Published online Oct 28, 2021. doi: 10.35712/aig.v2.i5.133
Peer-review started: June 30, 2021
First decision: July 28, 2021
Revised: August 9, 2021
Accepted: August 31, 2021
Article in press: August 31, 2021
Published online: October 28, 2021
Processing time: 118 Days and 16.9 Hours
Infectious or noninfectious liver disease has inexorably risen as one of the leading causes of global death and disease burden. There were an estimated 2.14 million liver-related deaths in 2017, representing an 11.4% increase since 2012. Traditional diagnosis and treatment methods have various dilemmas in different causes of liver disease. As a hot research topic in recent years, the application of artificial intelligence (AI) in different fields has attracted extensive attention, and new technologies have brought more ideas for the diagnosis and treatment of some liver diseases. Machine learning (ML) is the core of AI and the basic way to make a computer intelligent. ML technology has many potential uses in hepatology, ranging from exploring new noninvasive means to predict or diagnose different liver diseases to automated image analysis. The application of ML in liver diseases can help clinical staff to diagnose and treat different liver diseases quickly, accurately and scientifically, which is of importance for reducing the incidence and mortality of liver diseases, reducing medical errors, and promoting the development of medicine. This paper reviews the application and prospects of AI in liver diseases, and aims to improve clinicians’ awareness of the importance of AI in the diagnosis and treatment of liver diseases.
Core tip: Liver disease has inexorably risen as one of the leading causes of global death and disease burden. As a hot research topic in recent years, the application of artificial intelligence (AI) in medical fields has attracted extensive attention. The application of machine learning in the liver diseases can help clinical staff to diagnose and treat different liver diseases quickly, accurately and scientifically, which is of importance for reducing the incidence and mortality of liver diseases, reducing medical errors, and promoting the development of medicine. This paper reviews the application and prospects of AI in liver diseases.
- Citation: Li Q, Li JF, Mao XR. Application of artificial intelligence in liver diseases: From diagnosis to treatment. Artif Intell Gastroenterol 2021; 2(5): 133-140
- URL: https://www.wjgnet.com/2644-3236/full/v2/i5/133.htm
- DOI: https://dx.doi.org/10.35712/aig.v2.i5.133
Infectious or noninfectious liver diseases cause a significant disease burden. There were an estimated 2.14 million liver-related deaths in 2017, representing an 11.4% increase since 2012[1]. Traditional diagnosis and treatment methods have various dilemmas in different causes of liver disease. With the development of artificial intelligence (AI) technology, new technologies have brought more ideas for the diagnosis and treatment of some liver diseases.
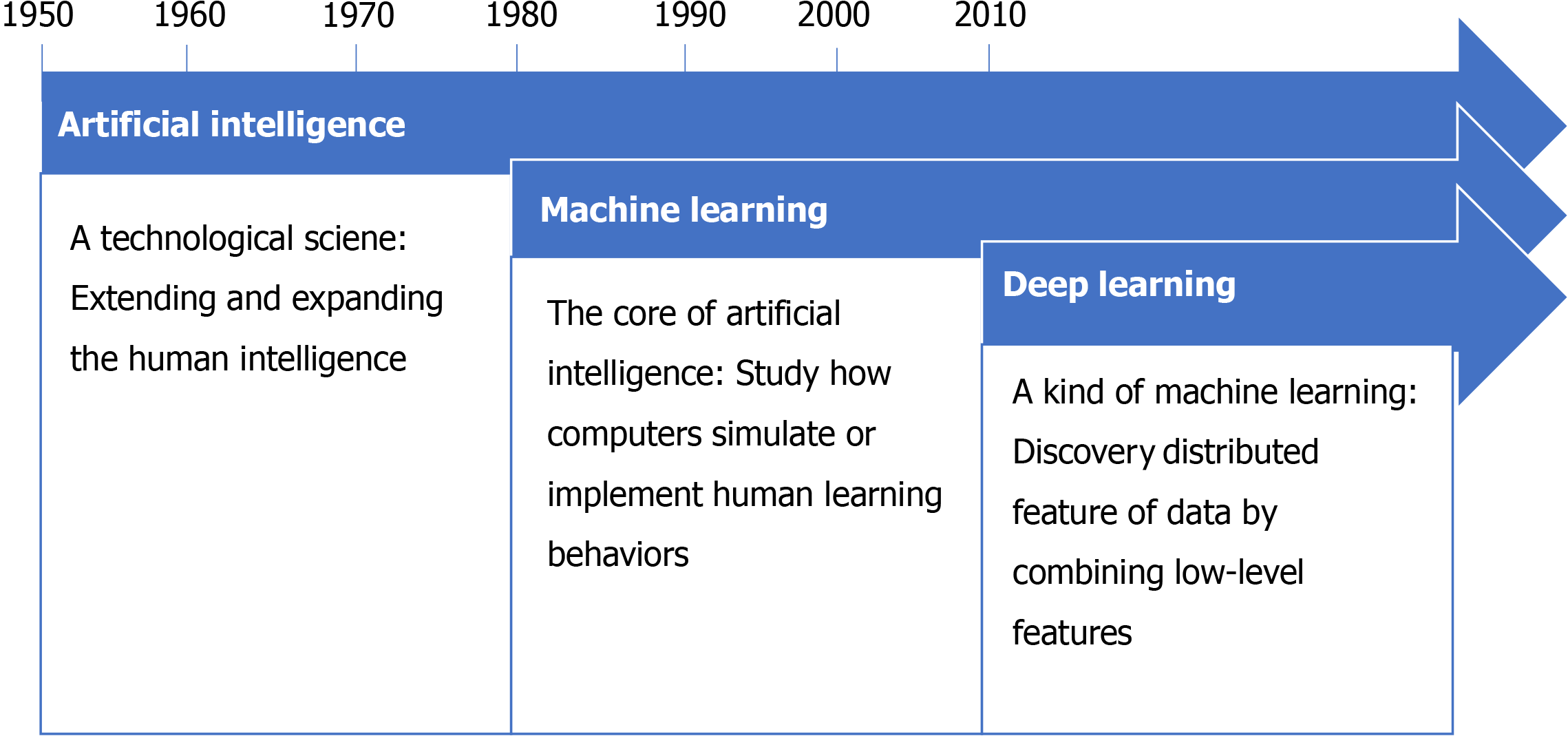
AI is an algorithm-based application field that simulates human mental processes and intellectual activities, enabling machines to solve problems with knowledge. In the information age, AI is widely used in the medical field and can provide accurate diagnosis and treatment for complex diseases, reduce medical errors, and promote the development of medicine[2]. For example, using deep learning architecture visual pattern analysis to detect basal cell carcinoma and distinguish malignant and benign lesions, the diagnosis accuracy rate is > 90% compared with experts[3]. There are two common types of AI. The first type is expert systems and the second is machine learning (ML), which is the core of AI and the basic way to make a computer intelligent (Figure 1). ML requires many data to train, which systematically improves computer performance in the process. By doing so, computers are able to shed light on previously unascertainable relationships that traditional statistical methods could not detect. ML is also capable of analyzing data types that were previously unavailable for advanced computer analysis, such as image and text data.
The area offering the most exciting new applications in healthcare is ML. Many studies in recent years have suggested that ML technology has many potential uses in hepatology, ranging from exploring new noninvasive means to predict or diagnose different liver diseases to automated image analysis. From the identification of liver areas at risk of radiation toxicity to the use of drug structures to predict the risk of liver injury, the accuracy of diagnosis and the effectiveness of treatment can be improved, and the efficiency can also be improved through automation. Although promising data from preclinical studies are now available, the application of AI in liver disease is far from being applied in clinical practice, so the application of AI in liver disease and other diseases remains challenging and deserves further study.
Liver disease is not an independent disease. Because the specific types of lesions are different, the diagnostic methods differ. Different examination methods can be selected according to the specific types of liver diseases to be examined. For example, at present, the common diagnostic method for nonalcoholic fatty liver disease (NAFLD) is liver ultrasound (US)[4,5]; the common diagnostic method for liver fibrosis is liver biopsy[4]; the diagnosis of liver cancer (LC) mainly uses imaging images and biomarkers, and the staging mainly uses the Barcelona staging system. However, due to subjective and invasive factors, the current examination methods have certain limitations in the diagnosis of some liver diseases. The sensitivity and specificity of liver US decrease with increasing body mass index because US is subjective. As a solid tumor, hepatocellular carcinoma (HCC) has significant temporal and spatial heterogeneity, which can predict the treatment response and prognosis of HCC[6]. The Barcelona staging system does not include the histological and molecular characteristics of tumors. The application of AI has filled the gaps in these respects. By designing noninvasive examination means to intelligently analyze images and pictures, AI has improved the diagnostic efficiency and accuracy of clinicians.
The prevalence of NAFLD is currently increasing, and there are currently no accurate diagnostic means or targeted medicines. The application of AI can realize the early diagnosis of NAFLD, which is expected to reduce the further deterioration of the disease. Current research has developed automatic liver segmentation based on deep learning tools used for quantitative abdominal computed tomography (CT) of liver fat. This fully automated CT tool provides rapid and objective assessment that can be used in a large retrospective cohort for future studies. If hepatic steatosis proves to be an independent risk factor for future adverse events, the automated tool can also be used for opportunistic NAFLD screening with any nonenhanced CT, including liver (abdomen or chest) scan, regardless of the clinical indications of imaging[7]. In addition, a technique that combines noninvasive markers with the ML approach is suitable for optimal identification of NAFLD risk assessment and can also be extended to predict other types of disease caused by metabolic syndrome[8]. The use of ML algorithms to establish a prediction model of NAFLD based on laboratory parameters is also a current research direction. A prediction model named the NAFLD ridge score, which can be easily calculated and obtain a high negative predictive value, is recommended as the simplest and most predictive ML model to exclude NAFLD[9].
Liver fibrosis, regardless of the etiology, is believed to be key to the progression of any form of chronic liver disease (CLD), and persistent fibrosis is widely believed to be a major driver of the eventual development of cirrhosis and liver failure[10,11]. Liver biopsy is considered to be the gold standard for staging liver fibrosis; however, it is invasive and is limited by sample error, interobserver variability and various potential complications[12]. Radiological and serum markers of fibrosis are also used to assess liver fibrosis[13], and it is not reliable to accurately distinguish the stages of fibrosis in these patterns. There is a clear need for safe, effective and reliable noninvasive assessment modalities. A study that aimed to develop and validate a deep learning system (DLS) for staging liver fibrosis by using portal venous phase CT images demonstrated that a DLS trained by using a large amount of CT data allowed for highly accurate staging of liver fibrosis. In this study, DLS was superior to radiologists and serum fibrosis tests in diagnosing significant fibrosis, advanced fibrosis and cirrhosis[14]. In addition, an existing model called deep learning radiomics of elastography has shown the best overall performance in predicting liver fibrosis stage, which has certain value and practical value for the accurate noninvasive diagnosis of liver fibrosis stage in hepatitis-B-virus-infected patients[15].
HCC is the most common primary liver cancer and has significant temporal and spatial heterogeneity. AI-based imaging, i.e., imaging omics, can quantitatively analyze tumor imaging to reveal the imaging manifestations of these heterogeneous characteristics. The concept of imaging omics was first proposed by Lambin et al[16] in 2012. It mainly extracts a large number of influential features from high-throughput radiological images and then uses statistics and AI algorithms to select the most valuable imaging omics to construct tumor predictive models. In essence, the significance of imaging omics is to dig deeper into the information of traditional medical images to compensate for the deficiency of the human eye.
Similarly, there is a need for better clinical classification of indeterminate liver nodules; however, the use of a single biomarker to predict the presence of cancer is difficult due to its multifactorial nature[17]. An AI-based predictive model of HCC reduced the misclassification rate by approximately half compared with that of a single tumor marker[18]. In addition, radiomics ML can be trained to diagnose hepatic nodules using the European Association for the Study of the Liver (EASL) guidelines in patients with HCC disease classified as uncertain cirrhosis[19]. According to EASL, indeterminate nodules include all nodules that do not provide arterial enhancement and washout [two major Liver Imaging Reporting and Data System (LI-RADS) features] and require biopsy regardless of LI-RADS; however, biopsies of cirrhosis carry life-threatening risks, including bleeding and tumor spread[20]. A study demonstrated that ML-based radiometric features using arterial and portal phase quantitative CT feature changes can enable the noninvasive diagnosis of HCC in patients with indeterminate nodules of cirrhosis. This feature will help to identify patients at high risk of HCC who should be prioritized for treatment to achieve significant clinical benefits[19].
Worldwide, CLD is a leading cause of morbidity and mortality[21]. There are a few therapeutic approaches for liver dysfunction, such as direct antiviral drugs (DAAs) for hepatitis C virus (HCV) and transarterial chemoembolization (TACE) for HCC[22]. Because some patients are resistant to DAAs and do not respond well to antiviral therapy and individualized responses to primary TACE vary among patients, AI seems to be an alternative option. AI has attracted attention for treatment of liver diseases in recent years, especially hepatitis C and LC[23]. AI can go beyond human reasoning to build drug-resistance predictive models from many complex combinations and overcome the limitations of traditional techniques, which may be effective in avoiding the emergence of a resistant virus, reducing medical costs and providing precise and personalized treatment advice for doctors and patients.
With the popularization of DAAs and the application of new detection technologies and service models, global progress has been made in the detection and treatment of HCV. However, some patients with HCV are resistant to DAAs and do not respond well to antiviral therapy, and the current lack of means to screen these patients may delay disease treatment. AI algorithms can go beyond human reasoning to build predictive models from many complex combinations. A current study identified all variants of HCV whole-genome sequences that could be evaluated, and a support vector machine (SVM) based on a machine algorithm was the best prediction model. Similar models can be used to determine the best treatment for other viral infections and cancers[24].
Coinfection with human immunodeficiency virus 1 and HCV is common in some populations today; however, treating coinfections is a challenge. A previous study demonstrated that a multiple quantitative structure–activity relationship model showed high performance in predicting multitarget inhibitors with anti-HIV and -HCV activity[25]. The application of ML methods enables us to identify variables associated with reduced HCV treatment intake. The most recent variable, people who inject drugs (PWIDs), was identified as a major limiting factor associated with therapeutic intake deficit, even when priority criteria were met. PWIDs refers to people who have been injected at some point but are not currently using oral contraceptives or abusing drugs. In fact, intelligent network interruption analysis has been used as a targeted strategy to effectively interrupt HCV transmission between PWIDs[26]. Its application in clinical decision-making of infectious diseases should be expanded to optimize treatment and prevention strategies.
Due to the well-known limitations of TACE, AI seems to be an alternative treatment option for HCC. Some studies have reported the use of fusion imaging (FI) techniques to overcome the limitations of traditional techniques. FI is an AI-based technology that allows the fusion of two different imaging modes[27]. A prospective randomized study conducted by Huang et al[28] showed that the technical response rate of FI in ablation for hepatic nodules < 5 cm was close to 100% and reported the special usefulness of FI in tumors at less obvious and dangerous sites, not only to accurately delineate the target lesion and critical organs, the structures that may be close to the target area of ablation can also be accurately delineated.
The clinical decision support system (CDSS) is the software that is designed to be a direct aid to clinical decision-making, in which the characteristics of an individual patient are matched to a computerized clinical knowledge base and patient-specific assessments or recommendations are then presented to the clinician or the patient for a decision[29]. One study applied AI technology to clinical realworld data of patients with primary HCC, explored the precise treatment of disease and built up the AIbased CDSS, HCC CDSS. In the internal use verification process of HCC CDSS in West China Hospital, the matching accuracy rate between HCC CDSS and the multidisciplinary team treatment scheme reached 95.10%. This scheme is conducive to optimizing the clinical treatment decision of LC and can provide precise and personalized treatment advice for doctors and patients[30].
Drug-induced liver injury (DILI) is a serious problem in clinical treatment and a common cause of drug development failure or withdrawal from the market[31]. Therefore, compound hepatotoxicity is important to determine.
Accurate estimation of the prognosis of patients with liver disease can help clinicians make appropriate treatment plans for different individuals; however, due to the complex process of CLD, the extensive impact on the systemic system and organs, and the lack of an adequate understanding of the nature of the development of liver disease, the understanding of the prognosis of different liver diseases is still limited. In recent years, HCV infection among LC patients and the mortality rate of HCV have been on the rise. Therefore, prediction of the prognosis of HCV patients has also attracted attention. Cirrhosis is a common, high-risk disease with slow clinical progression, and readmission and death in patients with cirrhosis are common and unpredictable. None of the clinically available predictive scores for cirrhosis can account for the broad range of clinical and psychosocial factors that may be associated with cirrhosis mortality. Individualized responses to primary TACE vary among patients with HCC. In addition, identifying a robust survival subgroup for HCC would also significantly improve patient care. The application of the prediction model of disease prognosis based on AI can improve the understanding of the prognosis of some liver diseases to a certain extent and provide an auxiliary reference for doctors’ decision-making.
AI is a low-cost, fast method to collect information on potential toxicity, and great efforts have been made in hepatotoxicity prediction in recent years. A study proposed that the integration of the Top-5 model could significantly improve the performance of hepatotoxicity prediction. The integrated Top-5 model consists of five base classifiers: Random Forest (RF) using Substructure Count, SVM using Chemistry Development Kit Extended, SVM using Chemistry Development Kit, SVM using PubChem, and RF using Klekota–Roth Count[32]. The deep learning model is also a stable and highly accurate predictive model of DILI, which can provide very useful safety information for early drug discovery and rational clinical drug use[33].
The prediction of the prognosis of HCV patients has attracted attention in recent years. One study showed that the recurrent neural network model was superior to the logistic regression (LR) model in predicting HCC risk in patients with HCV-associated cirrhosis, including patients with supraventricular tachycardia following antiviral therapy; thus, it can be used to identify patients at high risk for HCV-associated cirrhosis to develop HCC and to inform risk-based HCC expansion and surveillance strategies[34].
None of the clinically available predictive scores for cirrhosis can account for the broad range of clinical and psychosocial factors that may be associated with cirrhosis mortality. ML techniques have been used to help fill these gaps in cirrhosis but are not yet widely available. In one study, three AI models were established, including LR, kernel SVM and RF classifier, and showed that these models had difficulty predicting readmissions and deaths in cirrhosis at 30 and 90 d. The accuracy of the AI model is comparable to that generated using the model for the end-stage liver disease-NA (MELD-NA) score alone, requiring additional biomarkers to improve the predictive power[35].
Another study developed and validated a cirrhosis mortality model (CIMM) using variables selected from ML algorithms. The results showed that ML can help select important variables for more transparent risk scoring while maintaining high accuracy. The synthetic hybrid CIMM performed better than the widely used model for MELD-NA score[36].
For patients with LC, individualized responses to primary TACE vary. An AI-based radiomics strategy quantitatively analyses contrast-enhanced US images to predict personalized responses to primary TACE in HCC. There is potential for better selection of Barcelona Clinical Liver Cancer stage B patients receiving hepatic TACE and for better optimization of treatment planning and follow-up monitoring in the HCC management process[37].
Identifying a robust survival subgroup for HCC would also significantly improve patient care. Currently, few studies have integrated multiomics data to definitively predict HCC survival in a multipatient cohort. The survival-sensitive subtype model-deep learning model is of importance for the prognostic prediction and treatment intervention of HCC[38].
AI has become an important part of liver disease research, improving diagnostic accuracy, improving decision-making by enhancing predictive power, increasing efficiency through automation, and even predicting liver disease prognosis. Analysis of key biomarkers using ML can also provide deeper insights into the pathophysiology of liver disease. Despite the challenges, the application of AI in the field of liver disease is promising and worthy of further study. Researchers need to further develop new models of AI in liver disease diagnosis and precise treatment and conduct clinical verification to improve the accuracy of the results and promote the clinical application of AI. However, we must also be wary of over-reliance on such algorithms. AI will support rather than replace doctors, although computers and healthcare workers will have to work together. Ultimately, healthcare workers will have to make decisions for their patients based on their preferences, circumstances and ethics.
The authors thank all the participants and staff who contributed to this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hanada E S-Editor: Liu M L-Editor: Kerr C P-Editor: Li JH
| 1. | Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology. 2020;72:1605-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 2. | Miller DD, Brown EW. Artificial Intelligence in Medical Practice: The Question to the Answer? Am J Med. 2018;131:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 307] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 3. | Mori K, Sakuma I, Sato Y, Barillot C, Navab N. Preface. The 16th International Conference on Medical Image Computing and Computer Assisted Intervention, MICCAI 2013 was held in Nagoya, Japan during September 22-26, 2013. Med Image Comput Comput Assist Interv. 2013;16:V-X. [PubMed] |
| 4. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4894] [Article Influence: 699.1] [Reference Citation Analysis (8)] |
| 5. | Mancini M, Summers P, Faita F, Brunetto MR, Callea F, De Nicola A, Di Lascio N, Farinati F, Gastaldelli A, Gridelli B, Mirabelli P, Neri E, Salvadori PA, Rebelos E, Tiribelli C, Valenti L, Salvatore M, Bonino F. Digital liver biopsy: Bio-imaging of fatty liver for translational and clinical research. World J Hepatol. 2018;10:231-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 6. | Lewis S, Hectors S, Taouli B. Radiomics of hepatocellular carcinoma. Abdom Radiol (NY). 2021;46:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Graffy PM, Sandfort V, Summers RM, Pickhardt PJ. Automated Liver Fat Quantification at Nonenhanced Abdominal CT for Population-based Steatosis Assessment. Radiology. 2019;293:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Perveen S, Shahbaz M, Keshavjee K, Guergachi A. A Systematic Machine Learning Based Approach for the Diagnosis of Non-Alcoholic Fatty Liver Disease Risk and Progression. Sci Rep. 2018;8:2112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Yip TC, Ma AJ, Wong VW, Tse YK, Chan HL, Yuen PC, Wong GL. Laboratory parameter-based machine learning model for excluding non-alcoholic fatty liver disease (NAFLD) in the general population. Aliment Pharmacol Ther. 2017;46:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Rosselli M, MacNaughtan J, Jalan R, Pinzani M. Beyond scoring: a modern interpretation of disease progression in chronic liver disease. Gut. 2013;62:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP. An appraisal of the histopathological assessment of liver fibrosis. Gut. 2006;55:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 13. | Masuzaki R, Kanda T, Sasaki R, Matsumoto N, Ogawa M, Matsuoka S, Karp SJ, Moriyama M. Noninvasive Assessment of Liver Fibrosis: Current and Future Clinical and Molecular Perspectives. Int J Mol Sci. 2020;21:4906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Choi KJ, Jang JK, Lee SS, Sung YS, Shim WH, Kim HS, Yun J, Choi JY, Lee Y, Kang BK, Kim JH, Kim SY, Yu ES. Development and Validation of a Deep Learning System for Staging Liver Fibrosis by Using Contrast Agent-enhanced CT Images in the Liver. Radiology. 2018;289:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, Wu C, Liu C, Huang L, Jiang T, Meng F, Lu Y, Ai H, Xie XY, Yin LP, Liang P, Tian J, Zheng R. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 345] [Article Influence: 57.5] [Reference Citation Analysis (1)] |
| 16. | Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 3782] [Article Influence: 290.9] [Reference Citation Analysis (2)] |
| 17. | Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15 Suppl 4:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 354] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 18. | Sato M, Morimoto K, Kajihara S, Tateishi R, Shiina S, Koike K, Yatomi Y. Machine-learning Approach for the Development of a Novel Predictive Model for the Diagnosis of Hepatocellular Carcinoma. Sci Rep. 2019;9:7704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Mokrane FZ, Lu L, Vavasseur A, Otal P, Peron JM, Luk L, Yang H, Ammari S, Saenger Y, Rousseau H, Zhao B, Schwartz LH, Dercle L. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur Radiol. 2020;30:558-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 20. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 5953] [Article Influence: 850.4] [Reference Citation Analysis (3)] |
| 21. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2253] [Article Influence: 375.5] [Reference Citation Analysis (0)] |
| 22. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 922] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 23. | Spann A, Yasodhara A, Kang J, Watt K, Wang B, Goldenberg A, Bhat M. Applying Machine Learning in Liver Disease and Transplantation: A Comprehensive Review. Hepatology. 2020;71:1093-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 24. | Haga H, Sato H, Koseki A, Saito T, Okumoto K, Hoshikawa K, Katsumi T, Mizuno K, Nishina T, Ueno Y. A machine learning-based treatment prediction model using whole genome variants of hepatitis C virus. PLoS One. 2020;15:e0242028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Wei Y, Li W, Du T, Hong Z, Lin J. Targeting HIV/HCV Coinfection Using a Machine Learning-Based Multiple Quantitative Structure-Activity Relationships (Multiple QSAR) Method. Int J Mol Sci. 2019;20:3572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Rivero-Juárez A, Guijo-Rubio D, Tellez F, Palacios R, Merino D, Macías J, Fernández JC, Gutiérrez PA, Rivero A, Hervás-Martínez C. Using machine learning methods to determine a typology of patients with HIV-HCV infection to be treated with antivirals. PLoS One. 2020;15:e0227188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Abi-Jaoudeh N, Kruecker J, Kadoury S, Kobeiter H, Venkatesan AM, Levy E, Wood BJ. Multimodality image fusion-guided procedures: technique, accuracy, and applications. Cardiovasc Intervent Radiol. 2012;35:986-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Huang Q, Zeng Q, Long Y, Tan L, Zheng R, Xu E, Li K. Fusion imaging techniques and contrast-enhanced ultrasound for thermal ablation of hepatocellular carcinoma - A prospective randomized controlled trial. Int J Hyperthermia. 2019;36:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Sim I, Gorman P, Greenes RA, Haynes RB, Kaplan B, Lehmann H, Tang PC. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001;8:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 343] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 30. | Yang J, Guo F, Lyu T, Yan LN, Wen TF, Yang JY, Wu H, Wang WT, Song JL, Xu H, Zhang QH. Research of artificial intelligence-based clinical decision support system for primary hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi. 2020;100:3870-3873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Segall MD, Barber C. Addressing toxicity risk when designing and selecting compounds in early drug discovery. Drug Discov Today. 2014;19:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Ai H, Chen W, Zhang L, Huang L, Yin Z, Hu H, Zhao Q, Zhao J, Liu H. Predicting Drug-Induced Liver Injury Using Ensemble Learning Methods and Molecular Fingerprints. Toxicol Sci. 2018;165:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Feng C, Chen H, Yuan X, Sun M, Chu K, Liu H, Rui M. Gene Expression Data Based Deep Learning Model for Accurate Prediction of Drug-Induced Liver Injury in Advance. J Chem Inf Model. 2019;59:3240-3250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Ioannou GN, Tang W, Beste LA, Tincopa MA, Su GL, Van T, Tapper EB, Singal AG, Zhu J, Waljee AK. Assessment of a Deep Learning Model to Predict Hepatocellular Carcinoma in Patients With Hepatitis C Cirrhosis. JAMA Netw Open. 2020;3:e2015626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 35. | Hu C, Anjur V, Saboo K, Reddy KR, O'Leary J, Tandon P, Wong F, Garcia-Tsao G, Kamath PS, Lai JC, Biggins SW, Fallon MB, Thuluvath P, Subramanian RM, Maliakkal B, Vargas H, Thacker LR, Iyer RK, Bajaj JS. Low Predictability of Readmissions and Death Using Machine Learning in Cirrhosis. Am J Gastroenterol. 2021;116:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Kanwal F, Taylor TJ, Kramer JR, Cao Y, Smith D, Gifford AL, El-Serag HB, Naik AD, Asch SM. Development, Validation, and Evaluation of a Simple Machine Learning Model to Predict Cirrhosis Mortality. JAMA Netw Open. 2020;3:e2023780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Liu D, Liu F, Xie X, Su L, Liu M, Kuang M, Huang G, Wang Y, Zhou H, Wang K, Lin M, Tian J. Accurate prediction of responses to transarterial chemoembolization for patients with hepatocellular carcinoma by using artificial intelligence in contrast-enhanced ultrasound. Eur Radiol. 2020;30:2365-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 38. | Chaudhary K, Poirion OB, Lu L, Garmire LX. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin Cancer Res. 2018;24:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 576] [Article Influence: 82.3] [Reference Citation Analysis (0)] |