Published online Jul 28, 2020. doi: 10.35712/aig.v1.i1.19
Peer-review started: June 12, 2020
First decision: June 18, 2020
Revised: July 12, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: July 28, 2020
Processing time: 45 Days and 0.7 Hours
Pancreatic cancer is a complex cancer of the digestive tract. Diagnosis and treatment can be very difficult because of unclear early symptoms, the deep anatomical location of cancer tissues, and the high degree of cancer cell invasion. The prognosis is extremely poor; the 5-year survival rate of patients with pancreatic cancer is less than 1%. Artificial intelligence (AI) has great potential for application in the medical field. In addition to AI-based applications, such as disease data processing, imaging, and pathological image recognition, robotic surgery has revolutionized surgical procedures. To better understand the current role of AI in pancreatic cancer and predict future development trends, this article comprehensively reports the application of AI to the diagnosis, treatment, and prognosis of pancreatic cancer.
Core tip: There are few unified reports on the use of artificial intelligence (AI) with regard to pancreatic cancer. By collating information on AI’s application in this field in recent years, this article systematically reports the use of AI for the diagnosis, treatment, and prognosis of pancreatic cancer. Accordingly, this article fully depicts the current status of AI in this field and predicts future development trends.
- Citation: Lin HM, Xue XF, Wang XG, Dang SC, Gu M. Application of artificial intelligence for the diagnosis, treatment, and prognosis of pancreatic cancer. Artif Intell Gastroenterol 2020; 1(1): 19-29
- URL: https://www.wjgnet.com/2644-3236/full/v1/i1/19.htm
- DOI: https://dx.doi.org/10.35712/aig.v1.i1.19
Pancreatic cancer has a high degree of malignancy. Given the difficulty of early diagnosis, pancreatic cancer often metastasizes after diagnosis. Despite significant progress in pancreatic cancer research in the past decade, treatment and prognosis still tend to be unsatisfactory[1,2].
Artificial intelligence (AI) has greatly progressed in recent years. AI can avoid the influence of subjective thinking; deal with large data volumes; and support diagnosis, treatment, and prognosis[3]. Therefore, medical AI is a topic of considerable interest. AI is also widely used in the field of pancreatic cancer. Applications include diagnosing cancer by processing image data[4] and using machine learning to accurately distinguish cancer subtypes[5]. Robotic surgery is also widely applied to make up for the shortcomings of traditional laparoscopic surgery[6]. Artificial neural networks (ANNs) are able to predict the maximum probability of survival of patients with pancreatic cancer 7 mo after surgery[7]. Therefore, AI has great prospects for further application in the diagnosis, treatment, and prognosis of pancreatic cancer. This article summarizes the current role and application of AI in medical work related to pancreatic cancer.
In bioinformatics research, researchers often need to collect, screen, process, and summarize large amounts of data. As such, the question of whether machine learning can simplify the process and achieve good results has been a hot research topic[8]. There are many specific molecules related to pancreatic cancer such as microRNA (miRNA) 10b[9], cell-free DNA[10], and ZIP4[11]. Research on the molecular mechanism and diagnosis of pancreatic cancer has become a mature, standardized field, with a large number of relevant articles in recent years[11,12]. However, the need to collect and process data manually can consume a great deal of time and energy.
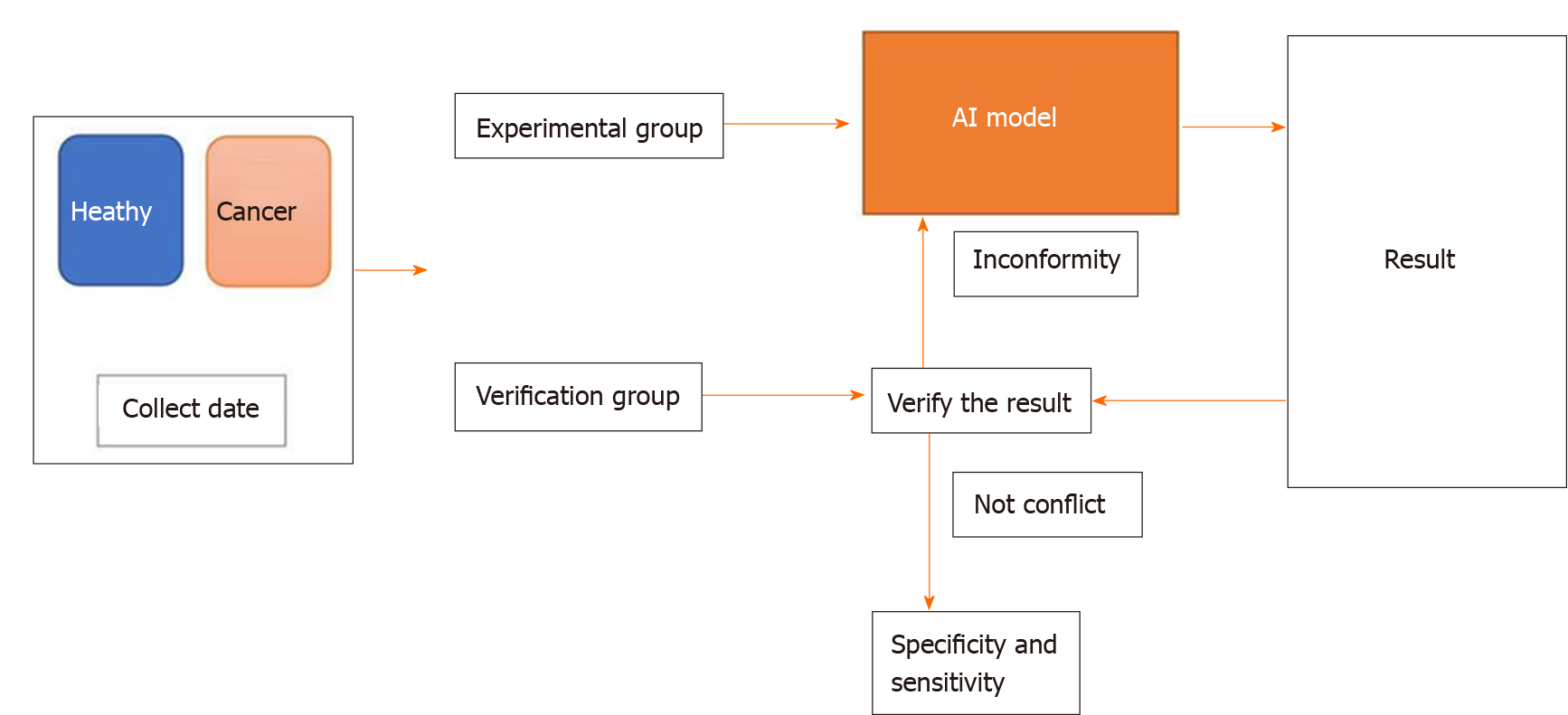
Machine learning helps researchers spend less time on data processing through one-time modeling. The steps for using machine learning typically include the following: Collecting the basic data, dividing data into an experimental group and a verification group, establishing a screening and processing model, inputting the experimental group data into the model, calculating the output results, and verifying the model’s feasibility using the verification group. The verification group can be used to test the specificity and sensitivity of the experimental group while the experimental group can make the model more intelligent[10]. The steps are illustrated in Figure 1.
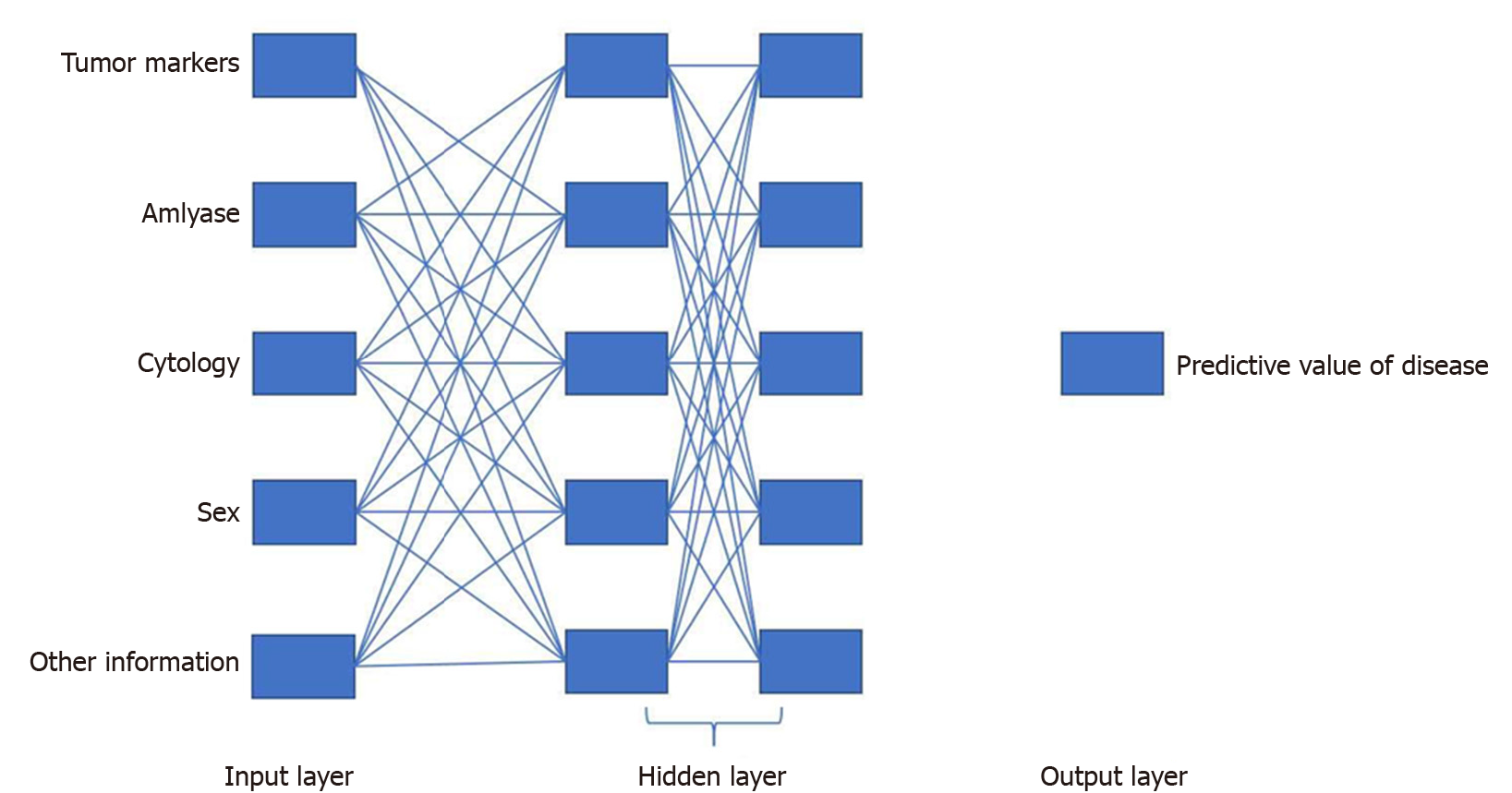
Using network representation learning and convolutional neural networks, the correlation between miRNA and pancreatic cancer disease can be analyzed, and the potential disease miRNA can be found[13]. Machine learning has been used to process large exocrine RNA data and generate predictive templates that can identify cancer in individuals[14]. An ANN can imitate the human neural meridian system. It is divided into three parts: Input layer, hidden layer, and output layer. “Deep learning” (Figure 2) refers to an ANN with multiple hidden layers. Using this technique, cyst tumor markers, amylase, cytology, and other information are inputted and then combined with two data; the output layer outputs whether the pancreatic cystic lesions are benign or malignant[15]. Some researchers have also proposed an extensible supervised classifier technical framework that can diagnose pancreatic cancer provided the expression profile of a single cell can be input to reveal its identity[9].
Although machine learning can save researchers a lot of time on data processing, machine learning still has many limitations. The first concerns data collection and processing. Specific input projects at the beginning of modeling are needed for machine learning and neural network analysis. However, for researchers who have not carried out data analysis, it is unknown which raw data are necessary and unnecessary. Useless data simply increase the workload and can also become the specificity and sensitivity of the model. Meanwhile, editing the model also poses a significant problem. Although AI can save time, the threshold and workload in the establishment of AI programs are prohibitive for nonprofessionals who lack a foundation in math and programming.
The occurrence and development of pancreatic cancer is complex and changeable, and the patient’s condition has a large degree of variability. In this regard, AI can be applied to the molecular diagnosis of pancreatic cancer and can obtain objective data processing results. However, AI is not independent and mostly can only be used as an auxiliary tool. Yet, with continuous development and improvement, AI might eventually have a more universal application.
AI algorithms (especially deep learning) have made great progress in medical image recognition; convolutional variational autoencoders and other methods have numerous applications in this field[16]. In fact, as early as 2001, neural networks were used to analyze endoscopic ultrasound images to distinguish pancreatic cancer from focal pancreatitis. A program was designed that could distinguish pancreatitis from pancreatic cancer by extracting pixel features from images, showing a high accuracy rate of 89%[17]. Given the state of image diagnosis technology at that time, the images were relatively simple, but with the help of computer neural networks, differential diagnosis became easier and achieved higher accuracy. Since then, neural network analysis images have been used in research to differentiate pancreatic cancer from chronic pancreatitis. This method involves collecting image data into a vector form and then converting it into a hue histogram. The sensitivity, specificity, and accuracy of this method in the differential diagnosis of benign and malignant pancreatic lesions were 91.4%, 87.9%, and 89.7%, respectively[18].
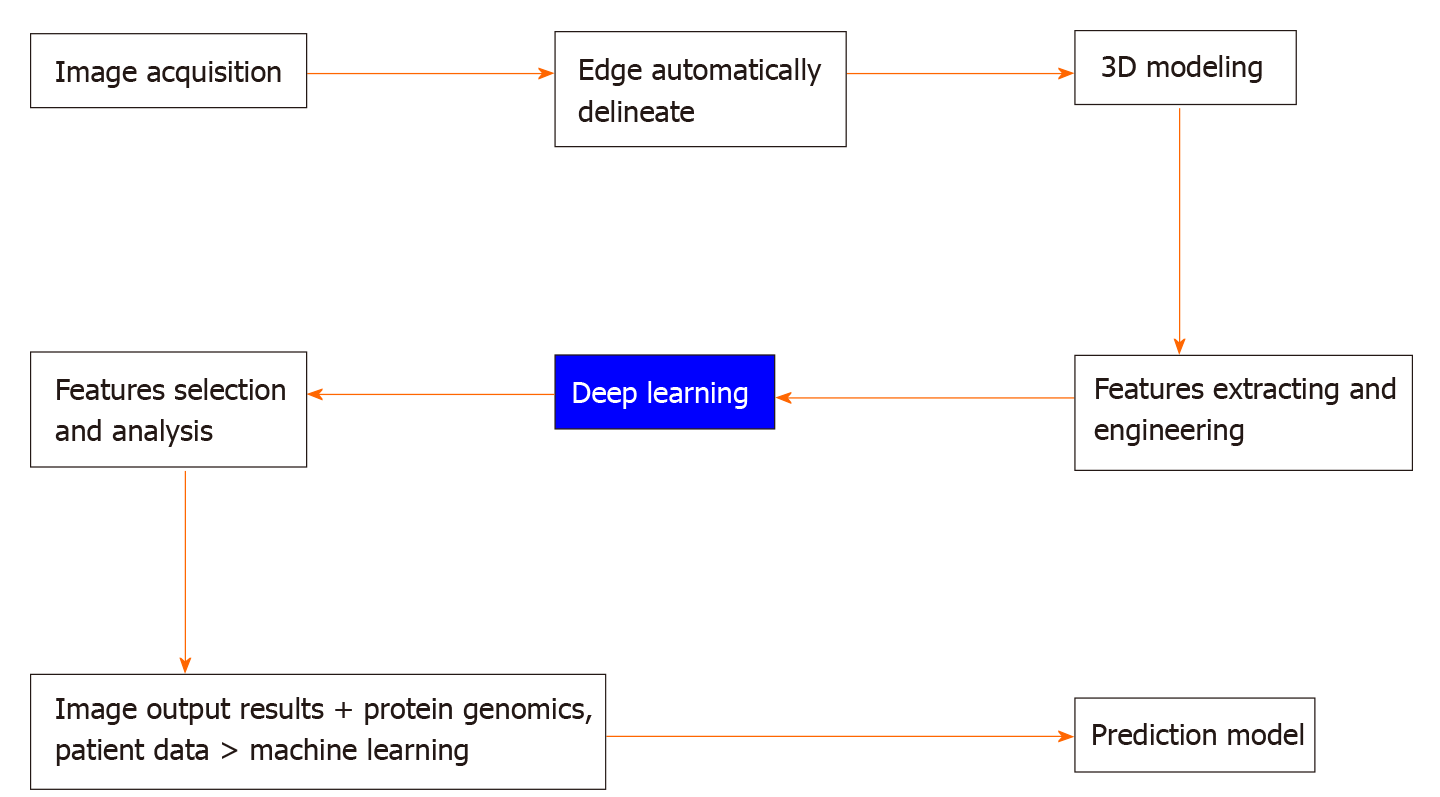
Pancreatic cystic lesions are often considered an important sign of pancreatic cancer. Machine learning is used to extract the imaging features of these cystic lesions, select and classify those features, and then use them to predict benign or malignant pancreatic cystic lesions. In this process, first, image acquisition is conducted uniformly, the edge of the suspected lesion object is delineated, and the three-dimensional (3D) shape of the variant is obtained. Then the features of suspected diseases in the image are extracted including the structure, density, and shape. AI software is used for in-depth learning, the features are screened and analyzed, and the imaging output results are obtained. The obtained results, proteomics, and patient data are entered into the machine learning model as the input layer to generate a predictive model, which can help clinicians in the differential diagnosis of benign and malignant pancreatic cysts[19].The entire process is shown in Figure 3.
Over the past 20 years, with the popularization and development of computed tomography (CT), magnetic resonance imaging, and positron emission tomography-CT, medical staff has been able to obtain more clear imaging data. However, because of human limitations, they cannot achieve zero errors, and diagnostic efficiency is not high. Furthermore, it is time consuming to train professional radiologists. Moreover, the image itself can only reflect the internal structure of the patient at a certain time and from a certain angle; thus, slight changes can be difficult to be detect with the naked eye. As such, reliable AI can improve the accuracy of image diagnosis.
As mentioned above, manual diagnosis has shortcomings such as subjective judgment, a lack of repeatability, and low accuracy. Recent research on using convolutional layer neural networks to recognize CT in pancreatic cancer diagnosis may provide a way to overcome such shortcomings. An AI designed for one related study consisted of two parts: training and verification. First, a patient data database is established, image data are collected, and an image database is established. Then the feature extraction, area generation (RPN), and classification and regression networks are established. In the AI network, the input image is first converted into a convolutional feature graph, and the RPN parameters are adjusted through the feature map to generate the ROI feature vectors. Then the RPN parameters are put into the convolutional layer, and a certain model is used for regression and classification. Next, the regression parameters are generated into new RPN parameters, and the two RPN parameters are updated only for the unique network layer of RPN through machine learning. The RPN parameters are then generated by the regression parameters to fine-tune the unique convolutional layer. Using a reserved verification group input model, the Secure Global Desktop network is trained by back-propagation and random gradient descent, and the network weights and parameters can be constantly updated and optimized. Finally, the final model is obtained as an AI diagnosis system. The receiver operating characteristic curve of the experimental results reached 0.9632. The AI in that study needed only 20 s to identify images and was more objective and effective than traditional diagnosis methods. It was noted, however, that while this method showed high accuracy in the diagnosis of pancreatic cancer, it does not mean AI can replace specialists; rather, it provides an auxiliary tool for diagnosis[20].
Although AI has good prospects for image diagnosis, it also has limitations, and the process of model training is inseparable from the assistance of artificial diagnosis. In theory, the ultimate goal of diagnostic accuracy is infinitely close to the imaging doctor. Therefore, how to make good use of this to make AI more intelligent may be an important problem to be solved in future research. In fact, the application of AI in imaging has been investigated by experts in many fields, and it also requires knowledge from many fields. Such projects create a platform for imaging experts to communicate with computer experts. The result is that an AI system is established that uses a deep learning algorithm to collect and analyze CT images of the pancreas. The experimental group image data and the normal control image data are imported into the program. Through two matrices and the application of a filter, statistics, texture, shape, and other data are obtained. Then the pancreatic ductal adenocarcinoma and the normal control are distinguished by data processing, statistical analysis, and the random forest model.
The relationship between AI and imaging involves knowledge from various fields such as pathology, radiology, oncology, and computer science. Thus, a more intelligent AI system may be built through the combined work of experts from multiple fields[21]. The AI image acquisition discussed above is based on segmenting the pancreas from the image. The traditional segmentation method is a top-down simulation fitting method based on a large amount of map input and fixed pancreatic label fusion. However, there is also a bottom-up pancreatic segmentation method that subdivides the aggregated image region into a pancreatic region and a nonpancreatic region. The segmentation is based on the visual features of the image itself, which can improve the accuracy of pancreatic segmentation. It has been reported that the bottom-up pancreas segmentation method has been optimized. With the improvement of deep convolutional neural networks, this method can deal with the highly complex appearance of the pancreas in CT images[22].
Based on the above, we can see that the application of AI in the imaging diagnosis of pancreatic cancer has made considerable advances and is constantly improving.
Pathologists need to identify diseased tissues in different tissue sections, which is a time-consuming and laborious process. Even experienced professionals may have the risk of subjective judgment. As with the application of AI in imaging diagnosis, AI is also important in the field of pathology, wherein tissue sections are digitized by a computer[23]. First, the AI system divides the lumen and nucleus from tissue fragments and extracts feature vectors from tenfold epithelial nuclei. Different cells have different feature vectors. An epithelial nucleus algorithm is used to identify epithelial nuclei. Then, the morphological features of the diseases that can be diagnosed are extracted. Finally, AI classifiers are used for classification. These classifiers include Bayesian classifiers, k-nearest neighbors, support vector machines, and ANNs[24]. Based on an automatic learning framework, cells can be segmented more accurately by combining bottom-up and top-down information. After collecting patient tissue samples, the tissue photographs are uniformly collected. A convolutional neural network model of a deep convolutional neural network is used to generate a probability map of tissue nuclear distribution. Then the iterative region merging method is used to initialize the shape of the probability graph. Next, combining a sparse shape model with stable selection and a local repulsive deformation model, a new segmentation algorithm is proposed to separate a single nucleus.
A significant advantage of this framework is that it is suitable for different stained histopathological images. Because of the feature-learning characteristics of deep cellular neural networks and the characteristics of high-level shape prior modeling, this proposed method is sufficiently universal and can be applied to different staining specimens and various types of histopathological identification. This model is not only less affected by the overlap of pathological tissues and cells but is also relatively insensitive to image noise and uneven intensity. Different tissue-staining datasets are tested, which can identify and label the concentrated area of the nucleus[25].
In addition to AI classifiers, neural networks also play an important role in image analysis to determine whether the pathology is benign or malignant. After collecting a certain amount of fine-needle aspiration pathology of the pancreatic tumor, a pathological image is captured for preprocessing (image gray conversion and noise reduction). Then, the K-means clustering algorithm is used to extract the highest value of pixels until all of the pixels are equal. The part of the image that needs to be identified is segmented so the tissue can obtain the basic nuclear features, which can be used to evaluate cellular morphological features. These features are input into the AI multilayer perceptron (a feedforward nonlinear neural network) as input vectors, and the decisions made by this perceptron are sent to the second layer perceptron using image evaluation. Because there are a certain number of validated cases, the diagnostic accuracy of benign and malignant lesions can be evaluated using statistical methods (logistic regression, multiple regression, area under the curve, and R-squared)[26]. Different from imaging diagnosis, pathological diagnosis pays more attention to accuracy. Thus, AI has a lot of room for improvement in the accuracy of auxiliary diagnosis, which will inevitably take a long time to develop.
It takes a long time to accurately delineate the target area of pancreatic cancer in radiotherapy. A recent study used machine learning to target unlabeled pancreatic cancer. The deep learning neural network included the following steps. Input of the complete X-ray image obtained by the vehicle imager, after which the image was processed by the computer. Then changes were simulated between the target tissue and normal tissue. Finally, the accuracy of the model was reevaluated through a retrospective study of patients with pancreatic cancer. The output was the position of the verified plan target in the projected image[27]. Target planning can also be conducted by imitating the human brain through AI. This AI is based on abdominal magnetic resonance (MR)-ART for automatic contour rendering through two steps. The first step is to compare the patient’s MR image with a normal MR image. Because of the high MR resolution, it can roughly outline the object. In the second step, information is directly obtained from the pixel data through a supervised, adaptive, active, learning-based support vector machine, and the target is sketched out from the features of the pixels. The information obtained through these two steps is then integrated by the AI, resulting in the final output. This approach can obtain data science institute values of more than 0.86[28].
Since the pancreas is located deep in the abdomen, radiation therapy requires not only a standard and accurate location but also an appropriate dose. An ANN dose model can be used to determine the appropriate dose. The data are processed by the input + the hidden layer + the output, which is continuously weighted. After training, errors are understood, and the weight distribution of the hidden layer is adjusted. The inputs in ANN data mining are geometric planning parameters [including CT images, treatment plans, structures, and dose distribution calculated by treatment planning systems (TPS)]. A single output is a prediction of the dose calculated by TPS for the voxel[29].
Different subtypes of pancreatic cancer cells are sensitive to different chemotherapy regimens[30]. The best way to determine cell subtypes is to make a diagnosis through pathology. However, invasive access to pathology will undoubtedly cause some pain for the patient. Due to the heterogeneity and cystic structure of pancreatic cancer tumors, the puncture results are often not ideal, sometimes even producing false-negative results. Machine learning has been applied for the noninvasive determination of pancreatic cancer cell types, including revealing the disease subtypes and molecular characteristics of pancreatic cancer. Using machine learning, pancreatic cancer-related protein expression, mRNA transcription, DNA methylation, and miRNA are integrated. Pancreatic cancer is divided into two categories. The determined subtypes have a clear response to the corresponding drug therapy and can therefore guide chemotherapy[5].
In a retrospective observation cohort study matched with histopathological tumor subtypes, after collecting the patients’ clinical and imaging data, the images were classified using a double-blind method. After image processing, feature extraction, feature preprocessing, feature engineering, and machine learning modeling, 70% of the queues were used for training, and 30% of the queues were tested. This study showed that radiological analysis combined with machine learning modeling can make high-sensitivity, high-specificity distinctions between the two groups of pancreatic ductal adenocarcinoma (PDAC) molecular subtypes defined by histomorphology. The analysis of radiological characteristics through machine learning can predict the subtypes of PDAC. This is highly related to responses to chemotherapy and patient survival[31]. AI can also simulate the effect of tumor targeted therapy drugs on tumor targeted genes. By combining machine learning, pharmacogenomics, and metabolomics, the efficacy of targeted drugs does not depend solely on the status of individual genes. It is also related to the degree of quantification of the Wahlberg effect, which leads to the emergence of the treatment window in vivo[32].
The first reports of laparoscopic pancreatectomy were published in the early 1990s[33], and the first laparoscopic pancreaticoduodenectomy was reported in 1994[34]. Laparoscopic distal pancreatectomy (LDP) is feasible and safe. Compared to open distal pancreatectomy, LDP has the advantages of less bleeding, shorter hospital stays, lower postoperative complication rates, and short-term oncology effects. LDP is also increasingly used for patients with high BMI, a history of abdominal surgery, complications, and large tumors[35]. Compared to open surgery for pancreatic cancer, laparoscopic surgery also has some limitations, such as a two-dimensional surgical field of vision, a limited range of motion, the fulcrum effect, and the enhanced tremor of effectors[6]. However, robot-assisted pancreatic cancer surgery (RDP) can make minimally invasive surgery more effective while maintaining the advantages of laparoscopic surgery (e.g., less trauma, rapid postoperative recovery, and little bleeding).
There is a question of whether robot-assisted surgery for pancreatic cancer can optimize laparoscopic surgery without increasing the side effects. Some studies have found that RDP is as safe and feasible as laparoscopic DP. The intraoperative blood loss, hospital stay, incidence of postoperative complications, perioperative mortality, and incidence of postoperative pancreatic fistula in RDP were similar to those in LDP[36-38]. Compared to open distal pancreatectomy, the probability of pancreatic fistula in RDP was not increased, and the probability of operative complications, readmission rate, mortality, and hospitalization days were similar. Robot-assisted distal pancreatectomy and pancreatectomy are comparable to traditional surgery in terms of safety and almost all outcome indicators[39,40]. RDP is relatively safe, but compared to traditional surgery and laparoscopic minimally invasive surgery, it improves the preservation rate of splenic vessels and reduces the risk of conversion to open surgery[38,41].
The Da Vinci robotic surgery system has unique characteristics, reflecting the main advantages of laparoscopic surgery. There is a stable 3D view, a wrist-like movement of the effector instrument (seven degrees of freedom), no fulcrum effect, no tremor, and no proportional adjustment of instrument motion[41]. Although robot-assisted distal pancreatectomy has potential benefits for spleen preservation, the cost of robotic surgery is very high, which is one of the obstacles to its widespread use[42]. Robotic surgery also lacks tactile sensory feedback and has a higher learning curve. Studies have shown that it takes 80 cases for a chief surgeon to reach a skilled level, and the experience of laparoscopic surgery can shorten this process[41,43]. In some complex cases, robotic surgery performs better than traditional laparoscopic surgery, such as spleen-preserving surgery with the preservation of splenic vessels[37,44]. For example, for patients with high BMI, robotic surgery may reduce intraoperative blood loss and shorten hospital stay[45]. The summary is revealed in Table 1.
| Class | ODP | LDP | RAP |
| History | Oldest | Modern | Recently |
| Bleeding | More | Less | Less |
| Hospital stay | Long | Short | Short |
| Postoperative complication rates | High | Low | Low |
| Short-term oncology effects | Normal | Litter | Litter |
| Trauma | More | Less | Less |
| Application prospect | Patients with high BMI, a history of abdominal surgery, complications, and large tumors | Approach to ODP | Preserve splenic vessels, for patients with high BMI |
| Vision | 3D | 2D | 3D |
| Tactile sensory feedback | Good | Worse | None |
| Learning curve | Low | High | Higher |
Pancreatic cancer is highly malignant. Although it can be cured through radical resection, the 5-year survival rate is still very low[46]. One study used population models and machine learning algorithms to predict the risk of recurrence in patients with pancreatic cancer 2 years after surgical resection. After collecting features considered having the most influence on recurrence, the most representative feature variables were selected, which were then used to train the machine learning algorithm. After repeated training, logistic regression was found to be the best prediction algorithm after cross-validation. This model had high accuracy in predicting the recurrence probability for a patient 2 years after surgery, suggesting that the machine learning algorithm may be helpful for identifying high-risk patients and developing adjuvant treatment strategies[47]. However, the sample size of that study was small, and there was no unified standard for treatment. Thus, this machine learning algorithm could be improved in future research by using larger samples and unified treatment.
Machine learning can also be used to develop prognostic classifiers to predict the survival of pancreatic cancer patients by integrating multiple DNA methylation statuses of pancreatic cancer–related mucin genes[48]. As a nonparametric machine learning method, ANN is also used to evaluate the survival rates of patients with pancreatic cancer. Similar to the working mode of the brain, patient variables are collected as processing elements, and interrelated processing elements are arranged and connected layer upon layer. Each connection has a related weight, each weight value can be transferred to the next ganglion layer, each lower layer can aggregate the input values of the upper layer, and the last layer is the output value. The output value is generally binary and can be used to determine whether the patient survives after 7 mo[7]. The malignancy degree of pancreatic cancer is closely related to the invasiveness of its tumor cells. Mathematical modeling represents the growth process of the tumor as a physiological and biomechanical model and personalizes the model according to the clinical measurements of target patients. The volume of the whole tumor, including its size, shape, and involved area, can be predicted[49].
Pancreatic cancer is a major cancer that threatens human health. Although there are systematic treatment plans, the effect of radiotherapy is poor because of the deep location of the pancreas and the tissue characteristics of the cancer. The special characteristics of pancreatic cancer also lead to drug resistance after chemotherapy, and surgical treatment is difficult because of the large number of important organs around the pancreas and its anatomical complexity. AI has the ability to replace or assist people in clinical work. It has great application prospects for the diagnosis, treatment, and prognosis of pancreatic cancer. Regarding molecular diagnosis, imaging diagnosis, and chemotherapy, machine learning can help researchers process data, perform analysis, and obtain experimental results. In radiotherapy, AI is mainly used for the automatic planning of radiation targets and radiation dose prediction. The development of robotic pancreatic surgery has increased the accuracy of pancreatic surgery and reduced complications, but automation cannot be fully achieved without continuous training and verification. Therefore, for a long time in the future, most AI applications for pancreatic cancer will continue to be used as practical auxiliary tools.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Donadon M S-Editor: Wang JL L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Adamska A, Domenichini A, Falasca M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int J Mol Sci. 2017;18:1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 423] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 2. | Halbrook CJ, Lyssiotis CA. Employing Metabolism to Improve the Diagnosis and Treatment of Pancreatic Cancer. Cancer Cell. 2017;31:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 3. | Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20:e262-e273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 645] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 4. | Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, Mak RH, Tamimi RM, Tempany CM, Swanton C, Hoffmann U, Schwartz LH, Gillies RJ, Huang RY, Aerts HJWL. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019;69:127-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 796] [Article Influence: 132.7] [Reference Citation Analysis (3)] |
| 5. | Sinkala M, Mulder N, Martin D. Machine Learning and Network Analyses Reveal Disease Subtypes of Pancreatic Cancer and their Molecular Characteristics. Sci Rep. 2020;10:1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Hwang HK, Kang CM, Chung YE, Kim KA, Choi SH, Lee WJ. Robot-assisted spleen-preserving distal pancreatectomy: a single surgeon's experiences and proposal of clinical application. Surg Endosc. 2013;27:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Walczak S, Velanovich V. An Evaluation of Artificial Neural Networks in Predicting Pancreatic Cancer Survival. J Gastrointest Surg. 2017;21:1606-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Rogers MA, Aikawa E. Cardiovascular calcification: artificial intelligence and big data accelerate mechanistic discovery. Nat Rev Cardiol. 2019;16:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 9. | Xie P, Gao M, Wang C, Zhang J, Noel P, Yang C, Von Hoff D, Han H, Zhang MQ, Lin W. SuperCT: a supervised-learning framework for enhanced characterization of single-cell transcriptomic profiles. Nucleic Acids Res. 2019;47:e48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, Jensen SØ, Medina JE, Hruban C, White JR, Palsgrove DN, Niknafs N, Anagnostou V, Forde P, Naidoo J, Marrone K, Brahmer J, Woodward BD, Husain H, van Rooijen KL, Ørntoft MW, Madsen AH, van de Velde CJH, Verheij M, Cats A, Punt CJA, Vink GR, van Grieken NCT, Koopman M, Fijneman RJA, Johansen JS, Nielsen HJ, Meijer GA, Andersen CL, Scharpf RB, Velculescu VE. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570:385-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 797] [Cited by in RCA: 843] [Article Influence: 140.5] [Reference Citation Analysis (0)] |
| 11. | Liu M, Zhang Y, Yang J, Cui X, Zhou Z, Zhan H, Ding K, Tian X, Yang Z, Fung KA, Edil BH, Postier RG, Bronze MS, Fernandez-Zapico ME, Stemmler MP, Brabletz T, Li YP, Houchen CW, Li M. ZIP4 Increases Expression of Transcription Factor ZEB1 to Promote Integrin α3β1 Signaling and Inhibit Expression of the Gemcitabine Transporter ENT1 in Pancreatic Cancer Cells. Gastroenterology. 2020;158:679-692.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 12. | Grant RC, Denroche RE, Borgida A, Virtanen C, Cook N, Smith AL, Connor AA, Wilson JM, Peterson G, Roberts NJ, Klein AP, Grimmond SM, Biankin A, Cleary S, Moore M, Lemire M, Zogopoulos G, Stein L, Gallinger S. Exome-Wide Association Study of Pancreatic Cancer Risk. Gastroenterology. 2018;154:719-722.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Xuan P, Sun H, Wang X, Zhang T, Pan S. Inferring the Disease-Associated miRNAs Based on Network Representation Learning and Convolutional Neural Networks. Int J Mol Sci. 2019;20:3648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Ko J, Bhagwat N, Yee SS, Ortiz N, Sahmoud A, Black T, Aiello NM, McKenzie L, O'Hara M, Redlinger C, Romeo J, Carpenter EL, Stanger BZ, Issadore D. Combining Machine Learning and Nanofluidic Technology To Diagnose Pancreatic Cancer Using Exosomes. ACS Nano. 2017;11:11182-11193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 15. | Kurita Y, Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, Obata M, Koda H, Tajika M, Shimizu Y, Nakajima A, Kubota K, Niwa Y. Diagnostic ability of artificial intelligence using deep learning analysis of cyst fluid in differentiating malignant from benign pancreatic cystic lesions. Sci Rep. 2019;9:6893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1821] [Article Influence: 260.1] [Reference Citation Analysis (2)] |
| 17. | Norton ID, Zheng Y, Wiersema MS, Greenleaf J, Clain JE, Dimagno EP. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointest Endosc. 2001;54:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Dalal V, Carmicheal J, Dhaliwal A, Jain M, Kaur S, Batra SK. Radiomics in stratification of pancreatic cystic lesions: Machine learning in action. Cancer Lett. 2020;469:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Liu SL, Li S, Guo YT, Zhou YP, Zhang ZD, Li S, Lu Y. Establishment and application of an artificial intelligence diagnosis system for pancreatic cancer with a faster region-based convolutional neural network. Chin Med J (Engl). 2019;132:2795-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Weisberg EM, Chu LC, Park S, Yuille AL, Kinzler KW, Vogelstein B, Fishman EK. Deep lessons learned: Radiology, oncology, pathology, and computer science experts unite around artificial intelligence to strive for earlier pancreatic cancer diagnosis. Diagn Interv Imaging. 2020;101:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Fu M, Wu W, Hong X, Liu Q, Jiang J, Ou Y, Zhao Y, Gong X. Hierarchical combinatorial deep learning architecture for pancreas segmentation of medical computed tomography cancer images. BMC Syst Biol. 2018;12:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A. Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. 2019;16:703-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 822] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 24. | Song JW, Lee JH, Choi JH, Chun SJ. Automatic differential diagnosis of pancreatic serous and mucinous cystadenomas based on morphological features. Comput Biol Med. 2013;43:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Xing F, Xie Y, Yang L. An Automatic Learning-Based Framework for Robust Nucleus Segmentation. IEEE Trans Med Imaging. 2016;35:550-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 26. | Momeni-Boroujeni A, Yousefi E, Somma J. Computer-assisted cytologic diagnosis in pancreatic FNA: An application of neural networks to image analysis. Cancer Cytopathol. 2017;125:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Zhao W, Shen L, Han B, Yang Y, Cheng K, Toesca DAS, Koong AC, Chang DT, Xing L. Markerless Pancreatic Tumor Target Localization Enabled By Deep Learning. Int J Radiat Oncol Biol Phys. 2019;105:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Liang F, Qian P, Su KH, Baydoun A, Leisser A, Van Hedent S, Kuo JW, Zhao K, Parikh P, Lu Y, Traughber BJ, Muzic RF. Abdominal, multi-organ, auto-contouring method for online adaptive magnetic resonance guided radiotherapy: An intelligent, multi-level fusion approach. Artif Intell Med. 2018;90:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Campbell WG, Miften M, Olsen L, Stumpf P, Schefter T, Goodman KA, Jones BL. Neural network dose models for knowledge-based planning in pancreatic SBRT. Med Phys. 2017;44:6148-6158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1536] [Cited by in RCA: 1345] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 31. | Kaissis G, Ziegelmayer S, Lohöfer F, Steiger K, Algül H, Muckenhuber A, Yen HY, Rummeny E, Friess H, Schmid R, Weichert W, Siveke JT, Braren R. A machine learning algorithm predicts molecular subtypes in pancreatic ductal adenocarcinoma with differential response to gemcitabine-based versus FOLFIRINOX chemotherapy. PLoS One. 2019;14:e0218642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Liberti MV, Dai Z, Wardell SE, Baccile JA, Liu X, Gao X, Baldi R, Mehrmohamadi M, Johnson MO, Madhukar NS, Shestov AA, Chio IIC, Elemento O, Rathmell JC, Schroeder FC, McDonnell DP, Locasale JW. A Predictive Model for Selective Targeting of the Warburg Effect through GAPDH Inhibition with a Natural Product. Cell Metab. 2017;26:648-659.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 33. | Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery. 1996;120:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 307] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 34. | Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 655] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 35. | Liang S, Hameed U, Jayaraman S. Laparoscopic pancreatectomy: indications and outcomes. World J Gastroenterol. 2014;20:14246-14254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Fernandes E, Giulianotti PC. Robotic-assisted pancreatic surgery. J Hepatobiliary Pancreat Sci. 2013;20:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Liu R, Wakabayashi G, Palanivelu C, Tsung A, Yang K, Goh BKP, Chong CC, Kang CM, Peng C, Kakiashvili E, Han HS, Kim HJ, He J, Lee JH, Takaori K, Marino MV, Wang SN, Guo T, Hackert T, Huang TS, Anusak Y, Fong Y, Nagakawa Y, Shyr YM, Wu YM, Zhao Y. International consensus statement on robotic pancreatic surgery. Hepatobiliary Surg Nutr. 2019;8:345-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Kamarajah SK, Bundred J, Marc OS, Jiao LR, Manas D, Abu Hilal M, White SA. Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | McMillan MT, Zureikat AH, Hogg ME, Kowalsky SJ, Zeh HJ, Sprys MH, Vollmer CM. A Propensity Score-Matched Analysis of Robotic vs Open Pancreatoduodenectomy on Incidence of Pancreatic Fistula. JAMA Surg. 2017;152:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 40. | Girgis MD, Zenati MS, King JC, Hamad A, Zureikat AH, Zeh HJ, Hogg ME. Oncologic Outcomes After Robotic Pancreatic Resections Are Not Inferior to Open Surgery. Ann Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Hong S, Song KB, Madkhali AA, Hwang K, Yoo D, Lee JW, Youn WY, Alshammary S, Park Y, Lee W, Kwon J, Lee JH, Hwang DW, Kim SC. Robotic versus laparoscopic distal pancreatectomy for left-sided pancreatic tumors: a single surgeon's experience of 228 consecutive cases. Surg Endosc. 2020;34:2465-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Alfieri S, Butturini G, Boggi U, Pietrabissa A, Morelli L, Vistoli F, Damoli I, Peri A, Fiorillo C, Pugliese L, Ramera M, De Lio N, Di Franco G, Esposito A, Landoni L, Rosa F, Menghi R, Doglietto GB, Quero G; Italian Robotic pNET Group. Short-term and long-term outcomes after robot-assisted versus laparoscopic distal pancreatectomy for pancreatic neuroendocrine tumors (pNETs): a multicenter comparative study. Langenbecks Arch Surg. 2019;404:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, Bartlett DL, Zeh HJ, Zureikat AH. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg. 2015;150:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 44. | Najafi N, Mintziras I, Wiese D, Albers MB, Maurer E, Bartsch DK. A retrospective comparison of robotic versus laparoscopic distal resection and enucleation for potentially benign pancreatic neoplasms. Surg Today. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | He S, Ding D, Wright MJ, Groshek L, Javed AA, Ka-Wan Chu K, Burkhart RA, Cameron JL, Weiss MJ, Wolfgang CL, He J. The impact of high body mass index on patients undergoing robotic pancreatectomy: A propensity matched analysis. Surgery. 2020;167:556-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1350] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 47. | Sala Elarre P, Oyaga-Iriarte E, Yu KH, Baudin V, Arbea Moreno L, Carranza O, Chopitea Ortega A, Ponz-Sarvise M, Mejías Sosa LD, Rotellar Sastre F, Larrea Leoz B, Iragorri Barberena Y, Subtil Iñigo JC, Benito Boíllos A, Pardo F, Rodríguez Rodríguez J. Use of Machine-Learning Algorithms in Intensified Preoperative Therapy of Pancreatic Cancer to Predict Individual Risk of Relapse. Cancers (Basel). 2019;11:606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Yokoyama S, Hamada T, Higashi M, Matsuo K, Maemura K, Kurahara H, Horinouchi M, Hiraki T, Sugimoto T, Akahane T, Yonezawa S, Kornmann M, Batra SK, Hollingsworth MA, Tanimoto A. Predicted Prognosis of Patients with Pancreatic Cancer by Machine Learning. Clin Cancer Res. 2020;26:2411-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Zhang L, Lu L, Summers RM, Kebebew E, Yao J. Convolutional Invasion and Expansion Networks for Tumor Growth Prediction. IEEE Trans Med Imaging. 2018;37:638-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |