Published online Jun 28, 2007. doi: 10.3748/wjg.v13.i24.3374
Revised: April 3, 2007
Accepted: April 11, 2007
Published online: June 28, 2007
AIM: To investigate anti-tumor activities and apoptosis-regulated mechanisms of bufalin in the orthotopic transplantation tumor model of human hepatocellular carcinoma in nude mice.
METHODS: BEL-7402 cells of human hepatocellular carcinoma were inoculated to form subcutaneous tumors, and were implanted into the liver to establish orthotopic transplantation tumor models of human hepatocellular carcinoma in nude mice. Seventy-five animals were randomized divided into five groups (n = 15). Bufalin was injected intraperitoneally into three groups at doses of 1.5 mg/kg (BF1), 1 mg/kg (BF2) and 0.5 mg/kg (BF3) for d 15-24, respectively. The NS group was injected an equal volume of saline as above and adriamycin was injected intraperitoneally into the ADM group at a dose of 8.0 mg/kg for d 15. Ten mice in each group were killed at d 25 and the survival time in each group was calculated. We also observed the morphologic alterations in the myocardium, brain, liver, kidney and tumor tissues by pathology and electron microscopy, measured the apoptotic rate by TUNEL staining method, and detected the expression of apoptosis-regulated genes bcl-2 and bax by immunohistochemical staining and RT-PCR in tumor tissues.
RESULTS: The tumor volumes in each group of bufalin were reduced significantly (35.21 ± 12.51 vs 170.39 ± 25.29; 49.83 ± 11.46 vs 170.39 ± 25.29; 83.99 ± 24.63 vs 170.39 ± 25.29, P < 0.01, respectively), and the survival times were prolonged in group BF1-2 (31.8 ± 4.2 vs 23.4 ± 2.1 and 29.4 ± 3.4 vs 23.4 ± 2.1, P < 0.05, respectively), and necrosis was mainly in severe or moderate degree in group BF1-2. No morphological changes were detected in the myocardium, brain, liver and kidney tissues. Apoptotic characteristics could be seen in group BF1-2. The positive rates of bcl-2 and bax protein expression of each group by immunohistochemical staining were 10.0%, 10.0%, 20.0%, 10.0% and 20.0%; 90.0%, 80.0%, 80.0%, 40.0% and 30.0%, respectively. Loss of expression of bcl-2 mRNA in each group was to be found and the density of bax mRNA was increased progressively with increase of dose of bufalin by RT-PCR.
CONCLUSION: Bufalin has significant anti-tumor activities in the orthotopic transplantation tumor model of human hepatocellular carcinoma in nude mice with no marked toxicity and was able to induce apoptosis of transplanted tumor cells. This apoptosis may be mediated mainly via up-regulating the expression of apoptosis-regulated gene bax, which may be involved in its anti-tumor mechanism of bufalin.
- Citation: Han KQ, Huang G, Gu W, Su YH, Huang XQ, Ling CQ. Anti-tumor activities and apoptosis-regulated mechanisms of bufalin on the orthotopic transplantation tumor model of human hepatocellular carcinoma in nude mice. World J Gastroenterol 2007; 13(24): 3374-3379
- URL: https://www.wjgnet.com/1007-9327/full/v13/i24/3374.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i24.3374
Apoptosis occurs in several pathological situations in multicellular organisms and constitutes part of a common mechanism of cell replacement, tissue remodeling, and removal of damaged cells. Bcl-2 family plays a crucial role in the control of apoptosis and can be classified into two functionally distinct groups: antiapoptotic proteins and pro-apoptotic proteins. Bcl-2, an antiapoptotic protein, is known to regulate apoptotic pathways and protects against cell death. Bax, a pro-apoptotic protein of that family, is expressed abundantly and selectively during apoptosis and promotes cell death. Increasing the ratio of bcl-2 to bax has commonly been used to determine the induction of apoptosis in several tissues[1,2].
Bufalinfalin (Bufalin) is a toxic ligand extracted from a traditional Chinese medicine Secretia bufonis, the molecular formula of which is C24H3404 with a relative molecular weight 386.5. Bufalin has the activities of inducing differentiation and apoptosis of tumor cells. Research on bufalin mainly involves tumor spectra of leukemia, prostate cancer, gastric cancer and liver cancer, and is confined to in vitro studies[3-11]. The results of our previous studies[12] have showed that a μmol dose of bufalin was able to produce a potent killing effect on human liver cancer cells in vitro within 48 h.
The present study was to use a human hepatocellular carcinoma in situ transplantation model of nude mice to observe the anti-tumor activities of bufalin in vivo and investigate the relation between this apoptosis and expression of bcl-2 and bax and to provide the theoretical and methodological basis for its clinical application in the future.
Eighty male BALB/Cnu/nu nude mice (aged 4-5 wk and weighing 18-20 g) were provided by the experimental animal center of Fudan University. The nude mice were caged individually under specific-pathogen free (SPF) conditions. The code number of the animals was SCKK (Shanghai) 2004-0010. Eighteen male ICR mice (aged 4 wk and weighing 10-22 g) were provided by the experimental animal center of the Second Military Medical University. Human hepatocellular carcinoma cell strain BEL-7402 (Shanghai Institute of Cell Biology of the Chinese Academy of Sciences) was generation cryopreserved in our laboratory.
Bufalin (10.0 mg/via) was purchased from American Sigma, and prepared to a 0.2 mg/mL concentration by adding 0.5 mL anhydrous alcohol and injection water. Adriamycin (ADM) (Wan Le Pharmaceuticals, Shenzhen, Batch No. 0505E1) was prepared into a 1.0 mg/mL concentration using normal saline.
The model was established by using the intrahepatic tunnel implantation[13]. Cryopreserved human liver cancer cell strain BEL-7402 was thawed, cultured in vitro to the log growth phase, centrifuged and washed with PBS to prepare to a concentration of 1 × 107 cells/mL. Each nude mouse was inoculated with 0.2 mL of the strain subcutaneously via the back. When the tumor grew to 1.0 cm in diameter, the fish meat-like fresh tumor tissue was cut into 1.0 mm × 2.0 mm pieces and implanted into the tunnel under the capsule of the left lateral liver lobe by using a pair of ophthalmologic forceps. The wound was slight pressed with a cotton swab for hemostasis and closed.
Eighteen ICR mice were equally divided into 3 dose groups: 2.0 mg/kg, 1.5 mg/kg and 1.0 mg/kg bufalin intraperitoneally daily for 10 d. Weight, appetite and behavior of the animals were observed. Anti-tumor dose of bufalin was initially defined as 1.5 mg/kg, 1.0 mg/kg and 0.5 mg/kg.
Exploratory laparotomy was performed in all animals 14 d after establishment of the model; the model success rate was 100%. The 75 models were equally randomized into five groups: the large-, middle- and small-dose groups (group BF 1-3), the positive group (ADM group) and the negative group (NS group). Drug administration was initiated at 15 d after establishment of the model. Group BF 1-3 was administered the drug according to the dose groups of the toxicity test intraperitoneally daily from 15 to 24 d. The NS group received the equivalent amounts of normal saline in the same way. The ADM group received 8.0 mg/kg intraperitoneally at d 15. At d 25, 10 of the 15 animals in each group were sacrificed. Blood sampling from the eye ball was performed to test liver and kidney functions and blood routine. Volume of the tumors was measured. Tumor tissue, heart, liver, lung and kidney specimens were taken for routine pathology and electron microscopy observation. The remaining 5 animals in each group were kept alive for observation of tumor-bearing survival.
Tumor volume and tumor inhibitory rate: The longest diameter (a) and shortest diameter (b) of the tumor body were measured by using a slide gaud, and the tumor volume was calculated according to the formula V = ab2/2; the tumor inhibitory rate = (1-mean tumor volume of the drug group/mean tumor volume of the control group) × 100%; prolonged survival = (mean days of survival of the drug group/mean days of survival of the control group-1) × 100%. The survival time was defined as the day from administering drugs to death. Histological examination: The remaining tissue of each group was formalin fixed, paraffin sectioned and HE stained for routine pathology to observe any change in morphology and tumor necrosis. Electron microscopic treatment: The tumor tissue was fixed with 3% glutaral for 1-3 h, washed with buffer solution, fixed with 1% osmium tetroxide for 1-2 h, gradient dehydrated with acetone, Epon812 embedded, uranyl acetate and lemon lead double stained, sliced, and then stained for transmission electron microscopy observation.
Tumor samples were cryopreserved in liquid nitrogen and cut into 8-μm thick slices. Slices were fixed in ice-cold 80% ethanol for 24 h, treated with proteinase K and 0.3% H2O2, labeled with fluorescein dUTP in a humid box for 1 h at 37°C. Slices were then combined with POD-horseradish peroxidase, stained with DAB and counterstained with methyl green. Controls received the same management except the labeling fluorescein dUTP. Cells were visualized with a light microscope. The apoptotic index (AI) was calculated as follows: AI = number of apoptotic cells/total number × 100%.
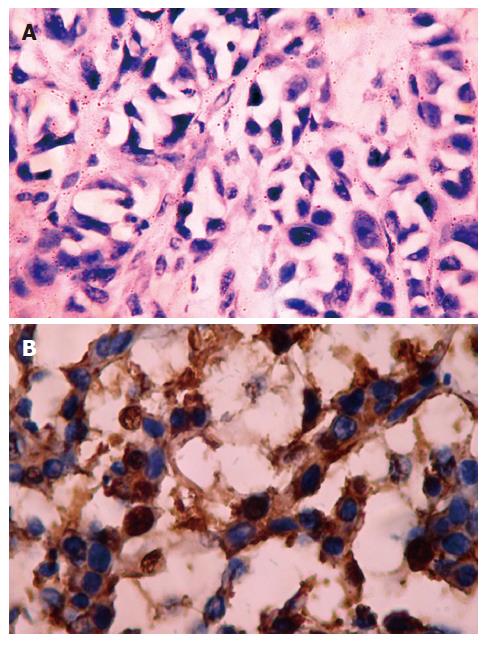
Tumor samples were cryopreserved in liquid nitrogen, cut into 8-μm thick slices and fixed by acetone. After washing with PBS, slices were incubated in 0.3% H2O2 solution at room temperature for 5 min. Slices were then incubated with anti-bcl-2 or anti-bax monoclonal antibody (purchased from Shanghai Biotechnology Co. Ltd) at a 1:300 dilution at 4°C overnight. After washed with PBS, the second antibody, biotinylated antirat IgG, was added and cells were incubated at room temperature for 1 h. After washing with PBS, ABC compound was added and incubated at room temperature for 10 min. DAB was used as the chromagen. After 10 min, the brown color signifying the presence of antigens bound to antibodies was detected by light microscopy. Controls were managed as the experimental group except for the incubation of primary antibody. The positive criterion was defined as the positive rate must exceed 5%, and the positive rate (PR) was calculated as follows: PR = (number of positive cells/total number) ×100%.
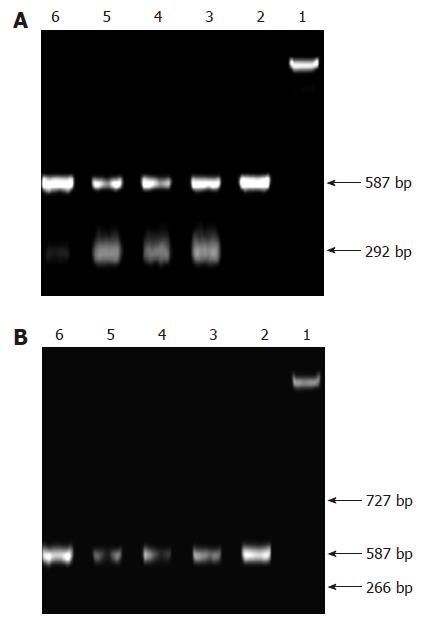
Tumor samples were cryopreserved in liquid nitrogen and total RNA was extracted. Concentration of RNA was determined by the absorption at 260 nm. The primers for bcl-2 mRNA, bax mRNA and β-actin were as follows: β-actin (587 bp) 5’ CCAAGGCCAACCGCGAGAAGATG 3’ (sense); 5’ AGCGTACATGGTGG -TGCCGCCA 3’ (antisense); bcl-2αmRNA (266 bp) 5’ CCGAGATGTCCA-GCCAGCT 3’ (sense); 5’ CAGTTCCACAAAGGCATCC 3’ (anti-sense); bcl-2βmRNA (727 bp) 5’ TACGACAACCGGGACATAGTG 3’ (sense); 5’ GAACGCTTTGTCCAGAGGAG 3’ (anti-sense) bax mRNA (292bp) 5’ CGAGTGGCAGTGACATGT 3’ (sense), 5’ TCTTCTTCCAGATGGTGAG 3’ (anti-sense). Polymerase chain reactions were performed in a 50 μL reaction volume. RT-PCR reaction was run under the following conditions: at 94°C for 1 min, 1 circle; at 94°C for 30 s, at 54°C for 40 s, at 72°C for 1 min, 32 circles; at 72°C for 5 min, 1 circle. 15 μL PCR products were placed onto a 15 g/L agarose gel and observed by EB staining using the Gel-Pro analyzer.
Data analysis of variance was performed by SPSS 11.0 and P < 0.05 was considered statistically significant.
Transient convulsion was observed 5 min after the first IP drug administration in half the animals of the 2.0 mg/kg bufalin groups, short-term listlessness was observed after the second injection, which was restored to normal 3-5 min later. No evident adverse reaction was observed in the 1.5 mg/kg and less groups. No evident change was observed in weight, appetite and behavior after 10 d of drug administration. There was no treatment-related death.
Table 1 shows that there was no significant difference in tumor volume between these five groups (P > 0.05), indicating that the tumor volumes before treatment were comparable between these five groups. Compared with the NS group, the tumors of the Bufalin and ADM groups shrank significantly (P < 0.01), especially in the BF1 group with a tumor inhibitory rate of 79.3%. The tumor inhibitory rate of the BF2 group, ADM and BF3 group was 70.7%, 67.6% and 50.7%, respectively. The difference between the ADM group and BF1 group and 2 was significant (P < 0.05). There was no significant difference in weight between the other groups and the NS group (P > 0.05). Compared with the BF1-3 groups, the weight of the ADM group decreased significantly (P < 0.05).
| Group | Tumor | Tumor inhibitory | weight | ||
| Volume (cm3) | rate | (g) | |||
| Pre-treatment | Post- treatment | (%) | Pre-treatment | Post-treatment | |
| BF1 | 43.50 ± 5.09 | 35.21 ± 12.51ac | 79.3 | 20.94 ± 1.00 | 21.39 ± 1.62e |
| BF2 | 42.92 ± 4.10 | 49.83 ± 11.46ac | 70.7 | 20.03 ± 1.16 | 21.48 ± 1.10e |
| BF3 | 43.00 ± 2.97 | 83.99 ± 24.63a | 50.7 | 21.06 ± 0.94 | 21.57 ± 1.14e |
| ADM | 42.93 ± 4.23 | 55.17 ± 16.13a | 67.6 | 21.00 ± 1.00 | 18.90 ± 0.77 |
| NS | 43.37 ± 4.82 | 170.39 ± 25.29 | - | 20.95 ± 1.07 | 20.40 ± 1.23 |
Tumor bearing survival of bufalin groups was prolonged, and the difference was significant between group BF1-2 and NS and ADM groups (P < 0.05), especially in group BF1. Survival of ADM group was not significantly prolonged as compared with the NS group (P > 0.05) (Table 2).
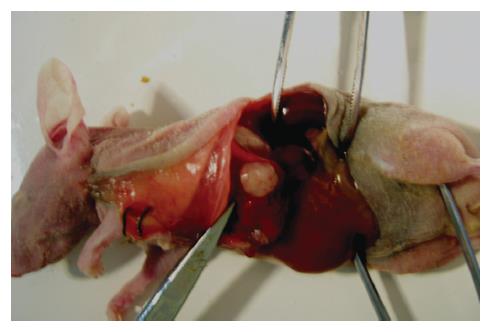
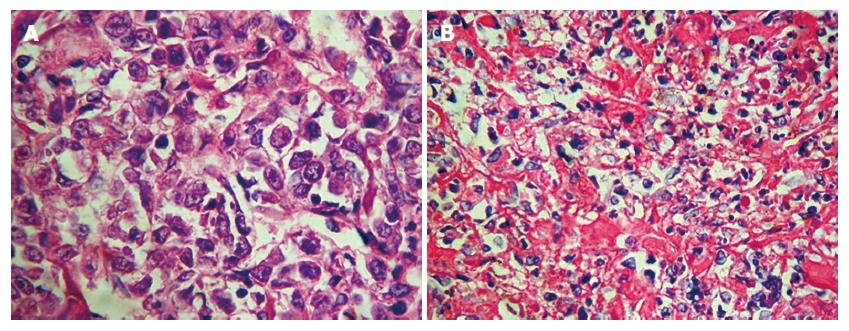
The in situ transplanted tumors were relatively regular, shaping round or oval with complete or incomplete capsules, mainly in the form of infiltrating growth (Figure 1). HE stain revealed defuse tumor tissue with rich blood vessels in the interstitial and infiltrative liver tissue around the edge of the tumor. The nucleus of the liver cancer cell was large oval-shaped; there was more chromatin; binucleolates were large and clearly visible; there were more free ribosomes and more pathological karyokinesis (Figure 2A). Severe necrosis was mainly seen in the BF1-2 and ADM group, and mild necrosis in the NS group (Figure 2B). There was less cytoplasm in tumor cells of group BF1-3, where cell apoptotic signs were seen, such as nuclei became pyknotic, chromatin was concentrated and collected around the edge, and nuclei were cleft.
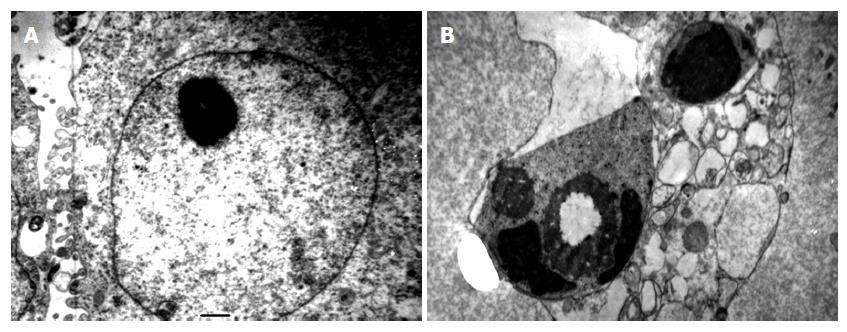
Transmission electron microscopy revealed that in the NS group the surface of the tumor cells was irregular; there was more chromatin; large and clear binucleolates were frequently seen; there was an increase in free ribosomes; rich mitochondrion and endoplasmic reticulum were seen in the cytoplasm (Figure 3A). In BF groups, cell shrinkage, cytoplasm concentration, collection of concentrated chromatin on the medial side of the nuclear membrane in the form of masses or crescents with bubbling of cytoplasm and apoptotic corpuscles (Figure 3B).
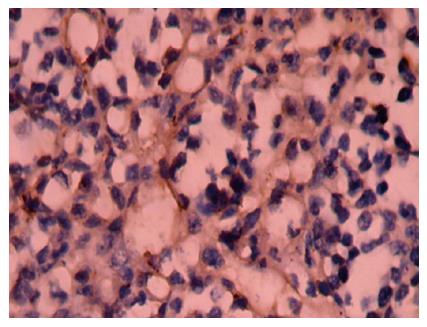
Positive staining was located in the nuclei (Figure 4). The apoptosis index of group BF1-3, ADM and NS group was 10.60% ± 3.42%, 8.86% ± 2.96%, 5.87% ± 2.13%, 4.26% ± 2.12% and 3.28% ± 0.98%, respectively (P < 0.01 or P < 0.05, vs the NS group).
Positive staining was located in the cytoplasm (Figure 5A and B). The positive rates of bcl-2 and bax protein of group BF 1-3, ADM and NS group was 10.0%, 10.0%, 20.0%, 10.0% and 20.0%; 90.0%, 80.0%, 80.0%, 40.0% and 30.0% respectively by immunohistochemical staining (P > 0.05, vs the NS group; P < 0.01 or P < 0.05, vs the NS group, Table 3).
Loss of expression of bcl-2 mRNA in each group was to be found and the density of bax mRNA was increased progressively with the increase of dose of bufalin by RT-PCR (Figure 6A and B).
Until now, few chemotherapeutic drugs are effective in the treatment of human hepatocellular carcinoma and it is necessary to look for new anti-hepatocellular carcinoma drugs. Bufalin is a toxic traditional Chinese medicine, mainly containing resibufogenin, cinobufagin, cinobufotalin, bufotalin and bufalin, of which bufalin has the strongest anti-tumor activities. Our previous studies showed that bufalin had marked anti-tumor activities on the human liver cancer cell lines SMMC-7721 and BEL-7402[12], on the basis of which the present study used the nude mice human liver cancer in situ transplantation model to study the anti-tumor activities of bufalin in vivo. The results show that tumor volumes were reduced significantly in group BF1-2 (as compared with the NS group, P < 0.01), tumor bearing survival was prolonged significantly (compared with the NS group, P < 0.05), and there was no significant change in weight (P > 0.05); tumor volumes of group BF1-2 were reduced significantly as compared with the ADM group (P < 0.05), and survival of the animals in the ADM group was not significantly prolonged (compared with NS group, P > 0.05) where weight of the animals decreased significantly (compared with the other groups, P < 0.05). Light electron microscopy showed moderate and severe necrosis of tumor tissues in group BF1-2, and moderate and mild necrosis in group BF1. No morphological changes were found in the heart, brain, liver, kidney and lung tissues; liver and kidney functions and blood were not significantly affected (data not shown). Transmission electron microscopy revealed more apoptotic changes of tumor tissues in group BF1-2. We assume that this is the result of the anti-tumor activities of bufalin.
In studies using 10 μmol bufalin of the above conce-ntrations to treat HL-60, ML-1 and U937 cell lines, Jing et al[14] found morphological changes seen in apoptotic cells, which included cell shrinkage, chromatin aggregation, cleavage, and formation of apoptotic corpuscles. No cell apoptosis was observed in the experiment using 1 μmol/L bufalin to treat normal human monocytes and polymorphic nuclear cells for 24 h. These results indicate that the actions of bufalin in selectively inhibiting tumor growth and inducing cell apoptosis are closely related. The results of the present study demonstrated that bufalin has the action of inducing apoptosis of in situ human liver cancer cells.
The Bcl-2 family plays a crucial role in the control of apoptosis. It has been found that the family includes a number of proteins which have homologous amino acid sequences, including antiapoptotic members such as bcl-2 and bcl-xL, as well as proapoptotic members including bax and bad[15,16]. Over expression of bax could promote cell death[1,2,17]. Conversely, over expression of antiapoptotic proteins such as Bcl-2 could repress the function of bax[18,19]. Thus, the ratio of bcl-2 /bax was a critical determinant of a cell’s threshold for undergoing apoptosis[20]. In this study, we evaluated the effectiveness of apoptosis in situ human liver cancer cells induced by bufalin in vivo, this apoptosis might be mediated by up-regulating the expression of apoptosis-regulated gene bax and decreased the ratio of bcl-2/bax of human liver cancer cells.
It is reported in the literature[21] that ID50 of bufalin is 2.2 mg/kg. Our pre-experiment also showed that 2.0 mg/kg bufalin caused spasmodic seizures but did not cause toxic death in mice. The results suggest that 1.5 mg/kg bufalin would prove to be the appropriate anti-tumor dose. The most appropriate dosage of bufalin for other tumors needs to be further observed.
The results of the present study showed that bufalin had marked anti-tumor activities and was able to induce apoptosis which might be mediated by up-regulating the expression of apoptosis-regulated gene bax and decreased the ratio of bcl-2/bax in the human in situ liver cancer transplantation model in nude mice with no evidence of toxicity to the heart, lungs, liver, kidneys and brain. Bufalin’s selective inhibition on tumor cell warrants further study with respect to its anti-tumor effect in vivo, the optimal dosage and the other related mechanisms.
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH
| 1. | Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 923] [Cited by in F6Publishing: 927] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 2. | Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, Kim J, Kim KH, Kim CJ. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull. 2006;29:1597-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Yamada K, Hino K, Tomoyasu S, Honma Y, Tsuruoka N. Enhancement by bufalin of retinoic acid-induced differentiation of acute promyelocytic leukemia cells in primary culture. Leuk Res. 1998;22:589-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Yeh JY, Huang WJ, Kan SF, Wang PS. Effects of bufalin and cinobufagin on the proliferation of androgen dependent and independent prostate cancer cells. Prostate. 2003;54:112-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Wu XX, Lu Q, Zhang M, Chen L, Xu RC, Chen XY. Apoptosis of gastric cancer cells induced by bufalin. J. ichu Yanjiu yu Linchuang. 2000;20:50-52. [Cited in This Article: ] |
| 6. | Chen XY, Hu WL, Xu RC, Chen L, Jin Q. Effect of buflin on cytotoxicity and growth related gene expression of human hepatoma cell line SMMC7721. Zhongguo Yaoli yu Dulixue Zazhi. 2001;15:293-296. [Cited in This Article: ] |
| 7. | Watabe M, Masuda Y, Nakajo S, Yoshida T, Kuroiwa Y, Nakaya K. The cooperative interaction of two different signaling pathways in response to bufalin induces apoptosis in human leukemia U937 cells. J Biol Chem. 1996;271:14067-14072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Masuda Y, Kawazoe N, Nakajo S, Yoshida T, Kuroiwa Y, Nakaya K. Bufalin induces apoptosis and influences the expression of apoptosis-related genes in human leukemia cells. Leuk Res. 1995;19:549-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Watabe M, Kawazoe N, Masuda Y, Nakajo S, Nakaya K. Bcl-2 protein inhibits bufalin-induced apoptosis through inhibition of mitogen-activated protein kinase activation in human leukemia U937 cells. Cancer Res. 1997;57:3097-3100. [PubMed] [Cited in This Article: ] |
| 10. | Zhu ZT, Jin B, Liu YP, Li YC, Lu XL, Tian X, Hou KZ. Enhancement of all-trans retinoic acid-induced differentiation by bufalin in primary culture of acute promyelocytic leukemia cells. Zhonghua NeiKe ZaZhi. 2006;45:314-317. [PubMed] [Cited in This Article: ] |
| 11. | Tian X, Luo Y, Liu YP, Hou KZ, Jin B, Zhang JD, Wang S. Downregulation of Bcl-2 and survivin expression and release of Smac/DIABLO involved in bufalin-induced HL-60 cell apoptosis. Zhonghua XueYeXue ZaZhi. 2006;27:21-24. [PubMed] [Cited in This Article: ] |
| 12. | Su YH, Yin XC, Xie JM, Gao B, Ling CQ. Inhibition effects of three kinds of buotoxins on human SMMC-7721 and BEL-7402 hepatoma cells lines. Dier Junyi Daxue Xuebao. 2003;24:393-395. [Cited in This Article: ] |
| 13. | Liang LJ, Lu MD, Huang JF, Lu HP, Peng BG, Zhou ZP. Antiandrogen treatment for nude ice model with ectopic transplanted human HCC. Zhonghua Yixue Zazhi. 1998;78:299-300. [Cited in This Article: ] |
| 14. | Jing Y, Ohizumi H, Kawazoe N, Hashimoto S, Masuda Y, Nakajo S, Yoshida T, Kuroiwa Y, Nakaya K. Selective inhibitory effect of bufalin on growth of human tumor cells in vitro: association with the induction of apoptosis in leukemia HL-60 cells. Jpn J Cancer Res. 1994;85:645-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713-1724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 304] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Bellosillo B, Villamor N, López-Guillermo A, Marcé S, Bosch F, Campo E, Montserrat E, Colomer D. Spontaneous and drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocytic leukemia. Blood. 2002;100:1810-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Chang WK, Yang KD, Chuang H, Jan JT, Shaio MF. Glutamine protects activated human T cells from apoptosis by up-regulating glutathione and Bcl-2 levels. Clin Immunol. 2002;104:151-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Chen GG, Lai PB, Hu X, Lam IK, Chak EC, Chun YS, Lau WY. Negative correlation between the ratio of Bax to Bcl-2 and the size of tumor treated by culture supernatants from Kupffer cells. Clin Exp Metastasis. 2002;19:457-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Jang MH, Shin MC, Shin HS, Kim KH, Park HJ, Kim EH, Kim CJ. Alcohol induces apoptosis in TM3 mouse Leydig cells via bax-dependent caspase-3 activation. Eur J Pharmacol. 2002;449:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87:555-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Jiangsu New Medical College. Dictionary of Chinese Materia Medica. Shanghai: Shanghai Sci and Tech Pub 1977; 84-85. [Cited in This Article: ] |