Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2140
Revised: February 11, 2004
Accepted: February 21, 2004
Published online: July 15, 2004
AIM: To investigate the effects and mechanism of d-limonene on the growth and metastasis of gastric cancer in vivo.
METHODS: Metastatic model simulating human gastric cancer was established by orthotopic implantation of histologically intact human tumor tissue into gastric wall of nude mice. One percent d-limonene was orally administered at dose of 15 ml/kg every other day for seven weeks. Eight weeks after implantation, tumor weight, inhibition rate, apoptotic index (AI), microvessel density (MVD), vascular endothelial growth factor (VEGF), variation of ultrastructure, and the presence of metastasis were evaluated, respectively, after the mice were sacrificed.
RESULTS: The tumor weight was significantly reduced in 5-FU group (2.55 ± 0.28 g), d-limonene group (1.49 ± 0.09 g) and combined treatment group (1.48 ± 0.21 g) compared with the control group(2.73 ± 0.23 g, P < 0.05). In 5-FU group, d-limonene group, combined treatment group, the inhibition rates were 2.60%, 47.58% and 46.84% and 0, respectively; AI was (3.31 ± 0.33)%, (8.26 ± 1.21)%, (20.99 ± 1.84)% and (19.34 ± 2.19)%, respectively; MVD was (8.64 ± 2.81), (16.77 ± 1.39), (5.32 ± 4.26) and (5.86 ± 2.27), respectively; VEGF expression was (45.77 ± 4.79), (41.34 ± 5.41), (29.71 ± 8.92) and (28.24 ± 8.55), respectively. The incidences of peritoneal metastasis also decreased significantly in 5-FU group(77.8%), d-limonene group (20.0%) and combined group (22.2%) compared with control group (100%) versus 62.5%, 30% and 22.2%) (P < 0.05). Liver metastasis was also inhibited and the incidences decreased significantly in 5-FU group, d-limonene group and combined group than that in control group (87.5% vs 55.5%, 20.0% and 22.2% respectively) (P < 0.05). The incidence of ascites in control group, 5-FU group, d-limonene group and combined group was 25.0%, 22.2%, 0, 0, respectively and 12.5%, 11.1% 0, 0, with respect to the metastasis rate to other organs.
CONCLUSION: d-limonene has antiangiogenic and proapoptotic effects on gastric cancer, thereby inhibits tumor growth and metastasis. Combination of d-limonene with cytotoxic agents may be more effective.
-
Citation: Lu XG, Zhan LB, Feng BA, Qu MY, Yu LH, Xie JH. Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by
d -limonene. World J Gastroenterol 2004; 10(14): 2140-2144 - URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2140.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2140
d-limonene is a monoterpene compound. In recent years, it was used for prevention and cure of various animal tumor models induced by chemical carcinogen and was proved to have anti-cancer activities[1-7]. Our previous study also suggested that d-limonene can inhibit the growth of human gastric cancer cell in vitro through a mechanism of inducing the apoptosis of tumor cells[8]. To make a further understanding of the anti-cancer effects and mechanism of d-limonene in vivo, an orthotopic transplantation model of gastric cancer in nude mice was employed. The d-limonene emulsion was administrated to investigate its inhibition effects on the growth and metastasis of gastric cancer dynamically, which will be the theoretical basis for the clinical application of d-limonene.
Experimental animals Forty male BALB/c nu/nu nude mice, purchased from Chinese Medical University, were raised under the SPF (specific pathogen free) condition for a week to adapt the surroundings before the initiation of the experiment.
Tumor tissues Gastric cancer cell line BGC-823 was obtained from Cell Research Institute (Shanghai, China). A 0.2 mL of cell suspension (2 × 106 cells) was subcutaneously injected into the anterior axilla of nude mice to form the entity of cancer. When the tumor grew to a diameter of 2-2.5 cm, the mouse was sacrificed to dissect the neoplasm and the capsule of tumor was removed and kept in saline containing 100 U/mL penicillin, 100 U/mL streptomycin (pH7.2). Pieces of fresh tumor tissue (1 mm3) near the margin were inoculated to another mouse by puncture method. The above procedure was repeated for 5 times and the last generation of the tumor tissue was be used for orthotopic transplantation.
Animal model Before transplantation, mice bearing tumor were killed. Then the tumor was dissected and put into saline containing penicillin (100 U/mL) and streptomycin (100 U/mL) aseptically. Pieces of intact, fish-meat-like tumor tissue of 3 mm3 in diameter from margin were prepared after the removing of tumor capsule. Mice anesthetized by the thialisobumalnatrium (40 mg/kg) injected into abdominal cavity were sterilized with caseoiodine on skin of abdomen and a transverse incision was made in left upper quadrant. After the exposure of greater curvature of stomach, the serosa and muscular layer were incised. Then, tumor tissues were transplanted under serosa of stomach by means of suture and embedding. Stomach was brought into abdominal cavity; the incision in belly was sutured delaminatedly. The whole operation procedure followed the doctrine of aseptic manipulation.
Drugs and groupsd-limonene with 97% purity (product number: 183164) was purchased from Sigma Company (USA). It was prepared as 10 g/L lecithin emulsion by Dalian Medical Science Institute. Mice were randomly divided into 4 groups: control group, 5-FU group, d-limonene group, and d-limonene + 5-FU group and administrated with saline (0.3 mL/d, gastric perfusion), 5-FU (30 mg/kg/d, intraabdominal injection), D-limonene (15 mL/kg/d, gastric perfusion), and 5-FU (30 mg/kg/d, intraabdominal injection) + d-limonene (15 ml/kg.d, gastric perfusion), respectively for 7 wk. The body mass and abdominal circumstances were measured on the 7th, 14th, 21st, 28th, 35th, 42nd, 49th d after transplantation and the status of eating, drinking and defecation of mice were recorded. The dying mice were executed and necropsy was performed. In the 8th wk, all the mice were killed; the neoplasm was resected and weighted; and the occurrence of distant metastasis and ascites were examined.
The measurement of tumor weight and inhibition rate of tumor growth Mice were killed by dislocation of cervical vertebra, the tumor weight was measured and the tumor inhibition rate was calculated. inhibition rate = [(tumor weight of control group - tumor weigh of treated groups)/weight of control] × 100%.
The occurrence of metastasis and ascites in nude mice The metastasis of gastric cancer was examined in abdominal cavity, peritoneum and liver. Tumor specimens were fixed in neutral formaldehyde, dehydrated by dimethylbenzene and embedded in paraffin for the hematoxylin and eosin (HE) staining and immunohistochemistry. The characters and volume of ascites were recorded.
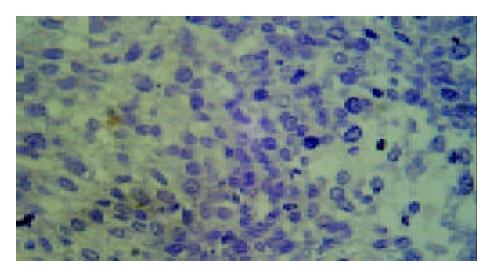
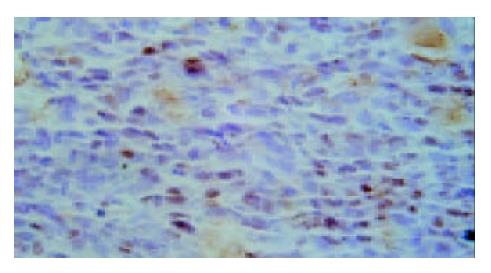
Apoptotic index (AI) TUNEL method was used to detect the apoptosis of tumor cell. The sections were fixed and embedded as usual. TUNEL test kit was purchased from Roche Company. DNase I (1 mg/mL) was used to digesting the positive control specimen for 10 min before being put into TUNEL response mixture. Only label liquor was added into the negative control response mixture. Cells with brown or yellow nuclei were assumed as apoptotic cells. the number of apoptotic cells and total cancer cells were counted under light microscope at 400 × magnification in 5 fields of vision and the average values were used for the calculation of apoptosis index (AI) according to the following formula: AI = (apoptotic cells/total cancer cells) × 100%.
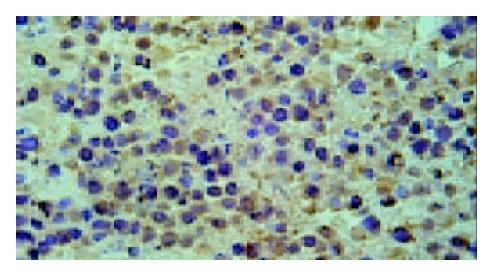
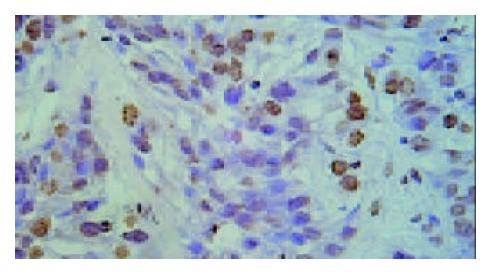
Microvessel density assay The polyclonal antibody for factor □-related antigen (F□-RAg, Santa Cruz Biotechnology, Inc, USA) was selected to detect the microvessel density in paraffin embedded tumor sections by immunohistochemical method according to the manufacturer’s instruction. Result determination: Sections were screened at 40 × magnification under a light microscope to identify three regions with the highest MVD. Then, blood vessels stained brown by antibody for F□-RAg was counted at 200 × magnification. For the determination of microvessels, first of all, the hemorrhage area and marginal region were eliminated. Those brown-stained endothelial cells or cell cluster that had interspaces with microvessel, cancer cells and connective tissue were regarded as a micro vessels. The average value of the three regions was regarded as MVD.
Imunohistochemical assay of VEGF Imunohistochemical staining was performed with mouse monoclonal antibody specific for human VEGF (C1, Santa Cruz Biotechnology, Inc, USA) in a dilution of 1:100 according to SP method specification. The presence of brown or yellow granules in plasma or nucleus was regarded as positive staining for VEGF protein expression. The sum of positive cells number was calculated in five fields per slide at 200 × magnification under light microscope. Results were ratified by fluorescence microscope and colorful microscopic figure analysis system (OLYMPUS BX51TR, Image-Pro Plus, Analysis Software).
Tumor weight, AI, MVD and VEGF were expressed as the mean ± SD. Comparison between groups was performed using analysis of variance; Differences of the metastasis in peritoneum, liver and other organs and the occurrence of ascites were examined by χ2 test (P < 0.05 was considered statistically significant).
At the end of the 7th wk, two mice-bearing tumor from control group, one from 5-FU group and one from combined group died of exhaustion while none from d-limonene group. During the whole process of the experiment, only one mouse in 5-FU group was found with loose stools, and no passage of bloody stools was found in any groups. Compared with the control and 5-FU group in which mice were found emaciation, inactive and lassitude, mice in d-limonene group and combined group had better appetite and increased body mass although statistically had no difference.
At necropsy, xenografts of gastric cancer were found in each groups of nude mice. The transplanted tumor entity was pink in color, ellipse or round in shape, with nodosity in surface and necrosis in central parts, and was confirmed as adenocarcinoma by pathological section. Compared with the control group, the tumor weight of mice in d-limonene group and combined group were decreased significantly (P < 0.05). Moreover, the inhibition effects of d-limonene or the combined effects of d-limonene and 5-FU on the growth of gastric cancer were more significant than that of 5-FU (Table 1).
| Groups | n | Tumor mass (g) | Inhibition rate (%) | AI (%) | MVD (n) | VEGF(%) |
| Control | 8 | 2.69 ± 0.32 | 0 | 3.31 ± 1.44 | 18.64 ± 2.81 | 45.77 ± 4.79 |
| 5-FU | 9 | 2.62 ± 0.35 | 2.60 | 8.26 ± 1.21a | 16.77 ± 1.39 | 41.34 ± 5.41 |
| d-limonene | 10 | 1.41 ± 0.58ac | 47.58ac | 20.99 ± 2.84ac | 5.32 ± 4.26ac | 29.71 ± 8.92ac |
| Combined | 9 | 1.43 ± 0.51ad | 46.84ac | 19.34 ± 3.19ac | 5.86 ± 2.27ac | 28.24 ± 8.55ac |
Morphologically, tumor cells in the control group were bigger, ellipse, with red plasma and big, deep stained nuclei. A clear imbalance ratio of nucleus to plasma was found also. No significant difference was observed between the control and 5-FU group. While in d-limonene group and combined group, relatively small tumor cells with vacuoles in plasma and clouding nucleus were observed in which the ratio of nucleus to plasma was relatively normal.
The major morphological changes of cancer cells in D-limonene and combined group were the shrinkage of cell, the condensation of cytoplasm, the aggregation of ribosome and mitochondrion, the occurrence of nuclear fragmentation, the peripheral masses of condensed chromatin. Apoptotic bodies derived from the shedding of condensed masses were found to be degradated by surrounding phagocytes and formed vacuoles in cytoplasm. Fusiform shape, morphological irregular and karyomegaly cancer cells were observed in control and 5-FU group with tight binding between cells, imbalance ratio of nucleus to plasma and the maldistribution of chromatin. But no obviously changes in cell morphology were detected.
Results of TUNEL demonstrated that apoptotic cells with yellow nucleus were seen occasionally in control group and 5-FU group while piles of apoptotic cells were observed in d-limonene and combined group. There was a significantly difference in AI in d-limonene and combined group compared to the control and 5-FU group (P < 0.05) (Table 1, Figure 1, Figure 2, Figure 3, Figure 4).
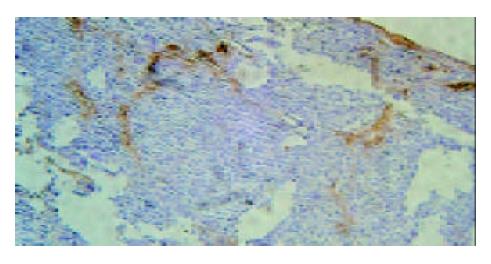
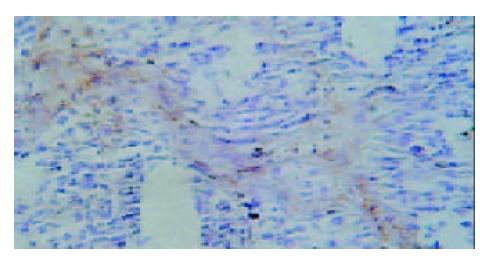
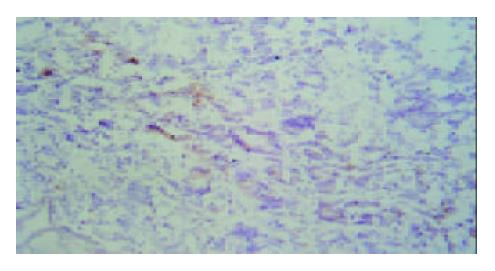
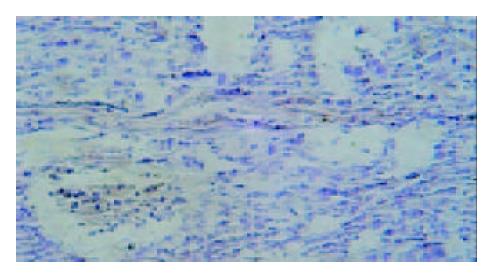
Under light microscope, the intensive brown-staining microvessels on stomach wall was observed without integrated basement membrane in the control group. Compared with the control and 5-FU group, almost no microvessels was found in the tumor of d-limonene and combined group for the cancer cells were in dormancy status, and the MVD of those two groups was statistically lower than that of the control and 5-FU group (Table 1, Figure 5, Figure 6, Figure 7, Figure 8).
The metastasis rate of gastric cancer to peritoneum (regional lymph node) was to 100% in the control group and the metastasis rates in other groups are displayed in Table 2. the metastasis to liver, peritoneum and other organs of gastric cancer and the formation of ascites were significantly inhibited in d-limonene and combined groups (P < 0.05) while no statistical inhibitory effects on the metastasis to liver and peritoneum were observed in 5-FU group (P > 0.05, Table 2).
Gastric cancer is the most common malignant tumor in digestive system. The cure rate of gastric cancer was increased year by year with the progress of surgical technique, chemotherapy and other means. But a five-year survival rate is still wandered below 10%, especially in middle or late phase patients[10]. Thus, to find an effective adjuvant chemotherapy drug became the focus of gastric cancer therapy besides emphasizing surgical operation.
Limonene (1-methyl-4-isopropyl-cyclohexene) is the essential component in citrus oil. d-limonene, the most common isomer of limonene, is a kind of monoterpene component. It has the ability of anti-oxygenation, anti-inflammation and drainage for gallstone. The anti-cancer activity of d-limonene was found recently. Therefore, d-limonene has been applied to precaution and treatment in chemical-induced animal model, such as colon cancer, breast cancer, gastric cancer, pancreatic cancer and hepatic cancer with promising results[1-7]. Most of those studies focus in the chemoprophylaxis of tumor. Our results demonstrated that in d-limonene and combined group, the tumor weight of xenograft was decreased drastically, the metastasis to liver, peritoneum and the occurrence of ascites were inhibited significantly compared with the control group. Thus, an inhibition effect of d-limonene on the growth and metastasis of gastric cancer cells was ratified. Taking the results of TUNEL assay into consideration, we found the main mechanism underlying the inhibition effects of d-limonene was to induce the apoptosis of cancer cells.
At present, it is thought that a phenotype change from no-vascular period to angiogenesis stage exists during the growth of tumor. In no-vascular period, the velocity of cancer cells apoptosis and proliferation maintain a dynamic balance and the tumor may survive for months or even years without the presence of metastasis. But the alterations of some important factors, such as heredity cause, over-expression of proangiogenic factors originated from cancer cells or down-regulation anti-angiogenic factors expressed by body tissues or cancer cells, the switch for tumor angiogenesis thereby activating and leading to vascularization of tumor. Once the tumor vascular is formed, the growth speed of tumor will be increased and the invasive growth and metastasis will be induced.
Microvessel density (MVD), is usually used to evaluate the vascularization level of tumor[10]. A large amount of experiments and clinical research results showed that MVD had a close relationship with the prognosis of almost all malignant tumors, a higher MVD suggested the lower differentiation and the higher reoccurrence and metastasis incidence[11]. We demonstrated that compared with control and 5-FU group, tumor MVD in d-limonene and combined group was decreased significantly, suggesting a inhibitive effect of d-limonene on tumor angiogenesis.
Although the mechanisms involved in tumor angiogenesis have not been understood exactly, the effect of VEGF on vascularization has been conformed. VEGF, a specific mitogen for vascular endothelial cells was isolated from follicular cell plasma of cattle and considered as the most important angiogenic factor of metastatic tumor[12-14]. The expression of VEGF was found very low in normal adult tissues. Once initiated in pathologic status of tumor, the angiogenesis was induced and promoted drastically by VEGF and resulted in the increase of MVD. Among vascular growth factors, VEGF was the only one to stimulate the proliferation of tumor endothelial cell and to induce the vascularization of tumor directly[15]. As the newly formed vessels may provide tumor with oxygen, nutrition, the growth of tumor will be invasive and uncontrollable[16]. It was reported that the over expression of VEGF had close relationship with tumor metastasis and poor prognosis. The invasion of blood vessels and lymph nodes has been often observed in patients with enhanced VEGF expression, which will result in the rapidly malignant change of tumor, easily relapse and short survival period after surgical operation. Here we showed that relatively high level of VEGF in the tumor of control and 5-FU group, especially in the areas around vessels, while notably decreased expression of VEGF in d-limonene and combined group. Thus, a positive correlation of VEGF expressiono with increased MVD was ratified, suggesting the anti-angiogenic mechanism of d-limonene via down-regulation of VEGF. Moreover, we found that the anti-cancer target of 5-FU might not on the inhibition of neovascularization.
5-fluorouracil, an antimetabolite pyramine, has been used as traditional chemitherapeutic drug for more than 40 years. For the anti-cancer target of 5-FU lies in the inhibition of thymidylic acid synthetase to prevent DNA synthesis but not the interference of tumor angiogenesis[17], no satisfied cure effect was obtained in our experiment. As to d-limonene, preventing tumor angiogenesis and increasing tumor cells apoptosis are the main anti-cancer mechanisms, which may diminish the incidence of drug resistance mutation for its character of physiological cell death. The effectiveness, none-toxicity, and lower drug resistance of d-limonene were ratified for a better eating, activity and living ability in d-limonene and combined groups than in control group within the 7-wk experiment. No statistical difference in body weight was observed among four groups for increased tumor weight and the formation of ascites in control and 5-FU group.
Further studies need to elucidate the apoptotic mechamisms of d-limonene before its clinical application.
Edited by Kumar M and Xu FM
| 1. | Uedo N, Tatsuta M, Iishi H, Baba M, Sakai N, Yano H, Otani T. Inhibition by D-limonene of gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1999;137:131-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Broitman SA, Wilkinson J, Cerda S, Branch SK. Effects of monoterpenes and mevinolin on murine colon tumor CT-26 in vitro and its hepatic "metastases" in vivo. Adv Exp Med Biol. 1996;401:111-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Kaji I, Tatsuta M, Iishi H, Baba M, Inoue A, Kasugai H. Inhibition by d-limonene of experimental hepatocarcinogenesis in Sprague-Dawley rats does not involve p21(ras) plasma membrane association. Int J Cancer. 2001;93:441-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Witschi H, Uyeminami D, Moran D, Espiritu I. Chemoprevention of tobacco-smoke lung carcinogenesis in mice after cessation of smoke exposure. Carcinogenesis. 2000;21:977-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Stratton SP, Dorr RT, Alberts DS. The state-of-the-art in chemoprevention of skin cancer. Eur J Cancer. 2000;36:1292-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Guyton KZ, Kensler TW. Prevention of liver cancer. Curr Oncol Rep. 2002;4:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Nakaizumi A, Baba M, Uehara H, Iishi H, Tatsuta M. d-Limonene inhibits N-nitrosobis(2-oxopropyl)amine induced hamster pancreatic carcinogenesis. Cancer Lett. 1997;117:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Lu XG, Feng BA, Zhan LB, Yu ZH. [D-limonene induces apoptosis of gastric cancer cells]. Zhonghua Zhongliu Zazhi. 2003;25:325-327. [PubMed] [Cited in This Article: ] |
| 9. | Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huangfu X, Sun J, Li L, Lu F. [1990-1992 mortality of stomach cancer in China]. Zhonghua Zhongliu Zazhi. 2002;24:4-8. [PubMed] [Cited in This Article: ] |
| 10. | Fanelli M, Locopo N, Gattuso D, Gasparini G. Assessment of tumor vascularization: immunohistochemical and non-invasive methods. Int J Biol Markers. 1999;14:218-231. [PubMed] [Cited in This Article: ] |
| 11. | El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239-2245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 13. | Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003;9:1421-1426. [PubMed] [Cited in This Article: ] |
| 14. | Ren J, Dong L, Xu CB, Pan BR. The role of KDR in the interactions between human gastric carcinoma cell and vascular endothelial cell. World J Gastroenterol. 2002;8:596-601. [PubMed] [Cited in This Article: ] |
| 15. | Risau W. What, if anything, is an angiogenic factor. Cancer Metastasis Rev. 1996;15:149-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Folkman J. The influence of angiogenesis research on management of patients with breast cancer. Breast Cancer Res Treat. 1995;36:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Rose MG, Farrell MP, Schmitz JC. Thymidylate synthase: a critical target for cancer chemotherapy. Clin Colorectal Cancer. 2002;1:220-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |