Published online Jun 14, 2006. doi: 10.3748/wjg.v12.i22.3512
Revised: February 8, 2006
Accepted: February 18, 2006
Published online: June 14, 2006
Basic and translational wound healing research in the biliary tree lag significantly behind similar studies on the skin and gastrointestinal tract. This is at least partly attributable to lack of easy access to the biliary tract for study. But clinical relevance, more interest in biliary epithelial cell (BEC) pathophysiology, and widespread availability of BEC cultures are factors reversing this trend. In the extra-hepatic biliary tree, ineffectual wound healing, scarring and stricture development are pressing issues. In the smallest intra-hepatic bile ducts either impaired BEC proliferation or an exuberant response can contribute to liver disease. Chronic inflammation and persistent wound healing reactions in large and small bile ducts often lead to liver cancer. General concepts of wound healing as they apply to the biliary tract, importance of cellular processes dependent on IL-6/gp130/STAT3 signaling pathways, unanswered questions, and future directions are discussed.
- Citation: Demetris AJ, III JGL, Specht S, Nozaki I. Biliary wound healing, ductular reactions, and IL-6/gp130 signaling in the development of liver disease. World J Gastroenterol 2006; 12(22): 3512-3522
- URL: https://www.wjgnet.com/1007-9327/full/v12/i22/3512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i22.3512
Wound healing in the biliary tract significantly contributes to the development of liver disease. For example, ineffectual wound healing, mural scarring, and stricture development in the large extra-hepatic bile ducts are responsible for diseases such as extra-hepatic biliary atresia and primary sclerosing cholangitis. These are leading indications for liver transplantation (http://www.optn.org). A similar problem occurs in 10%-15% of all liver allografts-the biliary sludge syndrome. Conversely, an exuberant wound healing response, or ductular reaction, contributes to the development of cirrhosis from a variety of causes[1-3].
BEC lining the extra-hepatic and large intra-hepatic bile ducts have a different embryologic origin and are distinct phenotypically from BEC lining small intrahepatic bile ducts. But considerations with respect to wound healing are similar to each other and to wound healing at other sites. Unique aspects of the biliary tract that have the potential to impact significantly on wound healing include: (1) anatomy and physiology; (2) exposure to high concentration of bile; and (3) triggering of reactions in the smallest intra-hepatic bile ducts by insults other than, or in addition to, direct injury and ulceration. In addition, chronic inflammation and persistent wound healing reactions in either the large or small bile ducts often precede the development of cancers.
In the skin and intestines, mechanisms of wound repair depend on the depth of injury. Superficial wounds or simple erosions are healed primarily by a two-step process: restitution and regeneration. Restitution begins immediately after creating a superficial wound in barrier epithelia. Cells near the edge of the defect lose close contacts with neighboring cells, undergo shape changes, spread, migrate, and then contract to close the hole. However, restitution is necessarily limited because remaining cells can cover only so much of the denuded surface area. In large wounds, regeneration or proliferation of the remaining epithelial cells is also needed. Eventually the epithelium is restored to a nearly original state[4,5], although surviving cells may carry a legacy of DNA damage and senescence-related changes[6]. Deeper wounds involve the epithelia and underlying stroma. Processes such as angiogenesis; activation, migration, and proliferation of (myo-) fibroblasts and endothelial cells; formation of granulation tissue; and wound contraction are needed to close these larger/deeper defects. These more extensive wounds are also frequently inflamed and, in general, stromal involvement and inflammation greatly increase the risk of subsequent scarring[7-10].
Epithelial aspects of wound repair are often studied, in vitro, by producing linear “wound” tracks in confluent epithelial monolayers. The restitution phase is isolated by treating the cultures with chemical mito-inhibitors to prevent cell proliferation from contributing to wound closure. Distances migrated by the epithelial cells from the edge of the wound at predetermined time points measure the effectiveness of restitution. We developed a BEC model using a collagen-matrix substrate to prevent premature BEC senescence, which occurs routinely when BEC are plated on plastic or collagen-coated plates, from interfering with the assay[11].
“Front row cells”, or those epithelial cells nearest the defect, experience dissolution of epithelial cell-cell contacts and changes in cell shape[5,12]. They can also acquire some mesenchymal characteristics and, under some circumstances, can undergo complete epithelial-mesenchymal transition (EMT)[13,14]. Changes in front row cells and EMT function to disaggregate epithelial units and reshape the epithelia for movement. Epithelia in transition lose polarity, adherens junctions, tight and gap junctions, desmosomes, and down-regulate cytokeratin intermediate filaments in order to rearrange their F-actin stress fibers and express filopodia and lamellopodia[5,12-14]. When wound closure is complete and proliferation has replenished the lost cells, re-establishment of inter-epithelial junctions restores the barrier. Coordinating these processes is critically important for barrier adaptation and wound healing[13,14].
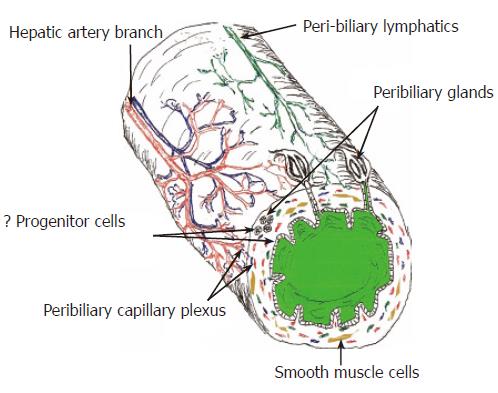
The biliary tree can be thought of as a delicate, relatively complex, self-contained organ that communicates with, and is enveloped by, the liver (Figure 1). It monitors, alters the composition of, and triages bile into the intestine. The tenuous only arterial blood supply can be damaged easily by diseases and by surgical procedures. A close relationship and cross-talk between BEC and periductal (myo-)fibroblasts exists throughout the entire biliary tree: damage to one population usually results in reactive changes in the other. For example, periductal (myo-) fibroblasts often undergo activation and proliferation in response to significant BEC growth, injury, and bile leakage from the small[15-21] or large bile ducts [22-24]. In extra-hepatic and large intra-hepatic bile ducts, this results in mural stricturing and luminal narrowing. In smaller intra-hepatic bile ducts, liver fibrosis and/or obliteration of the bile duct lumen can occur. A rich lymphatic network envelopes bile ducts that drains into regional hilar lymph nodes[25,26]. Numerous intramural peribiliary glands in the extra-hepatic bile ducts can become walled off after trauma and produce mucoceles. All of these potential sources of problems contribute to the well-deserved moniker of the biliary tree as the “Achilles heel” of liver transplantation.
Bile contains bile salts that can induce[27-30] or protect BEC from apoptosis[31], cross-activate EGFR via TGFα ligand binding[32], induce COX-2 expression[33], or trigger BEC IL-6 and other cytokine production[34]. Bile also normally contains several growth factors (e.g. HGF), cytokines (IL-6), and other molecules[35]. Understanding the effect of various bile constituents on wound healing (esp. restitution) is critical because bile composition can be altered therapeutically (e.g. treating patients with ursodeoxycholic acid) [36,37].
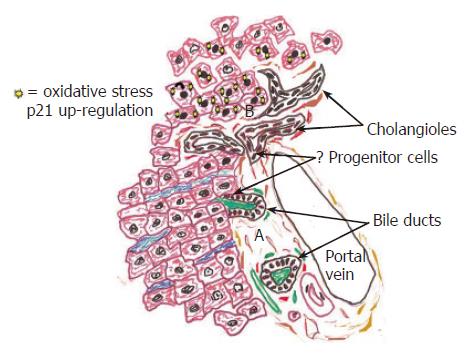
BEC lining the smallest intra-hepatic bile ducts are derived from hepatoblasts and are thought to contain a population of liver stem cells that can differentiate into either hepatocytes or BEC[38-41]. Changes in the intra-hepatic environment other than, or in addition to, direct injury and ulceration can trigger wound repair reactions in these smallest ducts. These “ductular reactions” are recognized as BEC and surrounding myofibroblasts at the interface zone of diseased livers[3,42,43]. Ductular reactions can be provoked by: (1) local BEC injury and inflammation[44,45]; (2) increased intra-biliary tract pressure, and (3) the combination of: (a) a strong liver regenerative stimulus, such as partial hepatectomy or chronic necro-inflammatory liver disease and (b) hepatocyte mito-inhibition because of carcinogen exposure or chronic oxidative stress[1-3]. Insufficient BEC regeneration in the smallest ducts leads to liver diseases such as chronic “ductopenic” rejection and drug-induced ductopenia[44].
Study of the biliary sludge syndrome in liver allografts has been particularly illustrative of pathophysiologic mechanisms involved in biliary wound healing. This relatively common and particularly frustrating complication affects about 10% of all liver allografts. There are many potential causes of ineffectual biliary wound healing including abnormal anatomy created by the operation; suboptimal arterial blood flow because of technical problems, anastomotic narrowing, thrombosis, or antibody mediated rejection; recurrent ascending cholangitis; recurrent primary sclerosing cholangitis; and ischemic-preservation injury[46]. Regardless of the cause, impediments to bile drainage results in progressive intrahepatic fibrosis, which in turn, increases morbidity and decreases organ half-life.
The extra-hepatic biliary tree is sustained only by the hepatic artery, which drains into three terminal classical capillary microvascular networks that supply: (1) the bile ducts (peribiliary plexus), (2) the connective tissue of the portal tracts, and (3) the hilar and perihilar structures[47]. The allograft biliary tree is especially vulnerable to arterial ischemia for the first several months after the operation. Preservation injury damages the microvasculature of the peribiliary plexus. The transplant operation can injure the arterial blood supply and it can also disrupt the normal collateral circulation typical of the arterial cascade arrangement supplying all gastrointestinal organs, including the liver[46]. Interference with arterial flow at any level can result in “ischemic cholangitis” - a succinct phrase used to describe the common association between poor arterial flow and biliary ischemia that manifests as persistent ulcers, inflammation, sludge, and strictures[48,49].
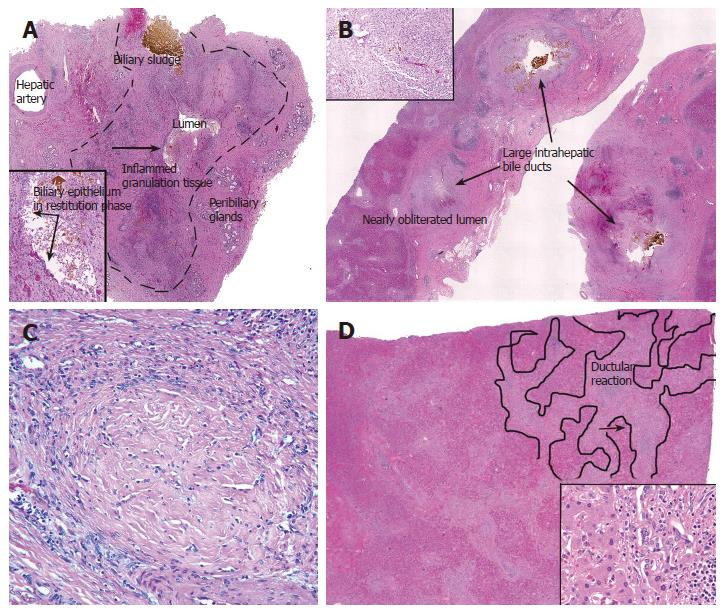
Cold ischemic-preservation injury depletes energy stores in microvascular endothelial cells and BEC. This results in activation of metalloproteinases, detachment of endothelium and BEC from the underlying matrix, and in the microvasculature, predisposition to thrombosis after reperfusion[50,51]. Reperfusion with blood after transplantation also delivers leukocytes that become activated by tissue damage. Activated leukocytes release effector molecules, which in turn, cause more tissue damage and further promote thrombogenesis[50,51]. Hydrophobic bile salts remaining in the biliary tree also further damage marginally viable BECs[50,52] already weakened by preservation injury. Damaged BEC are sloughed into the bile[53]. Although patient and allograft survival during the first several weeks after transplantation are dependent primarily on parenchymal function, long term allograft viability is determined primarily by biliary wound healing and adequate bile drainage[54,55]. Allografts that eventually fail show biliary sludge, mucosal ulcers, and inflamed granulation tissue and myofibroblast activation/proliferation in the wall of extra-hepatic and large intra-hepatic bile ducts[55] (Figure 2). Exposure of the underlying stroma to bile appears to serve as a nidus for crystallization of biliary sludge and a stimulus for inflammation and activation of myofibroblasts. This leads to wound contraction and fibrosis, and eventually, to strictures in large caliber ducts and to luminal obliteration of smaller caliber ducts (Figure 2C).
Two observations illustrate the critical and primary importance to wound healing of an adequate arterial blood supply. First, any insult that directly interferes with the arterial flow is usually associated with large bile duct ulcers and strictures. Second, perfusion of the hepatic artery and peribiliary plexus with low viscosity preservation solutions before transplantation dramatically decreases the incidence of biliary complications after transplantation in otherwise susceptible extended criteria donor livers with long cold ischemic times[56]. This maneuver is thought to flush thrombogenic material from the peribiliary plexus and facilitate reperfusion and oxygenation after transplantation. As with many clinical observations, confirmation of this mechanism is lacking. But it is reasonable to conclude that without sufficient arterial flow biliary wound healing is unlikely to proceed normally. Once adequate arterial flow is ascertained, other factors that also significantly contribute to BEC wound repair can then be studied in greater detail.
Deep wounds of extra-hepatic bile ducts precipitate stromal involvement in wound healing. Granulation tissue and inflammation, local production of interferon-γ[57] and transforming growth factor beta (TGF-β)[58] at this site are of importance in wound contraction and scarring. Our laboratory has focused primarily on BEC aspects of wound repair with an emphasis on interleukin-6/gp130 signaling-dependent cellular processes. This signaling pathway is also critically important for wound healing in the gastrointestinal tract[59] and skin[60,61].
IL-6/gp130 is pleiotropic cytokine signaling system that has diverse effects in many different organ systems and cell types(reviewed in[62,63]). gp130 is one of the most promiscuous cytokine receptors[62], binding to many different ligands including, interleukin (IL)-6, IL-11, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), and cardiotrophin-like cytokine (CLC)[62,63]. Ligand binding to gp130 generates two major signaling pathways: (1) Jak/STAT3 and (2) SHP2/ERK/MAPK and there is reciprocal negative feedback regulation between them[63,64]. Non-canonical STAT3 signaling pathways also exist, but are not well delineated[65].
In normal livers, IL-6 is produced at low levels by the BEC, perhaps stimulated by the bile salts[34], and secreted into the bile[66]. Active IL-6/gp130/STAT3 signaling can be detected in normal IL-6+/+ but not in normal IL-6-/- mice livers, as evidenced by the detection of phospho-STAT3 by Western blotting and immunohistochemistry. Biliary tree pSTAT3 in normal liver localizes to occasional BEC lining large bile ducts, but more prevalent expression is seen in BEC lining the peribiliary glands[11].
Virtually any bile duct insult, such as obstruction[67-69], infection[69,70], or immunologic damage[66,71,72] triggers sharp increases in IL-6 mRNA and protein production by BEC and peribiliary hematolymphoid cells[3]. This, in essence, alerts BEC to environmental stimuli and leads to subsequent autocrine, paracrine, and juxtacrine gp130/STAT3 signaling in BEC at the sites of injury[11,67,68,73]. As in the gastrointestinal tract[59] and skin[60,61], an absence of IL-6 in IL-6-deficient (IL-6-/-) mice leads to impaired wound healing[11] and biliary tree integrity[11,73,74]. For the last several years our laboratory has focused on cellular and molecular mechanisms that might contribute to impaired BEC wound healing and biliary barrier defects in the IL-6-/- mice and how the findings might apply to humans. Using a combination of knowledge gained from the gastrointestinal tract and skin, mRNA microarray expression analyses, and pathophysiologic studies, we first searched for genes that were: (1) expressed in BECs, (2) regulated by IL-6/gp130/STAT3 signaling, and (3) possibly involved in barrier function and/or repair. Two of the most interesting candidates, studied in greater detail, included intestinal trefoil family factors (TFF)[59,75-78] and small protein rich proteins (SPRR)[11,73]. The reader is referred to recent reviews of IL-6/gp130/ SHP2/ERK/MAPK signaling and other growth factors and cytokines involved in the regenerative phase of BEC wound healing[3,67,68,79-81].
Trefoil family factor (TFF) proteins are comprised of one or more trefoil motifs, which consist of 6 cysteine residues. TFF proteins increase mucous viscosity and thereby contribute to optimal protection of the intestinal mucosa from injury[77,82-84]. By enhancing intestinal epithelial spreading and migration, TFF proteins also stimulate the restitution phase of wound healing[76,85]. Each of the three known TFF proteins is differentially regulated in the gastrointestinal tract[59,75]: TFF1 and TFF2 are expressed primarily in the stomach[86,87]. TFF3 predominates in the small and large intestines[78] and in the biliary tree[11] of mouse and human livers[88-91]. IL-6/gp130/STAT3 signaling is crucial for BEC TFF3 expression. In normal liver, pSTAT3 and TFF3 mRNA and protein expression are significantly higher in IL-6+/+ than in IL-6-/- mice livers. Constitutive expression of TFF3 and pSTAT3 localizes to mucin secreting BEC lining large intrahepatic and extrahepatic bile ducts and peribiliary glands[11]. Medium and small-sized intra-hepatic bile ducts are generally negative for mucin secreting BEC, pSTAT3, and TFF3 expression[11,91,92].
IL-6+/+ BEC consistently show higher levels of TFF3 mRNA and protein expression and significantly better migration and wound healing than IL-6-/- BEC[11], in vitro. Defective migration in the IL-6-/- BEC can be partially, but significantly, reversed by treatment with recombinant TFF peptides[11]. In vivo, biliary TFF3 is dynamically regulated by various factors after bile duct ligation. Included are the reciprocal negative regulation known to exist between the STAT3 and MAPK signaling pathways[59], and other cytokines and growth factors, such as HGF and TGF-β, which can down-regulated BEC TFF3 expression[11]. However, a chronic deficiency of pSTAT3 signaling during bile duct injury, as seen in IL-6-/- mice after bile duct ligation, leads to a chronic deficiency of biliary TFF3 expression and impaired biliary barrier function[11]. In humans, p-STAT3 and TFF3 are newly co-expressed in BEC involved in florid duct lesions in primary biliary cirrhosis and at other sites of BEC injury, but not in similarly-sized normal bile ducts from the same livers[11,89,92]. This likely constitutes a primitive or innate mucosal defense system that guards against injury and stimulates repair.
Our BEC TFF3 studies are consistent with studies focused on the colon and carried out in mice harboring mutations that selectively block all gp130-mediated STAT activity (gp130ΔSTAT), but preserve gp130-mediated MAPK signaling. These mice show decreased colonic TFF3 expression, increased sensitivity to sodium dextran sulfate-induced colitis, and impaired mucosal wound healing[59,75]. Thus, it is reasonable to conclude that IL-6/gp130/STAT3 signaling contributes significantly to normal BEC cytoprotective mechanisms and to migration during wound healing, at least in part, by stimulating BEC TFF3 expression[11].
Small proline-rich proteins (SPRR) are encoded by a tandemly arranged four-member gene family contained within a 170-kilobase region of the epidermal differentiation complex (EDC). The EDC is a cluster of more than 50 genes located on chromosome 1q21[93-95] whose products are involved in terminal differentiation of the human epidermis. Included are formation of the cornified envelope that is an effective barrier against the external environment [93,96]. The four SPRR gene families, SPRR1-4, are distinguished on the basis of the number of amino acids in the repeats of the protein’s central domain and the consensus of that sequence[95]. There are two Sprr1 genes and one copy each of Sprr3 and Sprr4 genes[95]. SPRR2 genes are the most diversified family: there are seven in humans and eleven in mice[95]. In the skin and other squamous epithelia, SPRR genes are usually regulated coordinately as part of the EDC (i.e. high expression of most EDC genes, as in papillomas, or very low expression of most genes, as in newborn skin). SPRR genes encode for a series of highly homologous proteins that function primarily as critical cross-linkers. They form bridges among other EDC proteins, intermediate filaments, and cornified envelope constituents[94,95,97], such as desmoplakin, loricrin, and tricohyalin, through the catalytic action of transglutaminases[98] .
The diverse SPRR2 genes are also non-coordinately expressed, or expressed preferentially, without similar upregulation of other EDC family members[73,97]. This occurs most commonly in non-keratinizing epithelia in curious situations that cannot be explained by squamous differentiation or formation of a cornified envelope. Examples include greater than 100-fold increases of SPRR2A in the intestine after small bowel resection[99] or after introduction of commensal bacteria into germ-free mice[100,101] or after intentional infection with intestinal parasites[102]. In uterine epithelium SPRR2A mRNA and protein expression is, at least in part, regulated by estrogen[103]. Therefore, it is highly and non-coordinately upregulated during certain stages of the oestrous cycle[104,105] and is especially high at the blastocyst implantation site[103]. SPRR2A mRNA and protein are also expressed in bronchial and intestinal epithelium during allergic reactions[106]. Barrier remodeling, as a response to stress[94,105], inflammation, and/or growth, is a common condition of these diverse circumstances. Potential molecular and cellular processes affected by non-coordinate SPRR2A expression are currently under investigation in our laboratory.
In the liver, we have shown that SPRR2A mRNA and protein are not expressed in normal mouse liver, but are non-coordinately upregulated only in BEC after the stress of bile duct ligation[73]. Expression after bile duct ligation is not related to squamous metaplasia and shows strong dependence on IL-6/gp130/STAT3 signaling. In BEC lining the large bile ducts, SPRR2 protein localizes subjacent to the apical plasma membrane. SPRR2 expression is more diffusely distributed throughout the cytoplasm of cholangioles participating in ductular reactions and in large duct BEC engaged in the restitution phase of mucosal wound healing. Deficient BEC SPRR2A expression in IL-6-/- mice after bile duct ligation is associated with impaired barrier function[73]. IL-6 replacement therapy restores SPRR2A expression to levels seen in wild type controls and reverses the barrier defect in IL-6-/- mice. In a series of ongoing investigations, preliminary data suggest that BEC SPRR2A expression is associated with BEC restitution.
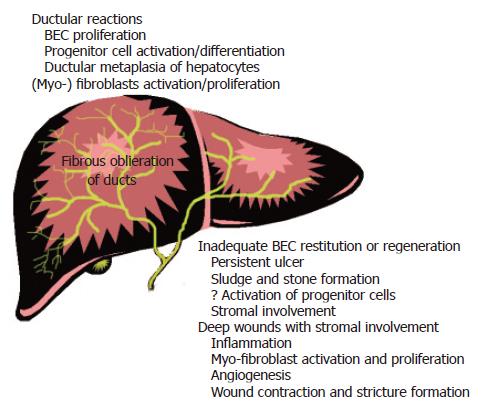
Wound healing responses can be triggered in BEC lining the smallest intrahepatic bile ducts by environmental changes other than, or in addition to, direct injury and ulceration. This often occurs in chronic necro-inflammatory liver disease regardless of the underlying cause. For several years we were puzzled by the observation that ductular reactions represent a survival advantage for BEC and myofibroblasts over hepatocytes, yet hepatocytes and BEC share the same responses to many cytokines and growth factors that are upregulated in chronic liver disease (e.g. HGF, EGF, IL-6, TGFβ, etc.[81,107]). Why then do BEC and myofibroblasts survive preferentially under these circumstances To answer this question it is helpful to view chronic necro-inflammatory liver disease as a “Darwinian” selection pressure applied to the liver. A survival advantage for BEC can occur because of a relative increase in the rate of proliferation, a relative decrease in the rate of death, transformation of hepatocytes into BEC, or various combinations of the above. Regardless of the mechanism, the end result is a relative decrease in volume percentage of hepatocytes and a relative increase in biliary epithelial cells and myofibroblasts-a pattern typical of evolving cirrhosis[1,74]. When combined with sufficient time[1,2], even a small deficit of hepatocyte survival is enough to evoke a ductular reaction that distorts the hepatic architecture.
Using an established mouse model of decompensated biliary cirrhosis[74] and p21-deficient mice, we tested the hypothesis that hepatocyte mito-inhibition combined with the regenerative stimulus of bile duct ligation would accentuate the ductular reaction and accelerate architectural distortion. Results showed that after long-term (12-wk) ligation mice prone to decompensation show significantly more oxidative stress and hepatocyte nuclear p21 expression, a cyclin dependent kinase inhibitor and important mediator of hepatocyte mito-inhibition[1]. As expected, mice prone to decompensation also showed less hepatocyte proliferation, an exaggerated ductular reaction, and accelerated architectural distortion compared with compensation-prone controls[1]. We next subjected p21 deficient mice to bile duct ligation for 12 wk with the expectation that p21 deficient mice would be better able than wild-type controls to compensate for long-term BDL because of significantly greater hepatocyte proliferation. Indeed, results of these experiments showed that p21-deficient mice showed a larger liver mass because of more hepatocyte proliferation, a less florid ductular reaction, and less architectural distortion than wild type controls[1].
We next wanted to determine whether this concept was applicable to other, non-cholestatic or non-biliary, liver diseases. To accomplish this task, we first showed that hepatocyte nuclear p21 expression in humans awaiting liver replacement directly correlated with pathological disease stage and model of end-stage liver disease scoring[1]. We also engaged in a collaborative study with Clouston et al who had previously shown that HCV-related liver disease progresses more rapidly when there is co-existent hepatic steatosis[108]. Liver biopsies from 115 patients with HCV scored for steatosis, inflammation, and fibrosis showed a strong correlation between (a) a ductular reaction and portal fibrosis and (b) steatosis and impaired hepatocyte replication[2]. Steatosis correlated with the ductular reaction and greater hepatic progenitor cell proliferation, but was not an obligate feature. The highly significant correlation between the ductular reaction area and fibrosis stage remained even after multivariate analysis[2]. Impaired hepatocyte replication, as determined by p21 expression, was independently associated with hepatic progenitor cell expansion, increased body mass index, and lobular inflammation.
The observation that ductular reactions often appear when hepatocyte mito-inhibition is combined with a liver regenerative stimulus is not new. It was made originally years ago while treating experimental animals with genotoxic carcinogens and then subjecting them to partial hepatectomy (reviewed in[3,109,110]). These maneuvers stimulate oval cell, or liver epithelial progenitor cell[111], expansion/proliferation at the interface zone-a ductular reaction. However, in carcinogenesis experiments, the ductular reaction eventually resolves without fibrosis.
Our studies show that this concept is applicable to ductular reactions associated with chronic necro-inflammatory liver diseases and the development of fibrosis/cirrhosis[1] (Figure 3). Hepatocytes are more susceptible to injury and mito-inhibition during chronic necro-inflammatory liver disease because they: (1) produce and secrete bile and are the major site of bile stasis; (2) are more complex metabolically and able preferentially to store lipids and metals, such as iron and copper, which are generators and/or catalysts for free oxygen radical formation; and, (3) support HBV and HCV replication, and (4) maybe most importantly, contain many more mitochondria than BEC and mitochondria are the major site of superoxide production[112]. Diverse disorders such as cholestasis[1], HCV replication[113,114], steatosis[2,115], copper deposition[116], and alcohol[117] preferentially stress or injure hepatocytes and this causes nuclear expression of the cyclin-dependent kinase inhibitor, p21[1,2], which in turn, inhibits hepatocyte proliferation.
When small intrahepatic bile ductules are destroyed (ductopenia), due to drugs or chronic allograft rejection, classical cirrhosis usually does not develop[45,109,118,119]. Despite ongoing immunologic liver injury and fibrosis, regenerative nodularity and the associated complications of portal hypertension rarely occur. BEC survival and proliferation in response to injury in these small ductules is related to a combination of immunologic injury, environmental influences, and importantly, arterial blood flow[120], as in the large bile ducts. Perhaps the arterial disease also inhibits regenerative nodule formation (Figure 4).
Persistent ductular reactions in human chronic necro-inflammatory diseases can activate progenitor cell popu-lations, as in the experimental animal carcinogenesis models, discussed above (Reviewed in refs 38, 39, 41). Several groups, including ours, have shown that oval cell expansion in mice is dependent significantly on IL-6/gp130/STAT3 signaling[121,122]. This raises the possibility that ductular reactions accelerate the development of cirrhosis and potentially increase the risk of hepatocellular carcinoma. Since oval cells eventually differentiate into hepatocytes[111], exposure to carcinogens or genotoxic damage from oxidative stress imprint genetic mutations in putative liver stem cells. These cells then divide, differentiate, and spread initiated cells more widely throughout the entire hepatocyte population. Liver cancers occur when initiated hepatocytes are subjected to tumor promoters that generally cause hepatocyte proliferation. Chronic liver disease is an excellent cancer-promoting environment.
In the skin, STAT3 signaling enables epidermal stem cells to the escape apoptosis induced by exposure to cutaneous carcinogens[123]. Initiated stem cells survive, divide, differentiate, and subsequently give rise to skin cancers in a promoting environment[123]. Similar processes might occur in hepatocellular and cholangiocarcinomas. Interleukin-6/gp130/STAT3 signaling might provide important survival signals for initiated liver epithelial progenitor cells that later give rise to liver cancers in the context of chronic necro-inflammatory disease. In evolutionary biology[124] this process is referred as “antagonistic pleiotropy” - a short term survival benefit at the expense of long-term increased risk of cancer.
IL-6/gp130/STAT3 signaling is indeed increased in a very wide variety of neoplasms, including hepatocellular carcinomas, melanomas, leukemias and myelomas, and lung, breast cancer, kidney, prostate, pancreatic, colon, gastric, cervical, ovarian, and head and neck cancers[125,126]. This signaling pathway participates and/or regulates many pathways important in oncogenesis including cell-cycle progression, apoptosis, tumor angiogenesis, tumor-cell invasion and metastasis, and tumor-cell evasion of the immune system (reviewed in[125,126]).
Whether this concept is applicable to cancers arising in extrahepatic and large intrahepatic bile ducts is uncertain because the mechanisms of BEC renewal at these sites have not been studied in any great detail. In the larger bile ducts there are two potential sources of new BEC: (1) proliferation of mature BEC; and (2) proliferation and/or maturation of progenitor cell populations. These potential sources are not mutually exclusive and only one study in the literature even indirectly addresses this topic. Koike et al[127] used pulse and continuous DNA labeling studies to show, in rats, that a proliferative zone, consistent with a BEC progenitor cell population, localized to peribiliary glands (called “crypts” in their study). Long-term follow-up of the animals showed that labeled BEC migrated gradually to the surface and were shed into the lumen with a transit time of about 30-40 d[127]. They concluded the arrangement and pattern of BEC renewal in the extra-hepatic bile ducts was similar to the intestine and colon, but kinetics of BEC turnover was slower[127]. More definitive work is needed in this area.
The following are a few examples of the many unanswered questions in the study of biliary wound healing that have stimulated research in our laboratory. For example, are there progenitor cell populations within the peribiliary glands or crypts of extra-hepatic and large intra-hepatic bile ducts If progenitor cells exist in the extra-hepatic biliary tree, where exactly do they reside, and how are they recognized Are they activated during wound repair, and do they contribute to the development of bile duct cancers
EMT contributes significantly to wound healing and to kidney fibrogenesis. Is EMT an important process in BEC wound healing and hepatic fibrogenesis Can, and do, BEC transform into myo-(fibroblasts) BEC appear to migrate and can acquire mesenchymal characteristics during the ductular reaction and hepatic fibrogenesis. They transform from polarized cuboidal epithelial cells into a spindle or dendritic-shaped vimentin-positive cells after bile duct ligation and in PBC[128,129]. In embryonic liver, ductal plate BEC “invade” or migrate into the portal tract connective tissue to form mature intrahepatic bile ducts. During migration BEC express vimentin[130] and down-regulate membranous E-cadherin[131] expression. Once mature bile ducts are formed BEC revert totally to an epithelial phenotype. Preliminary studies from our laboratory suggest that IL-6/gp130/STAT3 signaling triggers BEC changes under some circumstances that can induce BEC EMT. The extent to which EMT contributes to BEC wound healing, however, requires further study.
Several studies show that IL-6 pre-treatment can prevent liver parenchymal ischemic-preservation injury[132-136]. We have shown similar results in intestinal allografts: IL-6 pretreatment limits epithelial damage and promotes repair[137]. Would pretreatment of donor livers with IL-6, particularly in the aortic flush, have beneficial effects on preservation injury of the peribiliary plexus and promote wound healing in the biliary tree We would expect IL-6/gp130 signaling to help lessen the incidence of biliary strictures because it upregulates anti-apoptotic molecules in the microvascular endothelium[133,136], preserves epithelial integrity[11,137], and stimulates BEC restitution[11,73] and regeneration[79] after injury.
Several other strategies might be used to prevent biliary strictures[138,139], such as reducing eosinophil and mast cell accumulation with Tranilast[140,141] and Captopril[142-144], reducing activation and transformation of myofibroblasts with anti-oxidants such alpha tocopherol (vitamin E) and peroxisome proliferator-activated receptors-γ (PPAR-γ) ligands, such as the thiazolidinedione family of drugs[145-147].
S- Editor Pan BR E- Editor Bi L
| 1. | Lunz JG 3rd, Tsuji H, Nozaki I, Murase N, Demetris AJ. An inhibitor of cyclin-dependent kinase, stress-induced p21Waf-1/Cip-1, mediates hepatocyte mito-inhibition during the evolution of cirrhosis. Hepatology. 2005;41:1262-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Demetris AJ, Lunz JG, Subbotin V, Wu T, Nozaki I, Contrucci S, Yin X. Participation of cytokines and growth factors in biliary epithelial proliferation and mito-inhibition during ductular reactions. et al, editor. The pathophysiology of biliary epithelia. Georgetown, TX: Landes Bioscie 2003; 167-191. [Cited in This Article: ] |
| 4. | Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 449] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 5. | Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol. 2001;3:E117-E123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 272] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 368] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med. 2003;31:S532-S537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 2004;12:134-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost. 2003;90:993-1002. [PubMed] [Cited in This Article: ] |
| 11. | Nozaki I, Lunz JG 3rd, Specht S, Park JI, Giraud AS, Murase N, Demetris AJ. Regulation and function of trefoil factor family 3 expression in the biliary tree. Am J Pathol. 2004;165:1907-1920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Jacinto A, Martin P. Morphogenesis: unravelling the cell biology of hole closure. Curr Biol. 2001;11:R705-R707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776-1784. [PubMed] [Cited in This Article: ] |
| 14. | Boyer B, Vallés AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000;60:1091-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 351] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 15. | Miyazaki H, Van Eyken P, Roskams T, De Vos R, Desmet VJ. Transient expression of tenascin in experimentally induced cholestatic fibrosis in rat liver: an immunohistochemical study. J Hepatol. 1993;19:353-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Tang L, Tanaka Y, Marumo F, Sato C. Phenotypic change in portal fibroblasts in biliary fibrosis. Liver. 1994;14:76-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Libbrecht L, Cassiman D, Desmet V, Roskams T. The correlation between portal myofibroblasts and development of intrahepatic bile ducts and arterial branches in human liver. Liver. 2002;22:252-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 337] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Ramadori G, Saile B. Portal tract fibrogenesis in the liver. Lab Invest. 2004;84:153-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Jhandier MN, Kruglov EA, Lavoie EG, Sévigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280:22986-22992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Sobesky R, Chollet JM, Prat F, Karkouche B, Pelletier G, Fritsch J, Choury AD, Allonier C, Bedossa P, Buffet C. Inflammatory pseudotumor of the common bile duct. Endoscopy. 2003;35:698-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Dehner LP, Coffin CM. Idiopathic fibrosclerotic disorders and other inflammatory pseudotumors. Semin Diagn Pathol. 1998;15:161-173. [PubMed] [Cited in This Article: ] |
| 24. | Inaba K, Suzuki S, Yokoi Y, Ota S, Nakamura T, Konno H, Baba S, Takehara Y, Nakamura S. Hepatic inflammatory pseudotumor mimicking intrahepatic cholangiocarcinoma: report of a case. Surg Today. 2003;33:714-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Yamamoto K, Phillips MJ. Three-dimensional observation of the intrahepatic lymphatics by scanning electron microscopy of corrosion casts. Anat Rec. 1986;214:67-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Szabó G, Magyar Z, Szentirmai A, Jakab F, Mihaly K. Bile constituents in blood and lymph during biliary obstruction. II. The absorption and transport of bile acids and bilirubin. Lymphology. 1975;8:36-42. [PubMed] [Cited in This Article: ] |
| 27. | Tsuboi K, Tazuma S, Nishioka T, Chayama K. Partial characterization of cytoprotective mechanisms of lecithin against bile salt-induced bile duct damage. J Gastroenterol. 2004;39:955-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Barone M, Maiorano E, Ladisa R, Pece A, Berloco P, Strazzabosco M, Caruso ML, Valentini AM, Ierardi E, Di Leo A. Ursodeoxycholate further increases bile-duct cell proliferative response induced by partial bile-duct ligation in rats. Virchows Arch. 2004;444:554-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Patel T, LaRusso NF, Gores GJ. Interleukin-6 suppresses cholangiocyte apoptosis by down-regulation of Bax. Hepatology. 1997;26:226A. [Cited in This Article: ] |
| 30. | Que FG, Gores GJ, LaRusso NF. Development and initial application of an in vitro model of apoptosis in rodent cholangiocytes. Am J Physiol. 1997;272:G106-G115. [PubMed] [Cited in This Article: ] |
| 31. | Komichi D, Tazuma S, Nishioka T, Hyogo H, Une M, Chayama K. Unique inhibition of bile salt-induced apoptosis by lecithins and cytoprotective bile salts in immortalized mouse cholangiocytes. Dig Dis Sci. 2003;48:2315-2322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Werneburg NW, Yoon JH, Higuchi H, Gores GJ. Bile acids activate EGF receptor via a TGF-alpha-dependent mechanism in human cholangiocyte cell lines. Am J Physiol Gastrointest Liver Physiol. 2003;285:G31-G36. [PubMed] [Cited in This Article: ] |
| 33. | Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Lamireau T, Zoltowska M, Levy E, Yousef I, Rosenbaum J, Tuchweber B, Desmoulière A. Effects of bile acids on biliary epithelial cells: proliferation, cytotoxicity, and cytokine secretion. Life Sci. 2003;72:1401-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Kristiansen TZ, Bunkenborg J, Gronborg M, Molina H, Thuluvath PJ, Argani P, Goggins MG, Maitra A, Pandey A. A proteomic analysis of human bile. Mol Cell Proteomics. 2004;3:715-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 449] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 37. | Colombo C, Crosignani A, Assaisso M, Battezzati PM, Podda M, Giunta A, Zimmer-Nechemias L, Setchell KD. Ursodeoxycholic acid therapy in cystic fibrosis-associated liver disease: a dose-response study. Hepatology. 1992;16:924-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Strain AJ, Crosby HA, Nijjar S, Kelly DA, Hubscher SG. Human liver-derived stem cells. Semin Liver Dis. 2003;23:373-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis. 2004;24:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Thorgeirsson SS, Grisham JW. Overview of recent experimental studies on liver stem cells. Semin Liver Dis. 2003;23:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | POPPER H, KENT G, STEIN R. Ductular cell reaction in the liver in hepatic injury. J Mt Sinai Hosp N Y. 1957;24:551-556. [PubMed] [Cited in This Article: ] |
| 43. | Demetris AJ, Sakamoto T, Liu Z, Yokomuro S, Ezure T, Murase N, et al. The ductular reaction in liver disease emphasis on a type I response. Normal and Malignant Liver Cell Growth. Dordecht: Kluwer Academic Publishers 1999; 141–155. [Cited in This Article: ] |
| 44. | Lunz JG 3rd, Contrucci S, Ruppert K, Murase N, Fung JJ, Starzl TE, Demetris AJ. Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21(WAF1/Cip1) as a disease marker and the influence of immunosuppressive drugs. Am J Pathol. 2001;158:1379-1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Blakolmer K, Seaberg EC, Batts K, Ferrell L, Markin R, Wiesner R, Detre K, Demetris A. Analysis of the reversibility of chronic liver allograft rejection implications for a staging schema. Am J Surg Pathol. 1999;23:1328-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Demetris A, Crawford J, Nalesnik M, Randhawa P, Wu T, Minervini M. Transplantation Pathology of the Liver. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. Philadelphia: WB Saunders 2004; 909-966. [Cited in This Article: ] |
| 47. | Wanless I. Physioanatomic Considerations. Diseases of the Liver. Philadelphia: Lippincott Williams & Wilkins 1999; 3-37. [Cited in This Article: ] |
| 48. | Demetris AJ. Ischemic cholangitis. Mayo Clin Proc. 1992;67:601-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Ludwig J, Batts KP, MacCarty RL. Ischemic cholangitis in hepatic allografts. Mayo Clin Proc. 1992;67:519-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Kukan M, Haddad PS. Role of hepatocytes and bile duct cells in preservation-reperfusion injury of liver grafts. Liver Transpl. 2001;7:381-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 52. | Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993;56:495-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 130] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Carrasco L, Sanchez-Bueno F, Sola J, Ruiz JM, Ramirez P, Robles R, Rodriquez JM, Parrilla P. Effects of cold ischemia time on the graft after orthotopic liver transplantation. A bile cytological study. Transplantation. 1996;61:393-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 481] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 55. | Demetris AJ, Fontes P, Lunz JG, Specht S, Murase N, Marcos A. Wound healing in the biliary tree of liver allografts. Cell Transplant. 2006;15 Suppl 1:S57-S65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Pirenne J, Gunson B, Khaleef H, Hubscher S, Afford S, McMaster P, Adams D. Influence of ischemia-reperfusion injury on rejection after liver transplantation. Transplant Proc. 1997;29:366-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, Bezerra JA. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322-329. [PubMed] [Cited in This Article: ] |
| 58. | Geng ZM, Yao YM, Liu QG, Niu XJ, Liu XG. Mechanism of benign biliary stricture: a morphological and immunohistochemical study. World J Gastroenterol. 2005;11:293-295. [PubMed] [Cited in This Article: ] |
| 59. | Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 60. | Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73:713-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 61. | Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525-2531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 312] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 62. | Müller-Newen G. The cytokine receptor gp130: faithfully promiscuous. Sci STKE. 2003;2003:PE40. [PubMed] [Cited in This Article: ] |
| 63. | Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 337] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 64. | Ishihara K, Hirano T. Molecular basis of the cell specificity of cytokine action. Biochim Biophys Acta. 2002;1592:281-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Inghirami G, Chiarle R, Simmons WJ, Piva R, Schlessinger K, Levy DE. New and old functions of STAT3: a pivotal target for individualized treatment of cancer. Cell Cycle. 2005;4:1131-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased expression of interleukin-6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest. 1998;78:89-100. [PubMed] [Cited in This Article: ] |
| 67. | Liu Z, Sakamoto T, Ezure T, Yokomuro S, Murase N, Michalopoulos G, Demetris AJ. Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in mice: interactions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology. 1998;28:1260-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Liu Z, Sakamoto T, Yokomuro S, Ezure T, Subbotin V, Murase N, Contrucci S, Demetris AJ. Acute obstructive cholangiopathy in interleukin-6 deficient mice: compensation by leukemia inhibitory factor (LIF) suggests importance of gp-130 signaling in the ductular reaction. Liver. 2000;20:114-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Rosen HR, Winkle PJ, Kendall BJ, Diehl DL. Biliary interleukin-6 and tumor necrosis factor-alpha in patients undergoing endoscopic retrograde cholangiopancreatography. Dig Dis Sci. 1997;42:1290-1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Scotte M, Daveau M, Hiron M, Delers F, Lemeland JF, Teniere P, Lebreton JP. Interleukin-6 (IL-6) and acute-phase proteins in rats with biliary sepsis. Eur Cytokine Netw. 1991;2:177-182. [PubMed] [Cited in This Article: ] |
| 71. | Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725-731. [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 72. | Akiyama T, Hasegawa T, Sejima T, Sahara H, Seto K, Saito H, Takashima S. Serum and bile interleukin 6 after percutaneous transhepatic cholangio-drainage. Hepatogastroenterology. 1998;45:665-671. [PubMed] [Cited in This Article: ] |
| 73. | Nozaki I, Lunz JG 3rd, Specht S, Stolz DB, Taguchi K, Subbotin VM, Murase N, Demetris AJ. Small proline-rich proteins 2 are noncoordinately upregulated by IL-6/STAT3 signaling after bile duct ligation. Lab Invest. 2005;85:109-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Ezure T, Sakamoto T, Tsuji H, Lunz JG 3rd, Murase N, Fung JJ, Demetris AJ. The development and compensation of biliary cirrhosis in interleukin-6-deficient mice. Am J Pathol. 2000;156:1627-1639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Ernst M, Inglese M, Waring P, Campbell IK, Bao S, Clay FJ, Alexander WS, Wicks IP, Tarlinton DM, Novak U. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194:189-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Kanai M, Mullen C, Podolsky DK. Intestinal trefoil factor induces inactivation of extracellular signal-regulated protein kinase in intestinal epithelial cells. Proc Natl Acad Sci U S A. 1998;95:178-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 552] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 78. | Podolsky DK, Lynch-Devaney K, Stow JL, Oates P, Murgue B, DeBeaumont M, Sands BE, Mahida YR. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:6694-6702. [PubMed] [Cited in This Article: ] |
| 79. | Matsumoto K, Fujii H, Michalopoulos G, Fung JJ, Demetris AJ. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology. 1994;20:376-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Yokomuro S, Lunz JG 3rd, Sakamoto T, Ezure T, Murase N, Demetris AJ. The effect of interleukin-6 (IL-6)/gp130 signalling on biliary epithelial cell growth, in vitro. Cytokine. 2000;12:727-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Yokomuro S, Tsuji H, Lunz JG 3rd, Sakamoto T, Ezure T, Murase N, Demetris AJ. Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin A: comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology. 2000;32:26-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Tomasetto C, Masson R, Linares JL, Wendling C, Lefebvre O, Chenard MP, Rio MC. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology. 2000;118:70-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Gum JR Jr, Hicks JW, Gillespie AM, Carlson EJ, Kömüves L, Karnik S, Hong JC, Epstein CJ, Kim YS. Goblet cell-specific expression mediated by the MUC2 mucin gene promoter in the intestine of transgenic mice. Am J Physiol. 1999;276:G666-G676. [PubMed] [Cited in This Article: ] |
| 84. | Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995;109:516-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 252] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 331] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 86. | Tomasetto C, Rio MC, Gautier C, Wolf C, Hareuveni M, Chambon P, Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990;9:407-414. [PubMed] [Cited in This Article: ] |
| 87. | Rio MC, Bellocq JP, Daniel JY, Tomasetto C, Lathe R, Chenard MP, Batzenschlager A, Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988;241:705-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 268] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 88. | Hertel SC, Chwieralski CE, Hinz M, Rio MC, Tomasetto C, Hoffmann W. Profiling trefoil factor family (TFF) expression in the mouse: identification of an antisense TFF1-related transcript in the kidney and liver. Peptides. 2004;25:755-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Sasaki M, Tsuneyama K, Saito T, Kataoka H, Mollenhauer J, Poustka A, Nakanuma Y. Site-characteristic expression and induction of trefoil factor family 1, 2 and 3 and malignant brain tumor-1 in normal and diseased intrahepatic bile ducts relates to biliary pathophysiology. Liver Int. 2004;24:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Sasaki M, Tsuneyama K, Nakanuma Y. Aberrant expression of trefoil factor family 1 in biliary epithelium in hepatolithiasis and cholangiocarcinoma. Lab Invest. 2003;83:1403-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Srivatsa G, Giraud AS, Ulaganathan M, Yeomans ND, Dow C, Nicoll AJ. Biliary epithelial trefoil peptide expression is increased in biliary diseases. Histopathology. 2002;40:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Kimura Y, Leung PS, Kenny TP, Van De Water J, Nishioka M, Giraud AS, Neuberger J, Benson G, Kaul R, Ansari AA. Differential expression of intestinal trefoil factor in biliary epithelial cells of primary biliary cirrhosis. Hepatology. 2002;36:1227-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Marenholz I, Zirra M, Fischer DF, Backendorf C, Ziegler A, Mischke D. Identification of human epidermal differentiation complex (EDC)-encoded genes by subtractive hybridization of entire YACs to a gridded keratinocyte cDNA library. Genome Res. 2001;11:341-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Cabral A, Voskamp P, Cleton-Jansen AM, South A, Nizetic D, Backendorf C. Structural organization and regulation of the small proline-rich family of cornified envelope precursors suggest a role in adaptive barrier function. J Biol Chem. 2001;276:19231-19237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 95. | Patel S, Kartasova T, Segre JA. Mouse Sprr locus: a tandem array of coordinately regulated genes. Mamm Genome. 2003;14:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Mischke D, Korge BP, Marenholz I, Volz A, Ziegler A. Genes encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex ("epidermal differentiation complex") on human chromosome 1q21. J Invest Dermatol. 1996;106:989-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 382] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 97. | Elder JT, Zhao X. Evidence for local control of gene expression in the epidermal differentiation complex. Exp Dermatol. 2002;11:406-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Tarcsa E, Candi E, Kartasova T, Idler WW, Marekov LN, Steinert PM. Structural and transglutaminase substrate properties of the small proline-rich 2 family of cornified cell envelope proteins. J Biol Chem. 1998;273:23297-23303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Stern LE, Erwin CR, Falcone RA, Huang FS, Kemp CJ, Williams JL, Warner BW. cDNA microarray analysis of adapting bowel after intestinal resection. J Pediatr Surg. 2001;36:190-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1565] [Cited by in F6Publishing: 1424] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 101. | Mueller A, O'Rourke J, Grimm J, Guillemin K, Dixon MF, Lee A, Falkow S. Distinct gene expression profiles characterize the histopathological stages of disease in Helicobacter-induced mucosa-associated lymphoid tissue lymphoma. Proc Natl Acad Sci U S A. 2003;100:1292-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 102. | Knight PA, Pemberton AD, Robertson KA, Roy DJ, Wright SH, Miller HR. Expression profiling reveals novel innate and inflammatory responses in the jejunal epithelial compartment during infection with Trichinella spiralis. Infect Immun. 2004;72:6076-6086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Hong SH, Nah HY, Lee JY, Lee YJ, Lee JW, Gye MC, Kim CH, Kang BM, Kim MK. Estrogen regulates the expression of the small proline-rich 2 gene family in the mouse uterus. Mol Cells. 2004;17:477-484. [PubMed] [Cited in This Article: ] |
| 104. | Tan YF, Li FX, Piao YS, Sun XY, Wang YL. Global gene profiling analysis of mouse uterus during the oestrous cycle. Reproduction. 2003;126:171-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Song HJ, Poy G, Darwiche N, Lichti U, Kuroki T, Steinert PM, Kartasova T. Mouse Sprr2 genes: a clustered family of genes showing differential expression in epithelial tissues. Genomics. 1999;55:28-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Zimmermann N, Doepker MP, Witte DP, Stringer KF, Fulkerson PC, Pope SM, Brandt EB, Mishra A, King NE, Nikolaidis NM. Expression and regulation of small proline-rich protein 2 in allergic inflammation. Am J Respir Cell Mol Biol. 2005;32:428-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2495] [Cited by in F6Publishing: 2398] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 108. | Clouston AD, Powell EE. Interaction of non-alcoholic fatty liver disease with other liver diseases. Best Pract Res Clin Gastroenterol. 2002;16:767-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 109. | Demetris AJ, Sakamoto T, Liu Z, Yokomuro S, Ezure T, Murase N, Blakolmer K. The Ductular Reaction in Liver Disease emphasis on a type I response. Normal and Malignant Liver Cell Growth. Dordecht: Kluwer Academic Publishers 1999; 141-155. [Cited in This Article: ] |
| 110. | Sirica AE, Mathis GA, Sano N, Elmore LW. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58:44-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Zhang M, Thorgeirsson SS. Modulation of connexins during differentiation of oval cells into hepatocytes. Exp Cell Res. 1994;213:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol. 2002;21:71-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 113. | Marshall A, Rushbrook S, Davies SE, Morris LS, Scott IS, Vowler SL, Coleman N, Alexander G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology. 2005;128:33-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Kobayashi S, Matsushita K, Saigo K, Urashima T, Asano T, Hayashi H, Ochiai T. P21WAF1/CIP1 messenger RNA expression in hepatitis B, C virus-infected human hepatocellular carcinoma tissues. Cancer. 2001;91:2096-2103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 115. | Torbenson M, Yang SQ, Liu HZ, Huang J, Gage W, Diehl AM. STAT-3 overexpression and p21 up-regulation accompany impaired regeneration of fatty livers. Am J Pathol. 2002;161:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 116. | Sawada N, Kojima T, Obata H, Isomura H, Atsumi S, Sawaki M, Tsuzuki N, Tobioka H, Kokai Y, Satoh M. Expression of p21(waf-1/cip-1) is significantly induced in the livers of LEC rats with chronic liver injury. Jpn J Cancer Res. 1996;87:1102-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 117. | Koteish A, Yang S, Lin H, Huang J, Diehl AM. Ethanol induces redox-sensitive cell-cycle inhibitors and inhibits liver regeneration after partial hepatectomy. Alcohol Clin Exp Res. 2002;26:1710-1718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 118. | Blakolmer K, Jain A, Ruppert K, Gray E, Duquesnoy R, Murase N, Starzl TE, Fung JJ, Demetris AJ. Chronic liver allograft rejection in a population treated primarily with tacrolimus as baseline immunosuppression: long-term follow-up and evaluation of features for histopathological staging. Transplantation. 2000;69:2330-2336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, Fung J, Gouw A, Gustafsson B, Haga H. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31:792-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 351] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 120. | Oguma S, Belle S, Starzl TE, Demetris AJ. A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology. 1989;9:204-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 116] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Omori N, Evarts RP, Omori M, Hu Z, Marsden ER, Thorgeirsson SS. Expression of leukemia inhibitory factor and its receptor during liver regeneration in the adult rat. Lab Invest. 1996;75:15-24. [PubMed] [Cited in This Article: ] |
| 122. | Sakamoto T, Ezure T, Lunz J, Murase N, Tsuji H, Fung JJ, Demetris AJ. Concanavalin A simultaneously primes liver hematopoietic and epithelial progenitor cells for parallel expansion during liver regeneration after partial hepatectomy in mice. Hepatology. 2000;32:256-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 123. | Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N, Takeda J, DiGiovanni J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720-728. [PubMed] [Cited in This Article: ] |
| 124. | Campisi J. Cancer and ageing: rival demons. Nat Rev Cancer. 2003;3:339-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 333] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 125. | Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 126. | Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502-2512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 665] [Cited by in F6Publishing: 695] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 127. | Koike N, Saitoh K, Todoroki T, Kawamoto T, Iwasaki Y, Nakamura K. Cell proliferation kinetics of the bile duct epithelium of the rat. Cell Prolif. 1993;26:183-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 128. | Nakanuma Y, Kono N. Expression of vimentin in proliferating and damaged bile ductules and interlobular bile ducts in nonneoplastic hepatobiliary diseases. Mod Pathol. 1992;5:550-554. [PubMed] [Cited in This Article: ] |
| 129. | Milani S, Herbst H, Schuppan D, Niedobitek G, Kim KY, Stein H. Vimentin expression of newly formed rat bile duct epithelial cells in secondary biliary fibrosis. Virchows Arch A Pathol Anat Histopathol. 1989;415:237-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 130. | Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology. 1996;23:476-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 163] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 131. | Terada T, Ashida K, Kitamura Y, Matsunaga Y, Takashima K, Kato M, Ohta T. Expression of epithelial-cadherin, alpha-catenin and beta-catenin during human intrahepatic bile duct development: a possible role in bile duct morphogenesis. J Hepatol. 1998;28:263-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 132. | Camargo CA Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 317] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 133. | Gao B. Therapeutic potential of interleukin-6 in preventing obesity- and alcohol-associated fatty liver transplant failure. Alcohol. 2004;34:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 134. | Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40:933-941. [PubMed] [Cited in This Article: ] |
| 135. | Selzner M, Graf R, Clavien PA. IL-6: a magic potion for liver transplantation. Gastroenterology. 2003;125:256-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 136. | Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan HN, Jaruga B, Batkai S, Hoshino S, Tian Z. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125:202-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 137. | Kimizuka K, Nakao A, Nalesnik MA, Demetris AJ, Uchiyama T, Ruppert K, Fink MP, Stolz DB, Murase N. Exogenous IL-6 inhibits acute inflammatory responses and prevents ischemia/reperfusion injury after intestinal transplantation. Am J Transplant. 2004;4:482-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 138. | Safadi R, Friedman SL. Hepatic fibrosis--role of hepatic stellate cell activation. MedGenMed. 2002;4:27. [PubMed] [Cited in This Article: ] |
| 139. | Tillmann HL, Manns MP, Rudolph KL. Merging models of hepatitis C virus pathogenesis. Semin Liver Dis. 2005;25:84-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | Watanabe S, Matsuda A, Suzuki Y, Kondo K, Ikeda Y, Hashimoto H, Umemura K. Inhibitory mechanism of tranilast in human coronary artery smooth muscle cells proliferation, due to blockade of PDGF-BB-receptors. Br J Pharmacol. 2000;130:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Ikeda H, Inao M, Fujiwara K. Inhibitory effect of tranilast on activation and transforming growth factor beta 1 expression in cultured rat stellate cells. Biochem Biophys Res Commun. 1996;227:322-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 142. | Naftalin R. Alterations in colonic barrier function caused by a low sodium diet or ionizing radiation. J Environ Pathol Toxicol Oncol. 2004;23:79-97. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 143. | Wengrower D, Zanninelli G, Pappo O, Latella G, Sestieri M, Villanova A, Faitelson Y, Pines M, Goldin E. Prevention of fibrosis in experimental colitis by captopril: the role of tgf-beta1. Inflamm Bowel Dis. 2004;10:536-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 144. | Ramos SG, Montenegro AP, Goissis G, Rossi MA. Captopril reduces collagen and mast cell and eosinophil accumulation in pig serum-induced rat liver fibrosis. Pathol Int. 1994;44:655-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 145. | Yuan GJ, Zhang ML, Gong ZJ. Effects of PPARg agonist pioglitazone on rat hepatic fibrosis. World J Gastroenterol. 2004;10:1047-1051. [PubMed] [Cited in This Article: ] |
| 146. | Kawaguchi K, Sakaida I, Tsuchiya M, Omori K, Takami T, Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun. 2004;315:187-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 147. | Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF, Motomura K, Anania FA, Willson TM, Tsukamoto H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715-35722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 380] [Article Influence: 15.8] [Reference Citation Analysis (0)] |