167
Probiotics are mono or mixed cultures of live microorganisms that might beneficially affect the host by improving the characteristics of indigenous microflora[1]. The composition and the equilibrium of microbiota are known to influence important host activities, including the local immune response and several intestinal metabolic traits[2-5].
For therapeutic purposes, probiotics should have certain features: to be of human origin, safe for the host, and genetically stable[1,6]. Furthermore, it is important that probiotics, in order to be active, survive passage through the gastrointestinal (GI) tract irrespective of gastric acids, pancreatic enzymes, and bile acids so that they can reach the ileum and colon and colonize the intestinal mucosa and stools[6,7].
Although the effects of probiotic administration has been intensively investigated in vitro, in animal models, in healthy volunteers, and in some human GI diseases (i.e. inflammatory bowel diseases, alimentary allergy, infectious diarrhea, and pouchitis)[1-6] very little is known on the possible cross-interactions among probiotic administration, changes of intestinal flora, and local immune response in surgical patients. Probiotic administration in patients undergoing GI operations has been attempted to obtain a competitive action on microbiota that contain bacteria responsible for postoperative infections. The results of randomized clinical trials (RCTs) are inconsistent with significant reduction of infection rate in upper GI surgery[8-10] and show a lack of clinical benefits in other types of operations and clinical settings[11-13]. This might be due to the substantial differences in study design, probiotic dose and strain, duration, period and combination of treatments, and particularly, the paucity of preliminary phase II studies investigating in detail the relationship between probiotics and changes in intestinal pathophysiology.
The objectives of the study were to investigate whether peri-operative probiotic bacteria could adhere to the colonic mucosa, to assess whether this treatment could change the gut microflora by reducing potentially pathogenic bacteria, and whether the gut immune response could be modulated in a selected cohort of patients undergoing colorectal resection for cancer.
This was a prospective, randomized, controlled, double-blind trial of three groups in parallel. The study was carried out in two university hospitals (Department of Surgery, San Gerardo Hospital, Milano-Bicocca University, Monza, Italy and Department of Surgery, San Raffaele Hospital, Vita e Salute University, Milan, Italy).
Eligible patients were those with histological documentation of cancer of the colon or rectum. Inclusion criteria were: both genders, age between 18 and 80 years and candidate for elective colorectal resection. The exclusion criteria were: denied written informed consent, no collection of a stool sample 4 d before the operation, unresectable tumor, neoplastic ascitis, clinically relevant pulmonary, cardiovascular, hepatic and kidney dysfunction or failure, ongoing total parenteral nutrition, immunological disorders, ongoing or recent infections (within last 30 d), pregnancy, and participation to another clinical trial. After applying these criteria, patients were allocated by an individual random number into three study arms.
Concealment assignment was by central randomization by computer. Both probiotic and placebo preparations were in foil sealed sachets that were stored in identical numbered containers. The study products and the placebo were both white powders, identical in weight, smell, and taste. Thus, the identity of the specific product was blind to participants, support staff and investigators for the entire duration of the study period.
Individually numbered treatment packs were allocated to the subjects as per the randomization schedule. Randomization was done by a program trial balance. Balancing variables were sex (male and female) and age (18-54 years and 55-80 years). All data captured by the investigator were recorded directly on the case report forms (CRF). Data entering, from CRFs into a computer database, was blinded. The blind codes were broken after all the collected data were analyzed. All data were analyzed by intention-to-treat.
The Ethical Committees of both hospitals approved the protocol.
The trial was registered at ClinicalTrial.gov of the National Institute of Health with the identifier number NCT00936572.
Four days before the scheduled surgery, subjects were randomly assigned to one of three groups: Placebo (n = 10); oral treatment with low dose of probiotics every 12 h [total dose: 2 × 107 colony forming units (CFU)/d, n = 11]; oral treatment with high dose of probiotics (2 × 109 CFU/d, n = 10). Treatments were composed of a mixture 1:1 of Lactobacillus johnsonii (L. johnsonii) (La1) and Bifidobacterium longum (B. longum) (BB536) in spray-dried form and blended with maltodextrin. The placebo was maltodextrin only. Both probiotics and placebo were mixed in 100 mL of a nutritional supplement (Clinutren 1.5, Nestle Nutrition, Milan, Italy) before drinking. All groups received treatments or placebo for three consecutive days before surgery (from day -3 to day -1 included). Treatments or placebo resumed postoperatively on day +2 until day +4 for a total of 6 d of treatment (12 doses).
Two mucosal samples were collected during surgery for probiotic adherence testing, microbiological evaluation of microbiota, and immune parameters. Stool samples were collected before treatment initiation (day -4), during surgery (day 0) and postoperatively at day + 5 and analyzed as described below.
All patients received a single dose of prophylactic antibiotic (Cefoxitin, single dose, 30 min before incision).
Bowel preparation was done by an isosmotic solution (Macrogol; 3L) the evening before operation after the last preoperative dose of probiotics.
Weighed feces samples and mucosa (1 g) were homogenized for 1.5 min in a stomacher (PBI, Milan, Italy) before dilution in saline solution (9 g/L NaCl). Appropriate dilutions were plated using Rogosa Acetate agar (Difco) to enumerate Lactobacillus spp. Bifidobacteria isolates were enumerated using BSM media (MRS Broth, Bacto agar, Difco), 0.5 g/L of L-Cysteine Hydrochloride (Merck), while Enterobacteriaceae were counted on MacConkey Agar (Oxoid), enterococci on Bile Esculin Azide agar (Oxoid), and Clostridium perfringens (C. perfringens) was counted on Neomycin Nagler Agar (Eiken Chemical Co., Tokyo). Plates for Enterobacteriaceae and enterococci were incubated at 37°C for 24 h aerobically while lactobacilli, bifidobacteria and C. perfringens were incubated at 37°C for 48 h in anaerobic jars (GasPak, BBL, Coskeysville, MD, USA).
Counts of the CFU were performed for all countable plates (containing 30-300 CFU). Randomly selected CFU of lactobacilli and bifidobacteria (about 15% of colonies counted on readable plates) were isolated and cultivated in MRS broth and MRS broth with 0.5 g/L of L-Cysteine Hydrochloride, respectively to identify the L. johnsonii and B. longum species, and subsequent the identification of La1 and BB536 strains.
An overnight culture was collected by centrifugation at 5000 r/min for 10 min, the pellet was dissolve in 1 mL 0.9% NaCl and transferred to a tube containing 0.5 g of glass beads (Sigma, St. Louis, Mo.). Cell lysis was performed with the Mini-Beadbeater (Biospec product) for three min at maximum speed. Subsequently, the suspension was centrifuged for five min at 10 000 r/min and 1 μL of the supernatant was used directly for polymerase chain reaction (PCR).
Detection of L. johnsonii isolates was performed with primers LJ1 (GATGATTTTAGTTCTTGCACTAA) and P6 (CTACGGCTACCTTGTTACGA) using conditions described by Ventura et al[14], while B. longum isolates were detected with primers Blon1 (5'-TTCCAGTTGATCGCATGGTC-3') and Blon2 (5'-GGGAAGCCGTATCTCTACGA-3') with conditions described by Mullié et al[15]. All amplification reactions were performed in a total volume of 25 μL containing 200 μmol/L of each deoxynucleoside triphosphate, 2.5 U of Taq (Gold), 10 pmol of each primer, and 1 μL of the respective template DNA (which equaled about 20 ng of DNA). The PCR reactions were carried out in a Gene Amp 9700 thermal cycler (Applied Biosystem, Foster City, USA). The resulting amplicons were visualized under UV light in 1% and 1.5% (w/v) agarose (Bio-Rad Laboratories, Milan, Italy) electrophoresis gels, respectively for L. johnsonii and B. longum, followed by subsequent 0.5 μg/mL ethidium bromide staining.
Detection of L. johnsonii La1 strains was performed using the primers NCCE722 (GCATCATGCCCTTGAGTAGC) and NCCE723 (AATGCCCACTTTTTGGCCTC) while the B. longum BB536 strains detection was carried out using the primers NCC3001-A (5'-GAACAGGGTGTGCTGAGTGA-3') and NCC3001-B (5'-CAAGCGAGAAGATCATCGAA-3'), both of them provided by Nestec Ltd. All amplification reactions were carried out in a total volume of 25 μL containing 200 μmol/L of each deoxynucleoside triphosphate, 2.5 U of Taq (Gold), 10 pmol of each primer, and 1 μL of the respective template DNA. The PCR reactions were carried out in a Gene Amp 9700 thermal cycler. Amplification cycle for La1 was as follows: initial denaturation was performed at 94°C for five min, followed by 35 cycles of: 94°C for 30 s, 53°C for 30 s and 68°C for 1 min, and a final extension at 68°C for 7 min. The conditions for BB536 were: 94°C for five min, followed by 30 cycles of: 94°C for 30 s, 60 for 30 s and 72°C for 30 s, and a final extension at 72°C for 5 min. The 540 bp La1 PCR products were analyzed on 1.5% (w/v) agarose gels, while the 461 bp BB536 PCR products were analyzed on 1.5% (w/v) agarose gels, both at 80 V, using a 200 bp ladder (Promega Corporation, Madison, USA) for molecular weight standards.
Colon DCs and lymphocytes were isolated from specimens of healthy mucosa (> 10 cm from neoplasm) as previously described[16]. Briefly, after surgical excision of the colon, samples of mucosa and submucosa were separated mechanically from the muscular tunicae. Filtration through nylon mesh was used to isolate enterocytes. The enterocytes were discarded, and the remaining tissue was resuspended in medium with enzymes (liberase, DNAase, and hyaluronidase). The solution was centrifuged and the cells (containing lamina propria DCs and lymphocytes) were resuspended in HBSS before separation on a Percoll gradient. The Percoll gradient allowed separation of the lamina propria DCs from lymphocytes. Cells were washed with phosphate buffered saline and their phenotype was analysed by fluorescence activated cell sorter analysis.
DC and lymphocyte phenotypes were analyzed using antibodies to surface markers (CD11c: dendritic myeloid; CD123: dendritic plasmocytoid; CD HLA-DR: dendritic activated; CD83: dendritic mature; CD3: T cells; CD4: T helper; CD8: T suppressor; CD19: B cells; CD45RA: T naive; and CD45RO: T memory).
Discrete parameters, such as rate of colonization and adherence, were analyzed by a χ2 test or Fisher’s exact test. The Sidak procedure was used to correct for multiple testing.
The count of bacteria and the other continuous variables were compared and analyzed using non-parametric tests (Kruskal-Wallis and Wilcoxon tests) or using ANOVA after log10 transformed of the data.
The dose effect on La1 colonization was tested by the Cochran-Mantel-Hanzel test for a linear trend.
We also adopted another approach on data grouping according to La1 colonization by dividing colonized patients (La1+) from non-colonized (La1-), regardless of dose or treatment.
Categorical variables are described by frequency, while continuous variables by mean ± SD.
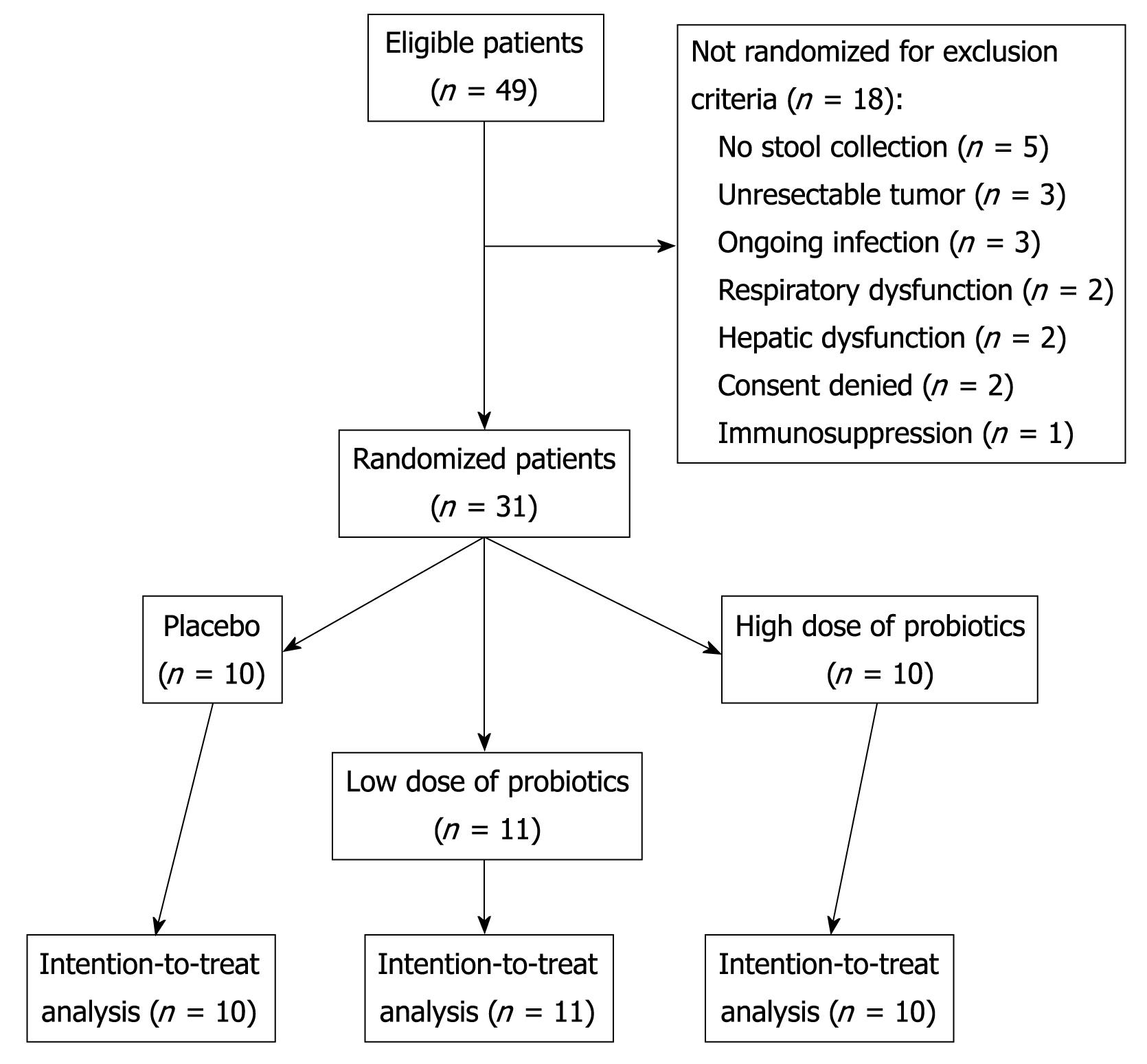
A total of 49 patients were eligible for the study. Due to exclusion criteria, 31 patients were randomized, completed the study, and were analyzed on an intention-to-treat basis (Figure 1). The three groups were well balanced for baseline characteristics and surgical procedures, as shown in Table 1.
| Placebo (n = 10) | Low dose (n = 11) | High dose (n = 10) | P | |
| Age (yr) | 63.3 ± 10.2 | 64.7 ± 4.8 | 62.7 ± 7.8 | 0.49 |
| Male/female | 7/3 | 8/3 | 7/3 | 0.86 |
| BMI (kg/m2) | 25.6 ± 2.6 | 26.5 ± 4.1 | 24.4 ± 3.7 | 0.24 |
| Hemoglobin (g/L) | 121 ± 21 | 131 ± 21 | 128 ± 17 | 0.30 |
| Leukocytes (cells/mm3) | 6.5 ± 1.9 | 7.5 ± 1.5 | 7.9 ± 2.3 | 0.15 |
| Blood glucose (mg/dL) | 109.2 ± 48.3 | 100.7 ± 19.5 | 103.4 ± 30.1 | 0.63 |
| Creatinine (mg/dL) | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.12 |
| Total protein (g/dL) | 7.1 ± 0.7 | 7.3 ± 0.7 | 7.6 ± 0.5 | 0.13 |
| Bilirubin (mg/dL) | 0.5 ± 0.4 | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.17 |
| ALT (IU/L) | 18.7 ± 5.5 | 26.3 ± 12.2 | 16.7 ± 5.7 | 0.08 |
| Type of operation | ||||
| Left colectomy | 4 | 6 | 5 | |
| Right colectomy | 3 | 2 | 2 | 0.72 |
| Rectal resection | 3 | 3 | 3 |
Before treatment (day -4) none of the patients was colonized by La1 or BB536. The effect of treatments on microbiota is shown in Table 2.
| Placebo (n = 10) | Low dose (n = 11) | High dose (n = 10) | |
| Patients colonized with La1 | 0 | 3 (27.2) | 6 (60)a |
| Patients colonized with BB536 | 0 | 0 | 0 |
| Lactobacillus count in stools, Log10 | 3.1 ± 1.1 | 4.2 ± 0.4 | 5.3 ± 0.9b |
| Enterobacteriaceae count in stools, Log10 | 4.5 ± 0.2 | 4.6 ± 0.6 | 2.4 ± 0.3c |
| Enterococci count in stools, Log10 | 4.3 ± 0.5 | 4.1 ± 0.4 | 3.4 ± 0.7 |
| Rate of enterobacteriaceae adherence to colonic mucosa | 7 (70) | 9 (81.8) | 3 (30)d |
| Rate of enterococci adherence to colonic mucosa | 6 (60) | 5 (45.5) | 3 (30) |
At day 0, the group receiving the high dose had a rate of adherence of 60% (6/10) vs 27.2% (3/11) of the low dose groups and none in the placebo group (P = 0.02 high dose vs placebo). Moreover, there was a significant linear positive correlation (P = 0.01) between the number of adherent La1 and the dose given (data not shown). BB536 was never found in mucosa and feces samples. We also evaluated the changes in count of enterobacteriaceae and enterococci. We observed that the group of patients receiving the high dose of probiotics had a lower count of enterobacteriaceae in stool samples than the groups treated with the low dose or placebo (P = 0.07). The same trend was observed for the enterococci count. The percent of patients with enterobacteriaceae adherent to colonic mucosa was 30% (3/10) in the high dose group, 81.8% (9/11) in the low dose group, and 70% (7/10) in the placebo group (P = 0.03 high dose vs low dose). Similar results were observed for enterococci adherence rate, but without reaching statistical significance among the groups (P = 0.372).
On postoperative day five, we didnot observe colonization/adherence in any of the three groups, for both La1 and BB536.
Specific stool cultures for Clostridium perfringens were always negative for both mucosa and stools in all three groups.
We also compared, regardless of treatment, patients who had colonization/adherence with La1 (La1+) with those who didnot have any La1 adherence (La1-) (Table 3).
| Patients colonized with La1 (n = 9) | Patients not colonized with La1 (n = 22) | |
| Enterobacteriaceae | ||
| Unchanged/increased | 2 (22.2) | 9 (40.9) |
| Decreased | 7 (77.8) | 13 (59.1) |
| Enterococci | ||
| Unchanged/increased | 1 (11.1) | 11 (50) |
| Decreased | 8 (88.9)a | 11 (50) |
By comparing day -4 to day 0, we found that only La1+ patients had a decrease (at least 1 log) of enterococci (P = 0.004) and of enterobacteriaceae colonization (P = 0.06).
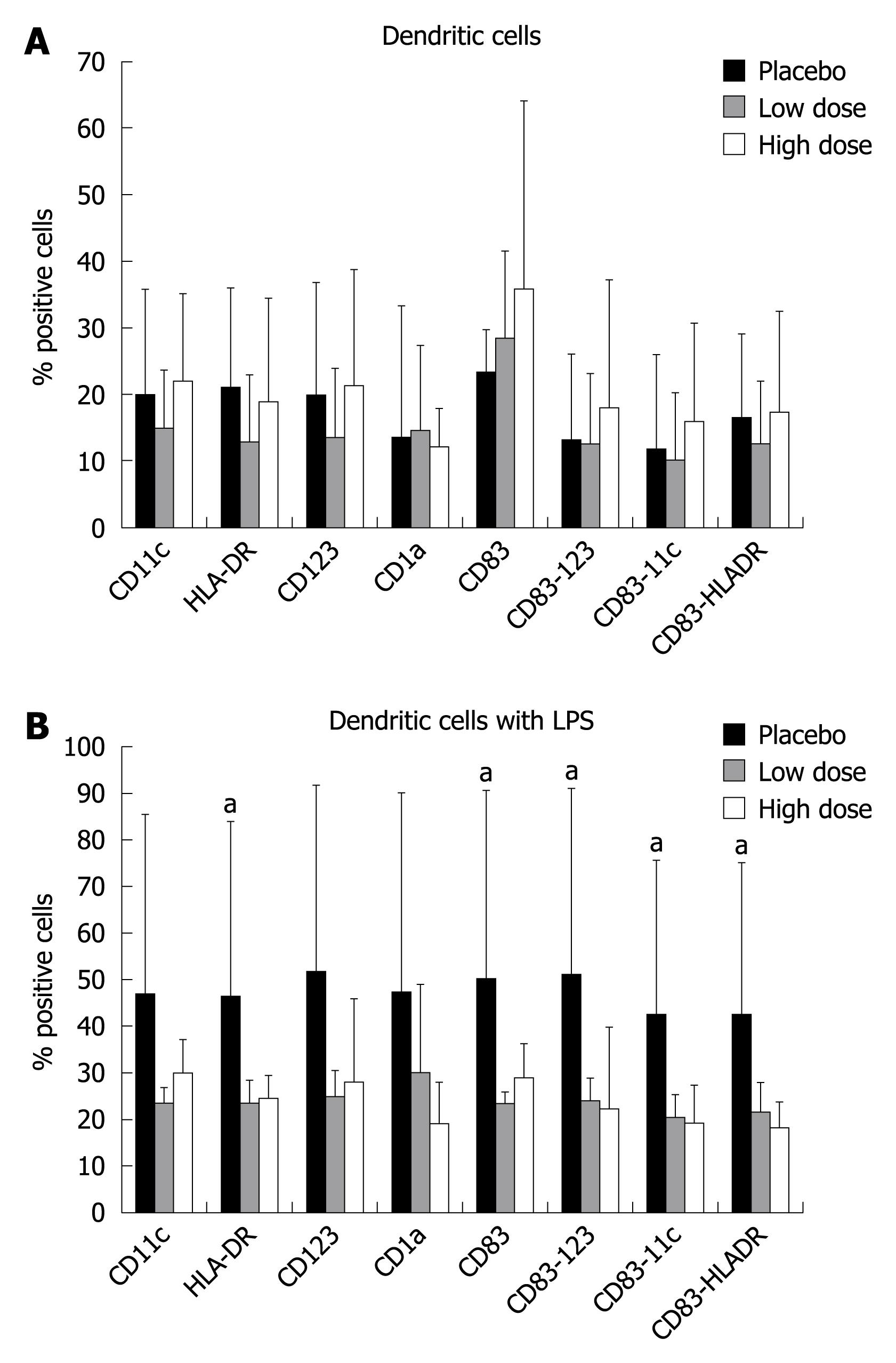
On day 0, the ex-vivo analysis of intestinal DC phenotypes didnot show any significant variation with respect to the type of treatment (Figure 2A). When DC were stimulated in vitro with lipopolysaccharide (LPS), there was a significant increase in proliferation of HLA-DR, CD83, CD83-123, CD83-11c, and CD83-HLA-DR subsets in the placebo group compared to the high dose and low dose groups. The same trend was observed for the other subsets, but without reaching a statistical difference (Figure 2B).
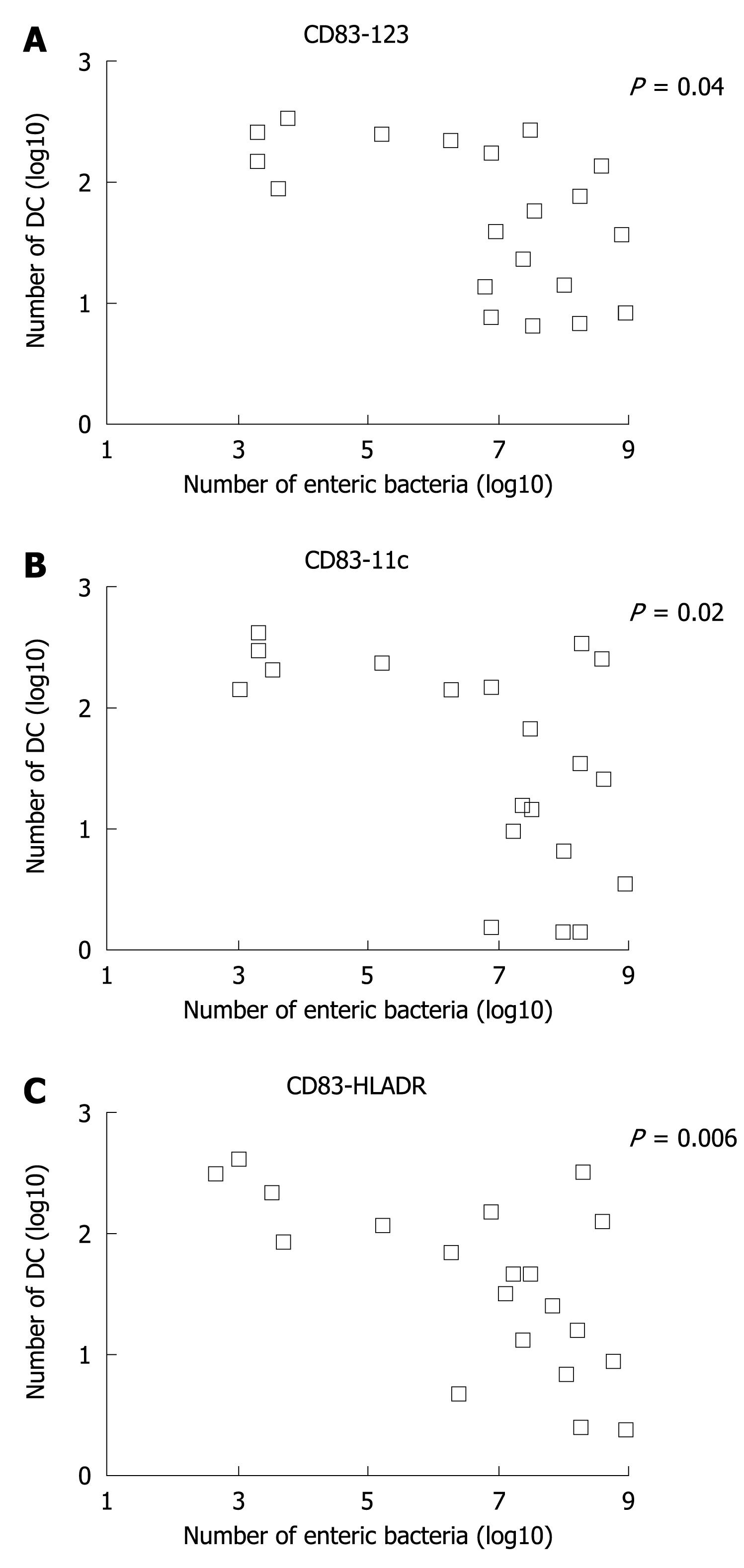
The Log number of dendritic subsets for CD83-123, CD83-11c, and CD83-HLADR was plotted in a linear correlation analysis with the total Log number of enteric bacteria in the stools, which includes also probiotics given. The analysis showed a significant inverse correlation between these two parameters (Figure 3).
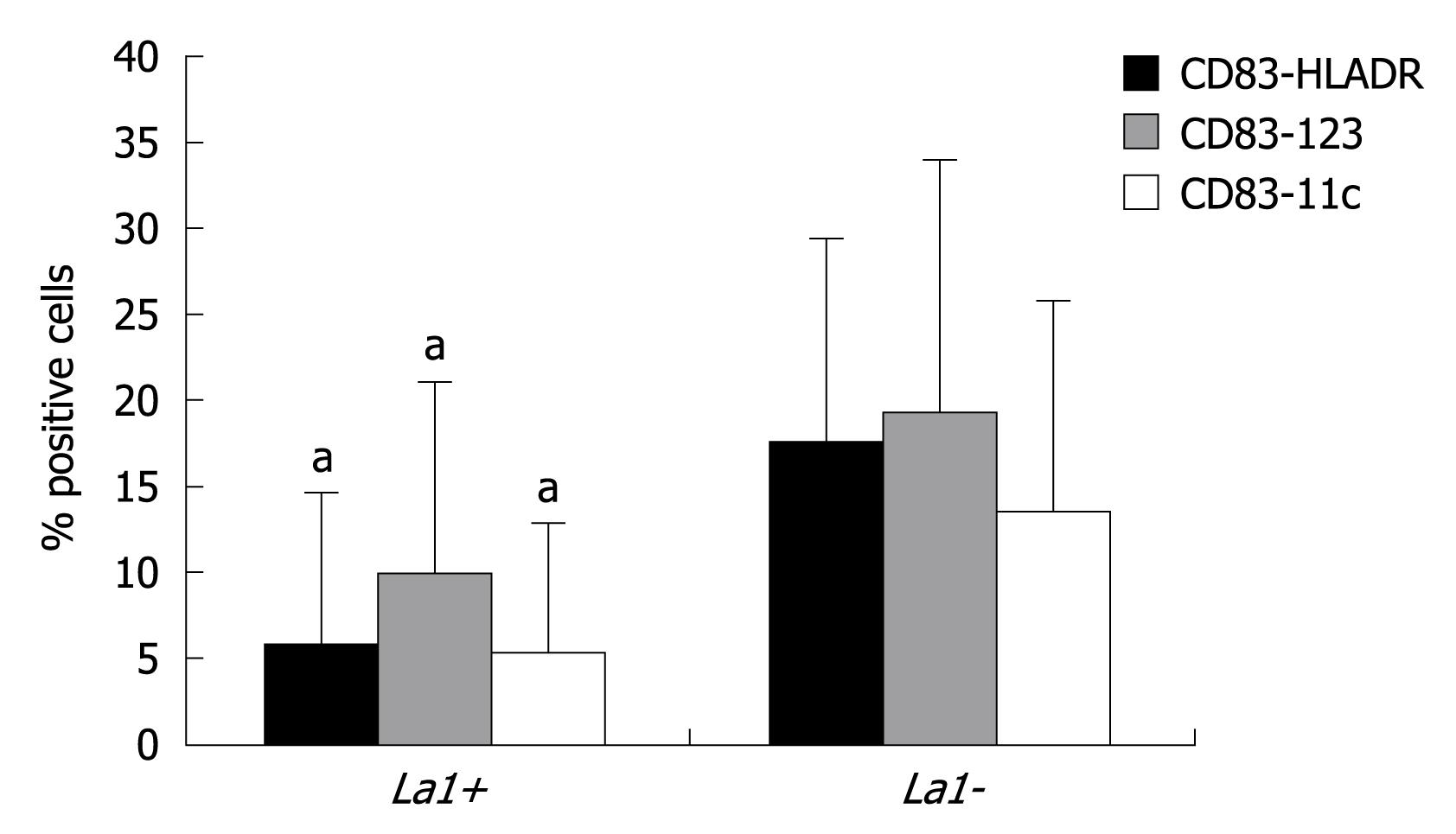
By grouping patients according to colonisation (La1+ vs La1-), we observed that those subjects with La1 adherence to colonic mucosa (La1+) had a significant blunted proliferation of CD83-123, CD83-11c, and CD83-HLA-DR subsets compared to La1- (Figure 4).
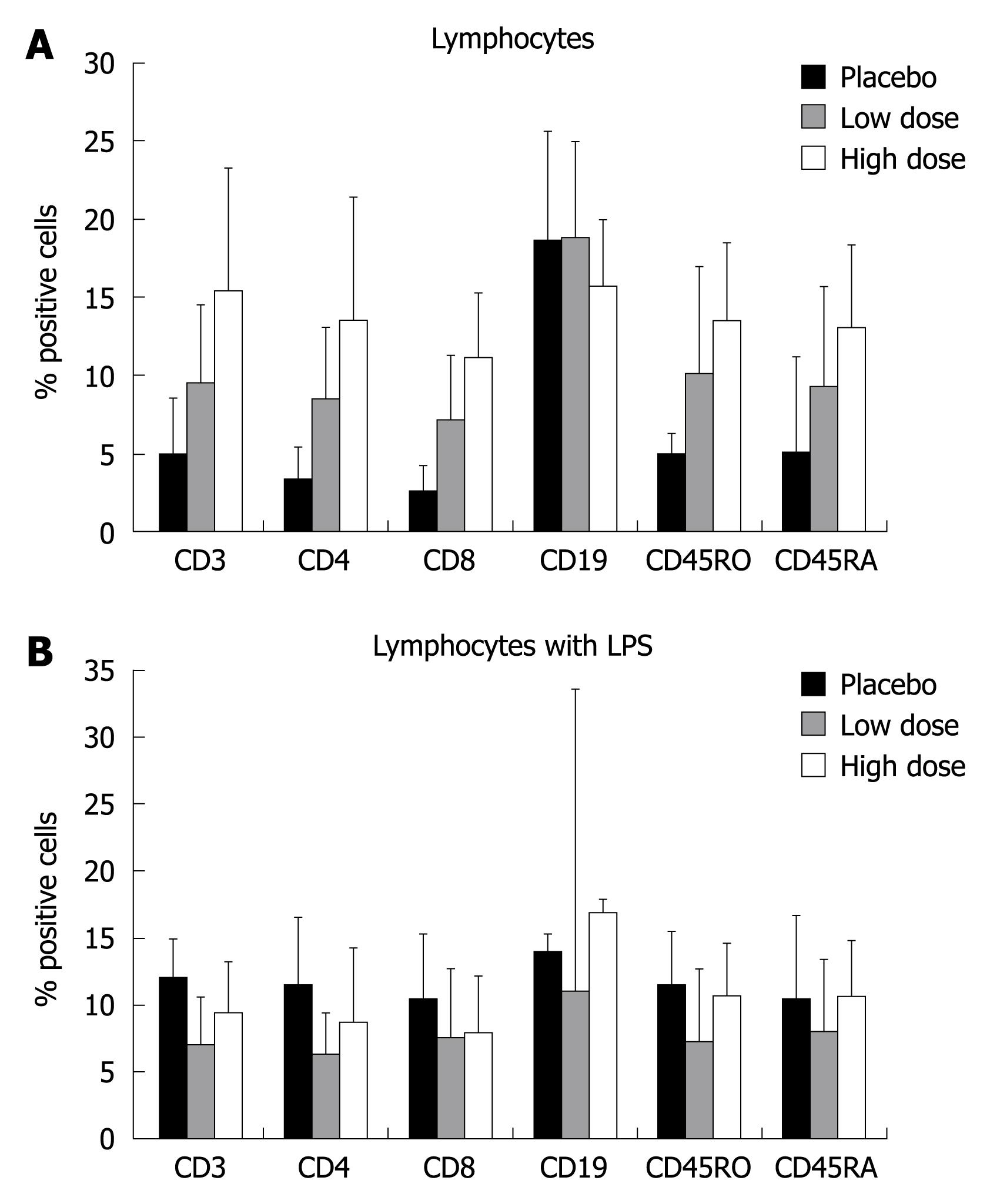
The ex-vivo analysis of lymphocytes suggested the ability of probiotics to stimulate a non-specific proliferation of all subsets, with the exception of CD19 (B cells). The increased percent of positive cells also seemed dependent on the dose of probiotics given (Figure 5A). However, these variations didnot reach statistical significance. LPS stimulation of lymphocytes didnot affect T cell proliferation (Figure 5B).
To our knowledge, this is the first clinical trial investigating the in vivo interaction among probiotic administration, variation of microbiota, and modulation of intestinal immune function in patients undergoing colorectal resection for cancer. This type of study might be important to better understand the potential mechanisms of action of probiotics and subsequently to design appropriate and safe phase III trials with relevant clinical endpoints. Our data suggest that of La1 (but not BB536) administered pre-operatively, is somehow able to adhere to colonic mucosa and colonize feces. This event seems to be correlated with a reduction of potentially pathogenic bacteria and modulation of the intestinal immune response. The dose given and the time of administration with respect to the operation appear to be key factors in obtaining these results.
Probiotic is a generic term that includes several species of bacteria. However, probiotic strains differ greatly in their mechanisms of action, survival during GI transit, modulation of intestinal metabolism, ability to affect the type of immune response, and competition with microbiota and pathogens[2,6]. Most of the data available are from animal or in vitro studies. Furthermore, the same probiotic strain might have dissimilar clinical effects and efficacy in different GI illnesses, and a specific disease might not be successfully treated by different probiotics[2,6,17].
We decided to test a mixture of two probiotics (La1 and BB536) because they have been previously and repeatedly shown to have positive effects, such as safety for clinical use, non-pathogenicity, genetic stability, survival during GI transit[18], antimicrobial properties, competitive antagonism with pathogens[19,20], and the ability to modulate the intestinal immune system[21-24]. In particular, BB536 has been shown, in single arm studies, to survive during intestinal passage and reach the colon intact, even though the experimental setting was different from ours[25]. These characteristics should make these probiotics suitable for testing in a trial with patients who are candidates for colorectal resection, because infectious complications are usually sustained by the subject’s own intestinal microbiota, more frequently than in other types of operation. In vivo, potential synergistic or antagonistic effects between these two probiotics are unknown in this kind of patients.
We believe that it is essential to have a comprehensive knowledge of the potential positive and negative interactions between defined probiotic strains and the host before designing a trial with a therapeutic strategy, such as reduction of surgical morbidity. This might avoid negative results or worse outcomes in specific clinical conditions, such as severe pancreatitis where the deleterious effect of probiotics on oxygen intestinal metabolism was not fully investigated a priori[13].
Several RCTs tested such therapeutic strategies in surgery. They have been recently reviewed by van Santvoort et al[26]. In most of these trials, synbiotics rather probiotics were used; the majority of the enrolled patients were candidates for hepato-biliary-pancreatic surgery, liver transplantation, or mixed cases. Most of the protocols were designed for exclusively postoperative treatment. Moreover, among these studies there were large variations of probiotic strain and dose, timing, duration, and route (oral vs enteral) of administration. Only one trial by Reddy et al[27] was selective for colorectal patients. They reported a synergistic positive effect of synbiotics, neomycin, and bowel preparation on the prevalence of enterobacteriaceae colonization and bacterial translocation, but these events were not associated with a significant reduction of septic morbidity. The different trial characteristics make it quite difficult to compare them and draw any firm conclusion on efficacy.
Our trial suggest that the rate of preoperative colonic adherence and colonization by La1 was suboptimal, reaching 60% with the higher dose tested, while BB536 was never recovered. Several factors might explain these results: all patients underwent bowel preparation, which might affect transit time and peristalsis, thereby reducing the capability of probiotics to adhere. In fact, it has been shown that preoperative intestinal washout might cause loss of superficial mucus and epithelial cells[28]. Other factors might have been the dose (109 not being enough), the preoperative timing of administration being too short, and the strains tested (particularly BB536) not being ideal in this type of patient. In any case, we did observe a reduction of pathogens and a modulation of the intestinal immune system in La1 colonized patients. Moreover, we didnot observe colonization and survival of both probiotics in the post-operative period. Our explanation is based on two hypotheses. First, the tolerance of probiotic administration in the postoperative period was quite low (less than 50%), in contrast to the tolerance of the pre-operative period (100%). This might explain why we could not recover live probiotics at day five postoperatively. Second, it might be due to the presence of postoperative ileus. This condition slows down the GI transit time and increased residence in the lumen might have killed the probiotics.
Animal and in vitro data strongly suggest that probiotics possess the ability to modulate the intestinal and systemic immune response[1-5]. DCs are bone-marrow-derived “professional” antigen-presenting cells. They can acquire antigens and then interact with lymphocyte populations. The different response after DCs - T cell cross talk is influenced by several factors, including the phenotype of DC and signals received in the local environment. Specific intestinal DCs acquire, on activation, signals that might drive the development of Th1, Th2, T regulatory, or Th0 cell responses[29]. The nature of T cell polarization is largely dependent on the type of microbial products. In particular, specific strains of lactobacilli, including La1, can modulate DC and T-cell specific responses via the release of anti-inflammatory cytokines[21]. This might explain the beneficial effect of probiotic treatment of a number of inflammatory bowel diseases, such as alimentary intolerance or allergy, infectious diarrhea, and pouchitis in Crohn’s disease[30-34]. Our data agree with the above findings and suggest that administration of probiotics can partially affect intestinal DC phenotype and activation. In fact, we observed an in vivo inverse correlation between number of enteric bacteria in feces (including probiotics administered) and number of mature and activated intestinal DCs. Thus, increases in microbiota appear to be correlated with a significant decrease of specific DC subsets. When DCs were stimulated “ex vivo” with LPS, only in placebo patients did we observe an enhanced ability of DCs to proliferate. Moreover, DCs isolated from patients colonized with La1 had a significantly blunted ability to proliferate. Although speculative, there results suggest that increasing the probiotic concentration in stools might modulate the immune activity of DCs. In particular, it is possible that probiotics might avoid an excessive activation of DCs with a possible Th1 driven pro-inflammatory response in the intestinal mucosa. In fact, DCs isolated from subjects receiving probiotics were not able to respond to a second inflammatory challenge, such as LPS.
We also observed an unexpected non-specific in vivo proliferation of all lymphocyte subsets, with the exception of B cells, in the patients receiving probiotics. This effect also appeared to be dose dependent. We speculate that in this specific setting, lymphocytes might have been activated by immune pathways that we didnot investigate, such as through monocyte or epithelial cell cross talk[35,36].
The clinical significance and impact of these observations remain to be elucidated. However, the results suggest a role for probiotics in blunting a surgery-induced over-inflammatory response at intestinal and distant sites[37].
In conclusion, our data suggest that La1, but not BB536, adheres to the colonic mucosa and colonizes stools, affects intestinal microbiota by reducing the concentration of pathogens, and modulates local immunity. Further trials are warranted to understand if, in this cohort of patients, better results might be obtained by different single probiotics or by a mixture of probiotic strains, increased dose, longer treatment period or timing of administration. The results of these future trials should be taken into consideration before designing phase III trials.
The therapeutic use of probiotic bacteria is generating great interest in several diseases, but extensive research is required to understand when their administration is really beneficial.
The administration of probiotics in patients undergoing resection for colon cancer has been tested for safety and efficacy.
The results of the present work suggest that giving probiotics before colon surgery might reduce the number of enteric bacteria that might be responsible for postoperative infectious complications. Moreover, probiotics are able to modulate the immune response of the intestine and there is an intensive cross-talk between immune cells and bacteria.
These data should be useful to design future clinical studies with a larger number of patients to understand if there is a correlation between metabolic, immunological and microbiological changes and reduction of surgery-related infections.
Probiotics: Mono or mixed cultures of live microorganisms that might beneficially affect the host by improving the characteristics of indigenous microflora.
This is the first clinical trial investigating in vivo the interaction among probiotic administration, variation of microbiota, and modulation of intestinal immune function in patients undergoing colorectal resection for cancer. Such studies might be important to better understand the potential mechanisms of action of probiotics and subsequently to design appropriate and safe trials with relevant clinical endpoints.
Peer reviewers: Dimitrios V Avgerinos, MD, Department of Surgery, Beth Israel Medical Center, 1st Ave. and 16th Str, 16 Baird Hall, New York, NY 10010, United States; Dr. Terence C Chua, Department of Surgery, St George Hospital, Level 3 Pitney Building, Sydney 2217, Australia
S- Editor Wang JL L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis in't Veld JH. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41:85-101. [Cited in This Article: ] |
| 2. | Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405-440. [Cited in This Article: ] |
| 3. | Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115-1118. [Cited in This Article: ] |
| 4. | Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512-519. [Cited in This Article: ] |
| 5. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [Cited in This Article: ] |
| 6. | Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658-672. [Cited in This Article: ] |
| 7. | Goossens DA, Jonkers DM, Russel MG, Stobberingh EE, Stockbrügger RW. The effect of a probiotic drink with Lactobacillus plantarum 299v on the bacterial composition in faeces and mucosal biopsies of rectum and ascending colon. Aliment Pharmacol Ther. 2006;23:255-263. [Cited in This Article: ] |
| 8. | Rayes N, Seehofer D, Theruvath T, Mogl M, Langrehr JM, Nüssler NC, Bengmark S, Neuhaus P. Effect of enteral nutrition and synbiotics on bacterial infection rates after pylorus-preserving pancreatoduodenectomy: a randomized, double-blind trial. Ann Surg. 2007;246:36-41. [Cited in This Article: ] |
| 9. | Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, Nomoto K, Nimura Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244:706-714. [Cited in This Article: ] |
| 10. | Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K, Asahara T, Nomoto K, Tanaka R, Nimura Y. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390:104-113. [Cited in This Article: ] |
| 11. | Anderson AD, McNaught CE, Jain PK, MacFie J. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut. 2004;53:241-245. [Cited in This Article: ] |
| 12. | McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211-219. [Cited in This Article: ] |
| 13. | Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659. [Cited in This Article: ] |
| 14. | Ventura M, Zink R. Specific identification and molecular typing analysis of Lactobacillus johnsonii by using PCR-based methods and pulsed-field gel electrophoresis. FEMS Microbiol Lett. 2002;217:141-154. [Cited in This Article: ] |
| 15. | Mullié C, Odou MF, Singer E, Romond MB, Izard D. Multiplex PCR using 16S rRNA gene-targeted primers for the identification of bifidobacteria from human origin. FEMS Microbiol Lett. 2003;222:129-136. [Cited in This Article: ] |
| 16. | Gianotti L, Sargenti M, Galbiati F, Nespoli L, Brivio F, Rescigno M, Nespoli A. Phenotype and function of dendritic cells and T-lymphocyte polarization in the human colonic mucosa and adenocarcinoma. Eur J Surg Oncol. 2008;34:883-889. [Cited in This Article: ] |
| 17. | Snelling AM. Effects of probiotics on the gastrointestinal tract. Curr Opin Infect Dis. 2005;18:420-426. [Cited in This Article: ] |
| 18. | Marteau P, Minekus M, Havenaar R, Huis in't Veld JH. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J Dairy Sci. 1997;80:1031-1037. [Cited in This Article: ] |
| 19. | Walker WA. Mechanisms of action of probiotics. Clin Infect Dis. 2008;46 Suppl 2:S87-S91; discussion S144-S151. [Cited in This Article: ] |
| 20. | Bengmark S. Bioecologic control of the gastrointestinal tract: the role of flora and supplemented probiotics and synbiotics. Gastroenterol Clin North Am. 2005;34:413-436, viii. [Cited in This Article: ] |
| 21. | Bernet MF, Brassart D, Neeser JR, Servin AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483-489. [Cited in This Article: ] |
| 22. | Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994;10:55-63. [Cited in This Article: ] |
| 23. | Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents -- physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22:495-512. [Cited in This Article: ] |
| 24. | Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47:79-87. [Cited in This Article: ] |
| 25. | Pochart P, Marteau P, Bouhnik Y, Goderel I, Bourlioux P, Rambaud JC. Survival of bifidobacteria ingested via fermented milk during their passage through the human small intestine: an in vivo study using intestinal perfusion. Am J Clin Nutr. 1992;55:78-80. [Cited in This Article: ] |
| 26. | van Santvoort HC, Besselink MG, Timmerman HM, van Minnen LP, Akkermans LM, Gooszen HG. Probiotics in surgery. Surgery. 2008;143:1-7. [Cited in This Article: ] |
| 27. | Reddy BS, Macfie J, Gatt M, Larsen CN, Jensen SS, Leser TD. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg. 2007;94:546-554. [Cited in This Article: ] |
| 28. | Bucher P, Gervaz P, Egger JF, Soravia C, Morel P. Morphologic alterations associated with mechanical bowel preparation before elective colorectal surgery: a randomized trial. Dis Colon Rectum. 2006;49:109-112. [Cited in This Article: ] |
| 29. | Bell SJ, Rigby R, English N, Mann SD, Knight SC, Kamm MA, Stagg AJ. Migration and maturation of human colonic dendritic cells. J Immunol. 2001;166:4958-4967. [Cited in This Article: ] |
| 30. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [Cited in This Article: ] |
| 31. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. [Cited in This Article: ] |
| 32. | D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324:1361. [Cited in This Article: ] |
| 33. | Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol. 1997;99:179-185. [Cited in This Article: ] |
| 34. | Isolauri E, Arvola T, Sütas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604-1610. [Cited in This Article: ] |
| 35. | Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507-514. [Cited in This Article: ] |
| 36. | Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P, Rescigno M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818-2826. [Cited in This Article: ] |
| 37. | Schiffrin EJ, Rochat F, Link-Amster H, Aeschlimann JM, Donnet-Hughes A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J Dairy Sci. 1995;78:491-497. [Cited in This Article: ] |