Published online Aug 21, 2006. doi: 10.3748/wjg.v12.i31.5091
Revised: June 10, 2006
Accepted: June 16, 2006
Published online: August 21, 2006
Hepatic infarction rarely occurs due to the double supply of arterial and portal inflow. A 53-year-old man with diabetes mellitus developed multiple hepatic infarctions after an episode of fever and diarrhea. The infarction was documented by pathology after partial liver resection. Several causes of hepatic infarction may present in this patient: dehydration and hypotension caused by fever and diarrhea, type 2 diabetes and administration of glibenclamide, diabetic ketoacidosis and widespread atherosclerosis. We suggest that diabetic patient with elevated liver enzyme should be considered the possibility of hepatic infarction.
- Citation: Deng YG, Zhao ZS, Wang M, Su SO, Yao XX. Diabetes mellitus with hepatic infarction: A case report with literature review. World J Gastroenterol 2006; 12(31): 5091-5093
- URL: https://www.wjgnet.com/1007-9327/full/v12/i31/5091.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i31.5091
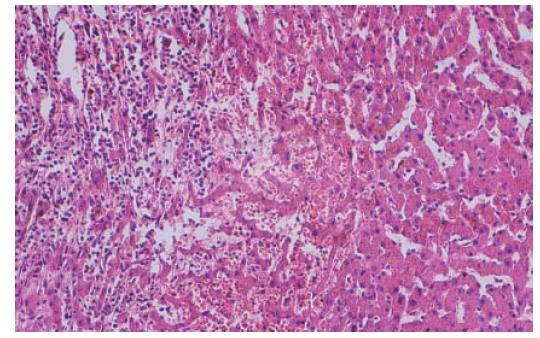
Hepatic infarction is a rare event because of the dual blood supply of the portal vein and hepatic artery, and extensive hepatic arterial collateral system. It is characterized by parenchymal necrosis involving at least two entire lobules, companied by a local circulation insufficiency[1].
Case reports of hepatic infarction in diabetes mellitus (DM) involved ketoacidosis[2] and nephrotic syndrome[3], in which upper abdominal pain was developed. We report a case of multiple hepatic infarction in diabetic patient, who was misdiagnosed with hepatic carcinoma.
A 53-year-old man was admitted to the hospital because of fever complicated with diarrhea for 4 d. There was a 4-year history of diabetes mellitus without proper control, although he was treated with glibenclamide 2.5 mg bid. His past medical history included hypertension for 2 years with the highest blood pressure of 150/100 mmHg. The patient did not complain of upper abdominal pain.
At first, he was admitted to the emergency ward. Blood routine examinations showed: hemoglobin 142 g/L, red blood count 4.58 × 1012/L, white blood count 11.7 × 109/L with neutrophils 93.1%, platelet count 71 × 109/L, mean corpuscular hemoglobin concentration 402 g/L; urine tests: ketone (+), protein (±), glucose (++++); stool routine examination: white blood cell 2-4/HP. He was treated with antibiotics because of suspicion of intestinal infection. After his condition became relative stable, he was transferred to the general ward.
On admission, physical examination revealed no abnormalities except for a slight fever (37.3°C). A second blood routine examination revealed: hemoglobin 144 g/L, white blood count 8.4 × 109/L, platelet count 77 × 109/L; urine routine examination: ketone and protein were negative, glucose (++++); stool routine examination: white blood cell 2-4/HP; liver and renal function tests and blood lipid were normal; blood glucose 13.9 mmol/L; blood potassium 3.1 mmol/L, blood sodium 127.8 mmol/L, blood chlorine 98.9 mmol/L, CO2 19.7 mmol/L; coagulation function was normal. The chest X-ray and electrocardiogram showed normal.
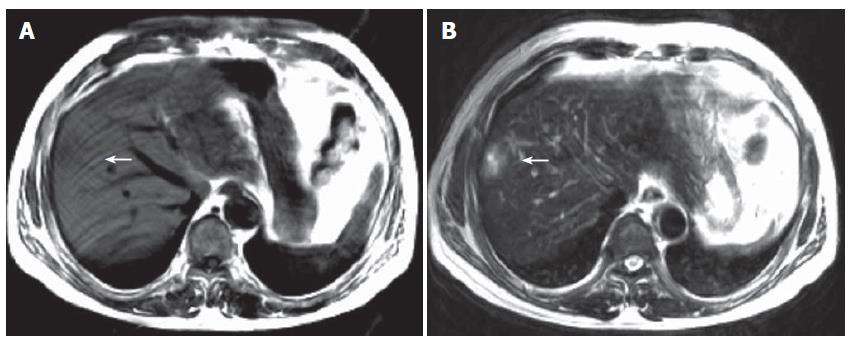
Treatment was instituted with insulin, antibiotics, and other drugs to correct microcirculation and electrolyte disturbance. After admission into the general ward for 3 d, the patient had a normal temperature and stool. But abnormalities of liver function tests were found: aspartate aminotransferase 50 U/L (normal value, < 40 U/L) and alanine aminotransferase 65 U/L (normal value, < 40 U/L). Doppler ultrasound disclosed an enhanced echo in the liver, suggesting angioma or carcinoma. Liver computerized tomography showed an abnormal signal in the right lobe (Figure 1). On the ninth day, right hepatic wedge resection was performed and the pathologic diagnosis was multiple hepatic infarction with infection (Figure 2). One month after the operation, the patient had a good recovery.
The hepatic artery supplies about 35% of hepatic blood flow and 50% of the oxygen required by the liver, while the portal vein supplies the remainder[3]. In addition, an extensive collateral system with 26 possible collateral arteries, and the hepatic arteries do not appear to be end arteries. These factors may explain why hepatic infarction is rare and usually requires impairment of both hepatic arterial and portal venous systems.
Hepatic infarction usually occurs when the hepatic artery or its branches are occluded or when the portal vein is thrombosed. Common cause of hepatic infarction is portal venous thrombosis, and hepatic infarction induced by isolated hepatic artery thrombosis is rare. Apart from vascular occlusion, other factors such as shock, sepsis, anaesthesia, biliary disease and diabetic ketoacidosis may result in hepatic infarction. Surgical factors, including abdominal surgery, laparoscopic surgery, liver transplantation, intra-arterial invasive procedures, transjugular intrahepatic portosystemic shunt, may be common causes of hepatic infarction in clinic[3,4].
The mechanism of hepatic infarction in this case is unclear. The following factors may contribute to infarction: (1) An episode of fever and diarrhea should be considered to induce dehydration and hypotension, which further decreased both portal and hepatic arterial inflows. (2) Elevated level of catecholamine in diabetic ketoacidosis might induce vasoconstriction causing ischemia and infarction[2]. (3) Oral antidiabetic agent glibenclamide taken by the patient exerted splanchnic vasoconstricting effects, which might reduce portal venous inflow or impair hepatic arterial buffer response[1]. (4) Widespread atherosclerosis is commonly seen in diabetic and hypertensive patients, and it may cause the thickening of blood vessels and arteriostenosis. Abnormalities of hemodynamics, endothelial dysfunction and hypercoagulable state possibly leading to increased thrombosis and decreased fibrinolysis have been found in diabetic patients with angiopathy. These factors could contribute to the hepatic infarction in diabetes.
Hepatic infarction is usually companied by the outbreak of sudden upper abdominal pain, fever, elevated white blood cell count, and markedly elevated liver enzymes[5]. Hyperglycemia may result in visceral automatic nerve lesion and contribute to the insensitiveness to ischemic pain. This may explain why the patient did not feel abdominal pain.
Computerized tomography scan of upper abdomen in patients with hepatic infarction usually show a well-marginated peripheral zone without any contrast capture in the corresponding hepatic lobe, wedge-shaped and extending to the liver capsule[4]. But in this case, liver computerized tomography scan exhibited untypical presentations, which led to the misdiagnosis of hepatic carcinoma and surgical operation and the diagnosis of hepatic infarction was finally established by liver pathology. MRI is the perfect method for the diagnosis of hepatic infarction because of its high resolution. In the early stage, the lesion shows slight long T1 and slight long T2 intensity. In the middle and late stages, it shows long T1 and long T2 intensity. The intensity is heterogenous due to various degrees of ischemia and infarction in the lesion. Generally speaking, the center of lesion is more apparent than the rim and shows prominent abnormal intensity as in the present case. The lesion shows no apparent enhancement and slight mass effect, which is the primary differentiation feature from hepatic mass.
In summary, we suggest that the diabetic patient with elevated liver enzymes should be suspected much of hepatic infarction and further liver computerized tomography scan should be considered to exclude the diagnosis.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Francque S, Condat B, Asselah T, Vilgrain V, Durand F, Moreau R, Valla D. Multifactorial aetiology of hepatic infarction: a case report with literature review. Eur J Gastroenterol Hepatol. 2004;16:411-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Ng RC, Sigmund CJ Jr, Lagos JA, Chernin M. Hepatic infarction and diabetic ketoacidosis. Gastroenterology. 1977;73:804-807. [PubMed] [Cited in This Article: ] |
| 3. | Martinez Vea A, Garcia Ruiz C, Sauri Conejero A, Mayayo Artal E, Oliver Rotellar J. Hepatic infarction: an unusual complication of nephrotic syndrome in a patient with diabetes mellitus. Postgrad Med J. 1990;66:968-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Shou Y, Wang X, Cheng HY, Xu AM, Chen D. Imaging analysis of hepatic infarction. Zhongguo Yixue Yingxiang Jishu. 2004;20:S58-60. [Cited in This Article: ] |
| 5. | Wu Z, Lu Y, Zhang XG, Wang HW, Li J. Liver infarction: analysis of two cases. Gandan Waike Zazhi. 2002;10:288-290. [Cited in This Article: ] |