Published online Feb 28, 2020. doi: 10.13105/wjma.v8.i1.4
Peer-review started: October 22, 2019
First decision: December 5, 2019
Revised: December 17, 2019
Accepted: February 15, 2020
Article in press: February 15, 2020
Published online: February 28, 2020
Non-alcoholic fatty liver disease (NAFLD) dominates the landscape of modern hepatology. Affecting 25% of the general population, there is critical unmet need to identify broadly available, safe and cost-effective treatments. Cumulative evidence in animal and human models suggests that intrahepatic and skeletal muscle fatty acid oxidation is impaired in NAFLD, such that lipid accretion is not matched by efficient utilisation. L-carnitine is a crucial mediator of fatty acid metabolism in vivo, promoting mitochondrial lipid β-oxidation and enhancing tissue metabolic flexibility. These physiological properties have generated research interest in L-carnitine as a potentially effective adjunctive therapy in NAFLD.
To systematically review randomised trials reporting effects of dietary L-carnitine supplementation on liver biochemistry, liver fat and insulin sensitivity in NAFLD.
Search strategies, eligibility criteria and analytic methods were specified a priori (PROSPERO reference: CRD42018107063). Ovid MEDLINE, Ovid EMBASE, PubMed, Web of Science and the Cochrane Library were searched from their inception until April 2019. Outcome measures included serum concentrations of alanine and aspartate aminotransferase (ALT and AST), liver fat and insulin sensitivity assessed by the homeostasis model of insulin resistance (HOMA-IR). A random effects meta-analysis was performed for, ALT, AST and HOMA-IR measures separately. Between-study heterogeneity was measured using I2 statistics.
Five eligible randomised trials were included in the qualitative and quantitative synthesis (n = 338). All of the 5 included trials assessed the effect of L-carnitine on serum ALT, identified from Italy, South Korea and Iran. Weighted mean difference (WMD) for ALT between L-carnitine and control groups after intervention was -25.34 IU/L [95%CI: -41.74-(-8.94); P = 0.002]. WMD for AST between L-carnitine and control groups was -13.68 IU/L (95%CI: -28.26-0.89; P = 0.066). In three studies (n = 204), HOMA-IR was evaluated. WMD for HOMA-IR between L-carnitine and control groups was -0.74 units [95%CI: -1.02-(-0.46); P < 0.001]. Two studies using validated outcome measures reported a significant reduction in liver fat in L-carnitine vs control groups post-intervention (P < 0.001).
Pooled results indicate that L-carnitine supplementation attenuates ALT, liver fat and insulin resistance in NAFLD cohorts, confirming a beneficial effect of L-carnitine for a highly prevalent condition with a growing economic burden.
Core Tip: Non-alcoholic fatty liver disease (NAFLD) presents a major public health challenge. As a leading cause of abnormal liver chemistry, rising in prevalence together with obesity and insulin resistance, there is critical unmet need to identify cost-effective, population-based treatment. We synthesised evidence from randomised trials published to date evaluating the effect of dietary L-carnitine supplementation on transaminases, liver fat and insulin resistance in NAFLD. We demonstrate a significant reduction in serum alanine aminotransferase, homeostasis model of insulin resistance and liver fat with dietary L-carnitine supplementation. L-carnitine could therefore present a novel therapeutic tool for NAFLD and its metabolic associations.
- Citation: Thiagarajan P, Chalmers J, Ban L, Grindlay D, Aithal GP. L-carnitine supplementation in non-alcoholic fatty liver disease: A systematic review and meta-analysis. World J Meta-Anal 2020; 8(1): 4-14
- URL: https://www.wjgnet.com/2308-3840/full/v8/i1/4.htm
- DOI: https://dx.doi.org/10.13105/wjma.v8.i1.4
Non-alcoholic fatty liver disease (NAFLD) has rapidly emerged as a leading cause of chronic liver disease and liver transplantation worldwide[1]. The global prevalence of NAFLD is estimated to be 25.24%[2], rising to 66% and 90% in those with type 2 diabetes and obesity, respectively[3]. Although progression to non-alcoholic steatohepatitis (NASH) is limited to approximately 30% of individuals with NAFLD, the high population prevalence of NAFLD heralds a looming socioeconomic burden due to the consequences of its progression, including end-stage liver disease[4]. In EU4 countries alone (France, Germany, Italy, United Kingdom), the annual cost associated with NAFLD is estimated to be €35 billion[5]. Diet and lifestyle modification remain current standard of care, and there is no specifically licensed disease-modifying therapy available.
L-Carnitine is a naturally occurring water-soluble quaternary amine which acts as a crucial mediator of fatty acid metabolism in vivo. The role of carnitine as a key regulator of intracellular bioenergetics has gained traction in the search for broadly applicable treatments for metabolic disorders, including obesity and type 2 diabetes. The ability of L-carnitine to regulate muscle mitochondrial fuel selection, through promoting both lipid oxidation and non-oxidative glucose disposal, renders it an attractive target for therapeutic intervention in the context of insulin resistance[6]. Cumulative evidence in both animal and human models suggests that intrahepatic and skeletal muscle fatty acid transport and oxidation is impaired in NAFLD and insulin resistance, such that excessive lipid accretion is not matched by efficient utilisation[7,8]. Thus, the effect of carnitine supplementation in the context of NAFLD specifically has been the focus of recent interest. This review aims to critically and systematically evaluate all human randomised trials investigating the effect of carnitine on liver fat and/or metabolic parameters in NAFLD.
Search strategies, eligibility criteria and analytic methods were specified a priori in the study protocol, which was registered with the PROSPERO database (CRD42018 107063).
We performed a systematic literature search for randomised trials reporting the effects of dietary L-carnitine supplementation on liver biochemistry and liver fat in adult individuals with NAFLD and NASH.
The databases searched were PubMed, Ovid EMBASE, Ovid MEDLINE, Web of Science Core Collection and the Cochrane Library. The full search strategy used in Ovid MEDLINE is provided in Appendix 1. Databases were searched from their inception until April 2019. No language restrictions were used. For each database, a comprehensive list of alternative terms for NAFLD were combined with alternative terms for L-carnitine, using the Boolean operator AND. Reference lists of studies ultimately selected for inclusion were searched to identify any other relevant research.
Diagnostic criteria for NAFLD varied significantly between studies, but eligible studies included adult individuals diagnosed with NAFLD on the basis of validated histological, imaging or biochemical tests, including the following, and where other causes of hepatic steatosis had been excluded: (1) Liver histology; (2) Magnetic Resonance Imaging with proton density fat fraction (MRI-PDFF) or proton magnetic resonance spectroscopy (1H-MRS); (3) Computed Tomography (CT); (4) Ultrasound and (5) Serum concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in conjunction with impaired glucose tolerance and in the absence of documented alcohol excess.
Primary outcome measures included change in serum concentrations of ALT, AST and liver fat (as assessed either by liver biopsy, cross-sectional imaging or ultrasound). Secondary outcome measures included changes in insulin sensitivity parameters (as assessed by the HOMA-IR) and, where available, markers of inflammation and oxidative stress.
Study selection was performed independently by two separate reviewers (PT and JC) with any disagreements revolved by a third researcher (GPA). Titles and abstracts of returned searches were evaluated against eligibility criteria. Those meeting eligibility criteria based on title and abstract were subsequently read in full. Wherever journal articles were found to contain insufficient information for critical analysis, attempts were made to contact the authors directly for clarification of missing details.
Eligible published studies included human randomised trials evaluating the effect of carnitine supplementation on liver fat, liver enzymes, glucose and markers of inflammation or oxidative stress in adult individuals with NAFLD. Only full reports were considered as eligible for inclusion on the basis that they provided sufficient data to permit critical analysis. Studies were considered eligible for inclusion if they: (1) Were randomised in design; (2) Evaluated L-carnitine versus placebo, L-carnitine plus another intervention versus that intervention alone, or L-carnitine versus no intervention; (3) Included a patient population diagnosed with NAFLD and/or NASH based on validated histological, radiological or biochemical parameters as listed above; and (4) Included only subjects aged 18 years or above. Studies were excluded if they: (1) Were non-randomised in design e.g., case reports, reviews or observational studies; (2) Included patients with another cause of hepatic steatosis e.g., alcohol, genetic or viral liver disease; (3) Were animal studies; and (4) Did not evaluate outcomes of interest as detailed above.
There were no restrictions based on dosage, formulation or frequency of administration. Interventions in the control group included active placebo supplementation, hypocaloric diet and metformin therapy. Trials evaluating L-carnitine supplementation together with other interventions, in the absence of a control group consisting of the other interventions alone, were excluded. No specific treatment duration was specified for inclusion in this review.
Data extracted from individual studies included (1) participant demographics; (2) intervention type and dose; (3) method of NAFLD diagnosis; (4) outcome measurements of liver fat, liver enzymes, glycometabolic profile and markers of inflammation and oxidative stress; (5) documentation as to whether informed consent was gained; (6) methods of randomisation; (7) allocation concealment; (8) participant and staff blinding; (9) blinding of outcome assessment; (10) presence of incomplete outcome data; and (11) evidence of any selective reporting. The Cochrane risk of bias tool[9] was then used to systematically appraise each included study in terms of methodological quality and validity according to the criteria of the Cochrane guidelines.
A random effects meta-analysis was performed for ALT, AST and HOMA-IR measures separately. Weighted mean difference (WMD) with 95%CI were calculated. Between-study heterogeneity was measured using I2 statistics, with I2 > 50% indicating significant heterogeneity. The analysis was performed using Stata software version 11.0 Stata (Version 11.2, StataCorp, College Station, Texas). Resultswere summarised using Forest plots.
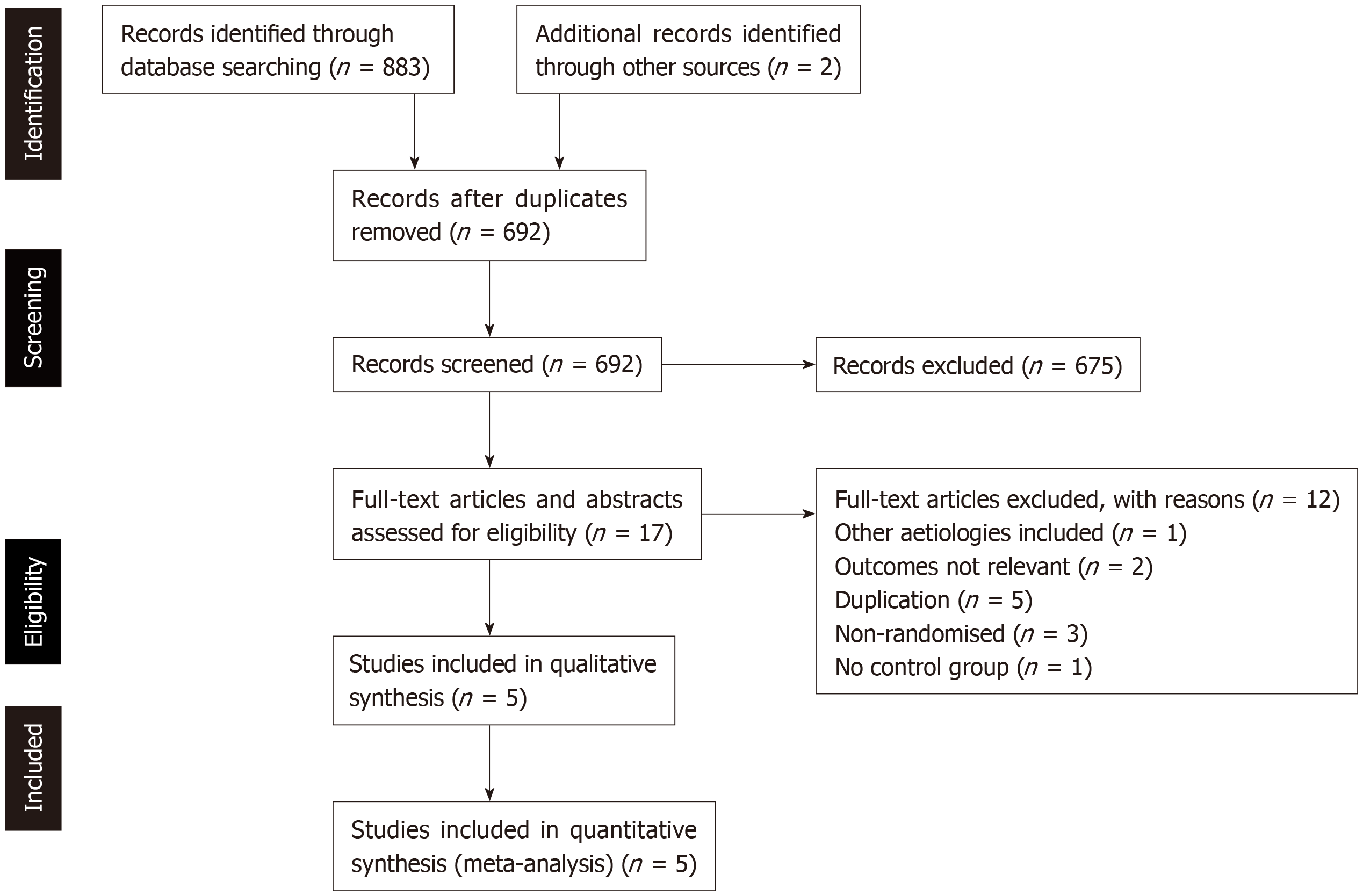
Figure 1 depicts the PRISMA flowchart process of identification and selection of eligible studies for inclusion in the qualitative and quantitative syntheses. Primary database searches yielded 883 citations. After de-duplication, 692 remaining citations were screened for eligibility by reading titles and abstracts. Of these remaining studies, 675 were excluded. Of the remaining 17 citations, full text articles retrieved were read in full to determine eligibility. Twelve studies were subsequently excluded.
Table 1 summarises study characteristics for the five randomised trials ultimately included in the qualitative and quantitative synthesis[10-14]. In total, these trials comprised 338 patients (234 men, 104 women). In 3 trials, non-diabetic patients with NAFLD were recruited and the other 2 trials recruited individuals with type 2 diabetes mellitus and NAFLD.
| Ref. | Study population (diagnosis); comorbidities | Sample size (M/F) Control/Carnitine | Age (yr) | BMI | Duration (wk) | Intervention (dose) | Outcome Measures | Control | Results |
| Malaguarnera et al[10] | Biopsy-proven NASH without diabetes | 74 (40/34) | 47.8 ± 5.8 (CTRL) | 26.5 ± 3.8 (CTRL) | 24 | L-carnitine (1000 mg BD) plus hypocaloric (1600 cal ) diet | Primary: Improvement in histological features of NASH | Placebo plus Hypocaloric (1600 cal) diet | Primary Outcome: ↓ NASH activity score1 (9.42-3.19), ↓ALT1 ↓AST1, ↓GGT1 ↓TC1, ↓LDL1, ↑HDL1, ↓GLC1, ↓HOMA-IR1, ↓CRP1, ↓TNFα1 |
| 38 (CTRL) | 47.9 ± 5.4 (CAR) | 26.6 ± 3.7 (CAR) | Other: ALT, AST, lipid profile, GLC, HOMA-IR, CRP, TNFα | ||||||
| 36 (CAR) | |||||||||
| Bae et al[11] | NAFLD with type 2 diabetes | 78 (54/24) | 52 ± 9.4 (CTRL) | 26.7 ± 3.7 (CTRL) | 12 | Carnitine orotate complex (824 mg TDS) | Primary: Change in ALT | Placebo | Primary outcome: ↓ALT (89.7% vs 17.9%)1 |
| 39 (CTRL) | 50.6 ± 9.3 (CAR) | 28.2 ± 2.6 (CAR) | Other: Liver attenuation index (CT) | Other: ↓Liver attenuation index1 (0.74 ± 8.05 vs 6.21 ± 8.96)1, ↓HbA1c, ↓GLC, ↓HOMA-IR2 | |||||
| 39 (CAR) | |||||||||
| Alavinejad et al[12] | NAFLD (ultrasound+ raised ALT) with T2DM | 54 (38/16) | 59 ± 9 (CTRL) | 29.5 ± 3.6 (CTRL) | 12 | L-carnitine (750 mg TDS) | Primary: AST, ALT | Placebo | Primary outcome: ↓ALT1, ↓AST1 Other: ↓TG2, ↓GLC2, ↓HbA1c2 |
| 26 (CTRL) | 60 ± 5 (CAR) | 28.6 ± 4.6 (CAR) | Other: TG, GLC, HbA1c | ||||||
| 28 (CAR) | |||||||||
| Hong et al[13] | NAFLD (plasma ALT 40-250) and impaired glucose tolerance (HbA1C ≥ 6.0%) | 52 (36/16) | 52.0 ± 9.6 (CTRL) | 27.0 ± 3.1 (CTRL) | 12 | Carnitine-orotate complex (300 mg TDS) + metformin | Primary: change from baseline ALT | Metformin alone | Primary outcome: ↓ ALT1 |
| 26 (CTRL) | 51.5 ± 9.4 (CAR) | 27.2 ± 2.6 (CAR) | Other: GLC, HbA1c, m t DNA copy number, urine 8-OHdG | Other: ↓HbA1c2, ↑ plasma mtDNA copy number1, ↓GLC2, ↓urine 8-OHdG | |||||
| 26 (CAR) | |||||||||
| Somi et al[14] | NAFLD (ultrasound + plasma ALT ≥ 40) | 80 (66/14) | 40.7 ± 8 | 29.4 ± 3.9 | 24 | L-carnitine (250 mg BD) | Primary: ALT, AST | No treatment | Primary outcome: ↓ALT1, ↓AST1 |
| Other: Sonographic change in liver fat, BMI | Other: ↓ liver fat on USS |
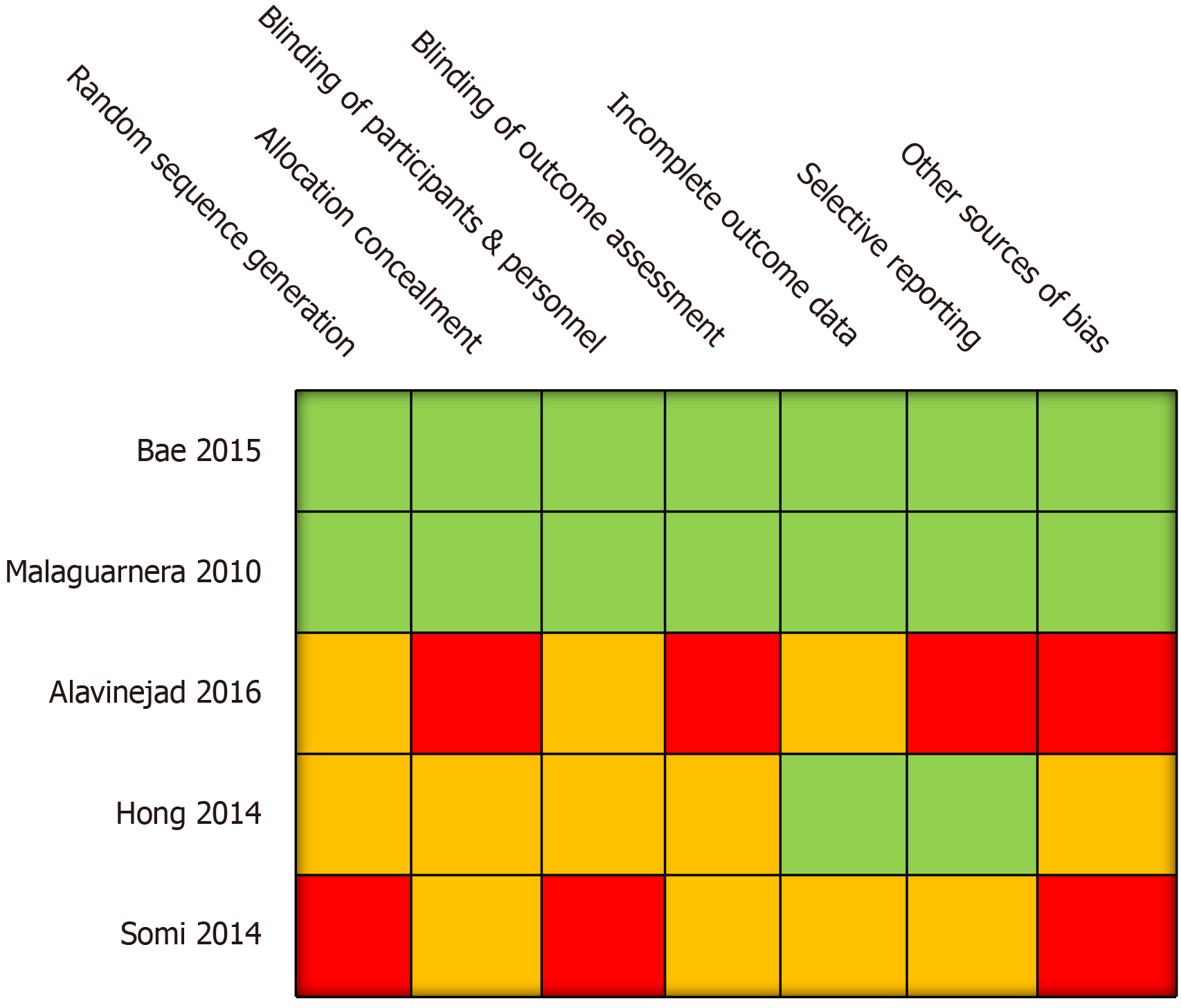
Methodological quality was assessed using criteria set out in the Cochrane Handbook for Systematic Reviews (Figure 2). All trials described randomising participants to L-carnitine and control arms. However, only two of the five trials[10,11] reported the methods used to generate random allocation sequence (i.e., computer generated tool) to a standard sufficient enough to be judged as having a low risk of bias. In the other 3 trials, no information was provided regarding methods of blinding and insufficient information was provided to enable informed decision-making regarding adequacy of randomisation. One trial reported randomising participants by using random numbers allocated to consecutive patients; this was considered to confer a high risk of selection bias[12].
Regarding allocation concealment, only two studies[10,11] reported efforts to conceal allocation sequences from personnel as well as patients. In two of the studies, genuine blinding was considered impossible due to lack of a placebo arm[13,14]. In the remaining two trials, insufficient information was given to determine whether a robust allocation concealment process was undertaken. Three of the five studies accounted for incomplete outcome data and clearly explained any loss to follow up and exclusions[10,11,13]. The other two studies lacked explanations for trial exclusion.
Selective reporting was considered to be present in two out of the five included studies. Alavinejad et al[12] (2016) with sonographic grade of liver fat as an outcome measure in their study, noted that “follow up ultrasonography did not show any significant change in comparison with baseline reports”. However no baseline or post-intervention values were reported. Somi et al[14] (2014) reported changes from baseline in sonographically-determined grade of NAFLD in patients in the L-carnitine arm of the study, with nine of the L-carnitine treated patients having no evidence of NAFLD on post-intervention ultrasound. However it was unclear how many patients had progressed or regressed in steatosis grade.
In three out of the five trials, funding sources and conflicts of interests were explicitly stated, whereas in the other two trials no mention was made of either[12,14]. Efficacy, safety and compliance measures were reported in 3 studies but no description of methods to evaluate compliance was made in the other two studies and Somi et al[12,14] (2014) did not report any safety visits for subjects during 24 wk of supplementation. Statistical analyses were reported in all five trials.
Estimates were made on the effect of L-carnitine on outcomes including liver transaminases, the HOMA-IR and liver fat (where measured).
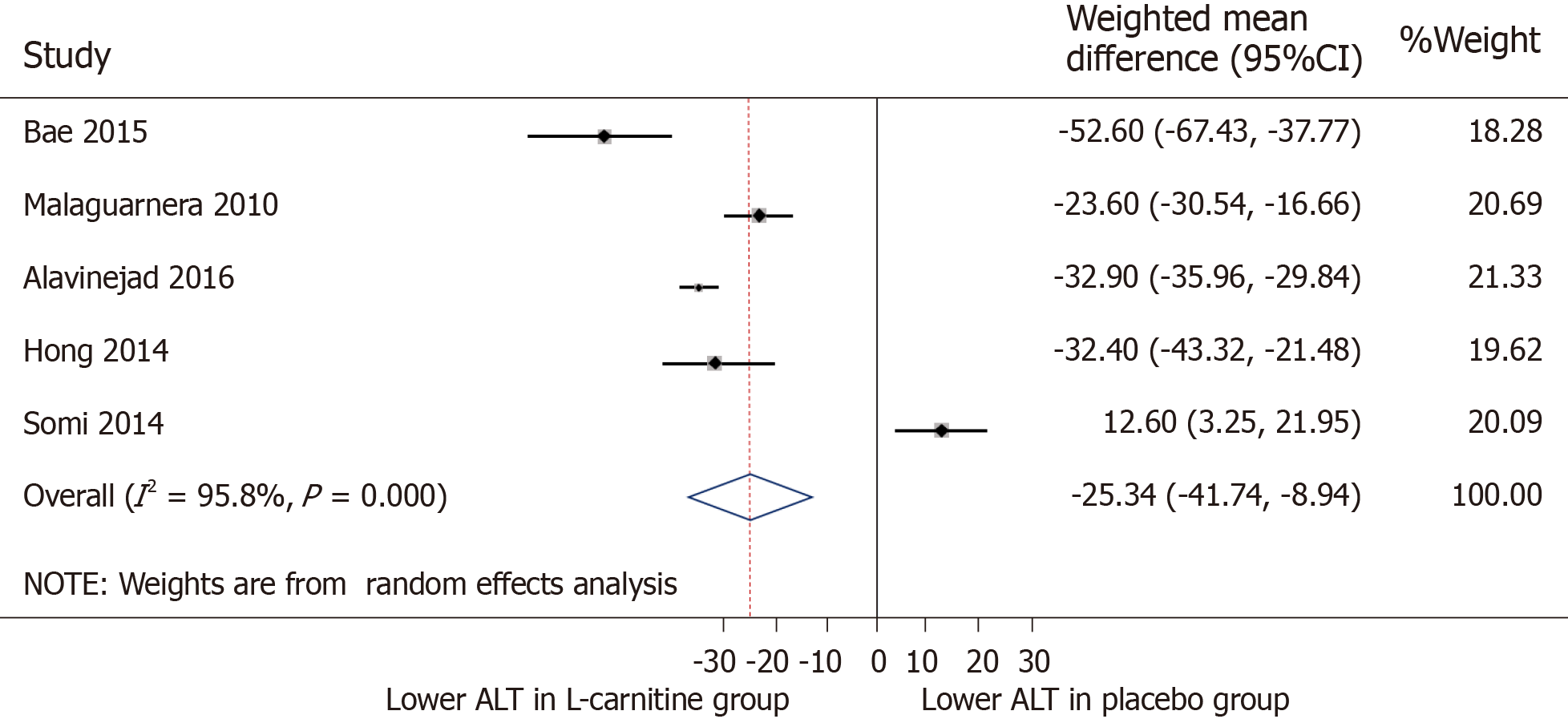
ALT: All of the five included trials were for meta-analysis of ALT measures after intervention (Figure 3). The WMD for ALT between the L-carnitine groups and the control groups after intervention was -25.34 IU/L [95%CI: -41.74-(-8.94), P = 0.002] (Figure 3). The I2 was 95.8%, indicating statistically significant heterogeneity between the studies.
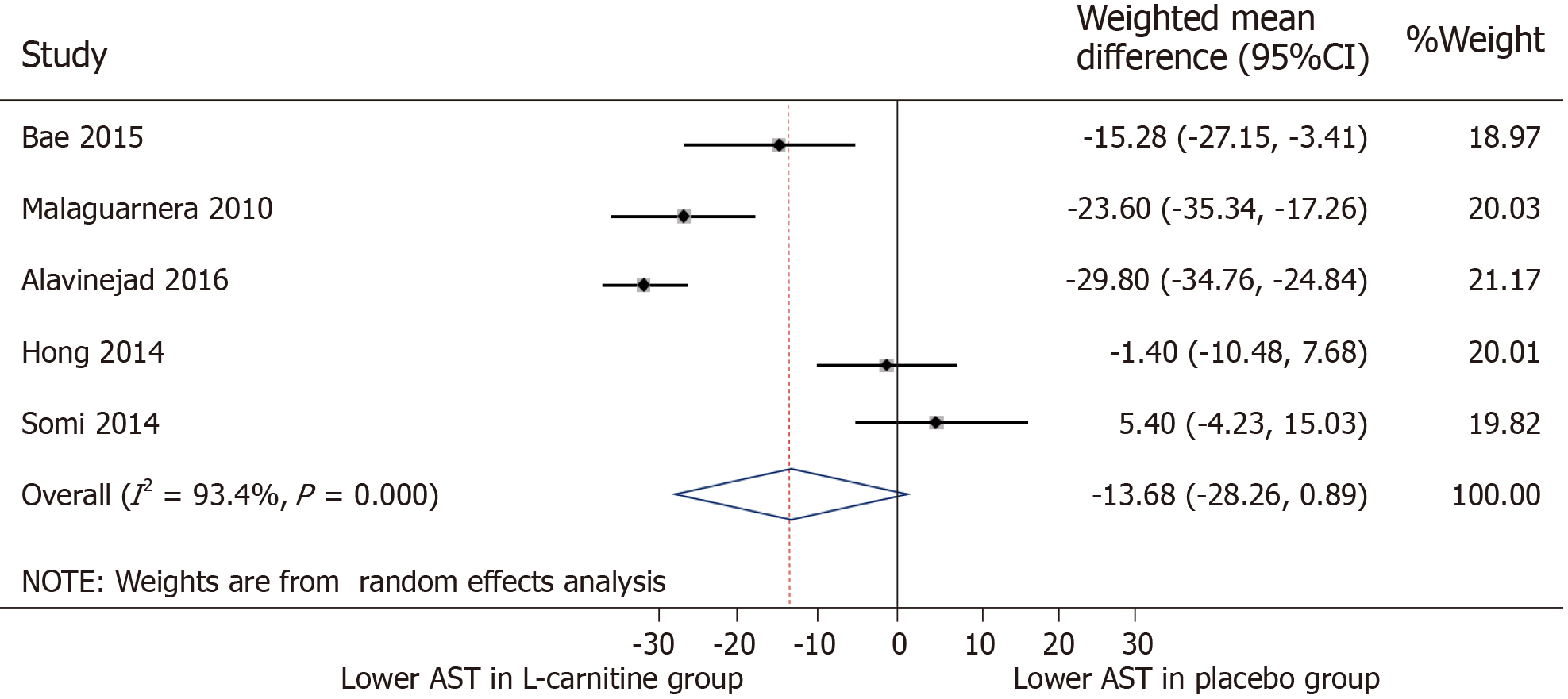
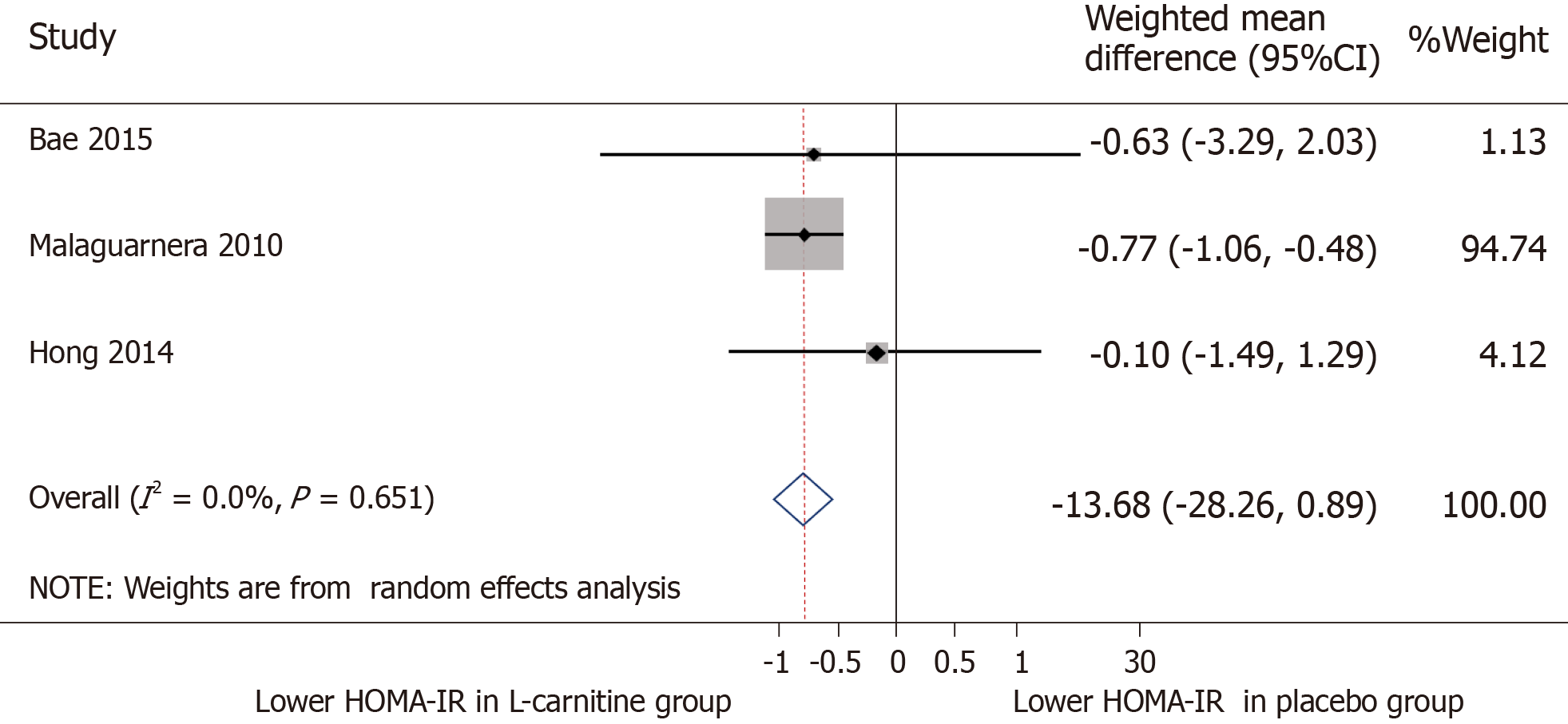
AST: All five trials were included for meta-analysis of AST measures after intervention. The WMD for AST between the L-carnitine group and the control group after intervention was -13.68 IU/L (95%CI: -28.26-0.89) (P = 0.066) (Figure 4). The I2 was 93.4%, indicating high statistical heterogeneity between the studies.
Liver fat: Four of the included studies evaluated hepatic steatosis at baseline and post-intervention. Outcome measures differed between the studies, precluding the possibility of a quantitative synthesis of results. In two studies, ultrasonography was used to grade liver fat at baseline and post-intervention[12,14]. In another study, CT imaging was used[11] and in the fourth study, liver fat was evaluated histologically[10].
Malaguarnera et al[10] (2010) assessed liver fat using paired biopsies; the group reported a significant reduction in steatosis in the group randomised to L-carnitine compared to placebo (1.68 ± 0.76 vs 0.94 ± 0.88; P < 0.001). However, within-group analysis also demonstrated that steatosis reduction was significant in the placebo group (hypocaloric diet + placebo) compared to baseline values (P < 0.001). In the same study, other histological features of NASH were shown to be significantly attenuated following 24 wk of L-carnitine therapy compared to placebo, including parenchymal inflammation (P < 0.001), hepatocellular injury (P < 0.05) and fibrosis (P < 0.05). However, it is again worth noting that within-group comparisons with baseline values also determined a significant reduction in these parameters following placebo supplementation.
Another study used hepatic CT with liver attenuation index (LAI) to evaluate steatosis before and after the study[11]. Authors reported that the patient group receiving L-carnitine complex supplementation had a mean increase in LAI values of 6.21 ± 8.96 Hounsfield Units (HU) (P < 0.001), indicating significant reduction in liver fat, whereas the placebo group showed no significant change (LAI increased by 0.74 ± 8.05 HU, P = 0.582). The changes in LAI were found to correlate inversely with changes in ALT.
Alavinejad et al[12] (2016) reported no significant difference between baseline and post-intervention sonographic liver fat in either the L-carnitine or placebo groups in their study. However, absolute values were not provided in the published article. Finally, Somi et al[14] (2014) reported a significant reduction in patients with sonographic grade 2 liver fat in the placebo but not the L-carnitine groups. In the L-carnitine group, 9 patients with sonographic evidence of fatty liver at baseline had resolution of fatty liver disease on post-intervention ultrasonography, suggesting a beneficial effect of L-carnitine on liver fat; however, this was reported to be non-significant. Thus, two high quality RCTs utilising histology and cross-sectional imaging for quantifying liver fat reported significant outcomes following L-carnitine supplementation, whereas the two trials utilising ultrasonography reported no significant difference in liver fat with L-carnitine.
HOMA-IR and glycometabolic profile: Three papers were included for meta-analysis of HOMA-IR measures after intervention. The WMD for HOMA-IR between the L-carnitine and control groups after intervention was -0.74 units [95%CI: -1.02-(-0.46)] (P < 0.001) (Figure 5). The I2 was 0%, indicating statistical homogeneity between the studies.
Adverse events: Adverse events (AE) were reported in 3 studies included in this review, including mild headache, musculoskeletal pain and gastrointestinal disturbance[10,11,13]. All AE rates were lower in the intervention versus placebo groups and no serious adverse events were reported.
Inflammation and oxidative stress: Malaguarnera et al[10] (2010) reported a significant reduction in hepatocellular injury (P < 0.05), parenchymal inflammation (P < 0.001), plasma C-reactive protein (CRP) and tumour necrosis factor alpha (TNFα) (P < 0.001) in L-carnitine treated patients compared to placebo. Hong et al[13] (2014) evaluated inflammation and oxidative stress at baseline and post-intervention in using high-sensitivity CRP (hs-CRP) and urine 8-hydroxy-2’-deoxyguanosine (8OHdG), respectively and report significant reduction in 8OHdG in the group treated with L-carnitine (P = 0.034).
To our knowledge, this is the first reported systematic review to evaluate the effect of dietary L-carnitine supplementation on liver fat, markers of liver injury and insulin resistance profiles in NAFLD populations. Pooled results from five randomised trials suggest that L-carnitine supplementation is associated with significant attenuation of liver fat, and reduction in serum ALT levels, the most commonly used surrogate biomarker of hepatocellular injury. A reduction in AST following L-carnitine therapy was also seen, though this did not reach statistical significance. Our results further suggest that L-carnitine can improve insulin sensitivity in NAFLD cohorts, as measured indirectly using the HOMA-IR index. These outcomes are potentially important to clinical practice, as they confirm a beneficial effect of a broadly available nutrient.
This review comprised a robust and comprehensive database search with no language restrictions. The search strategy was implemented by two reviewers separately, and both authors agreed on papers selected for inclusion as well as reasons for exclusion. Included studies were read and assessed for bias independently by each reviewer; any disagreements in the risk of bias tool were referred to a third reviewer for final arbitration. No publication bias was found to be present for selected outcomes entered into the meta-analysis.
There were several limitations associated with this review. Firstly, despite an extensive literature search, only five randomised studies were available evaluating L-carnitine on liver markers in NAFLD. Of these, only four evaluated change in liver fat as an outcome measure. Despite another trial being published, we were unable to obtain the full text after attempts to contact the authors of the paper.
Secondly, poor methodological quality was inherent in three of the included studies. For example, while all studies claimed randomisation, only Malaguarnera et al[10] (2010) and Bae et al[11] (2015) described robust methods of random sequence generation. Lack of placebo control use in the studies conducted by Somi et al[13] (2014) and Hong et al[14] (2014) further reduced methodological quality and reliability of reported results. Double-blind trial design is an important tool in minimising bias and maximising reliability of research outcomes. In two of the included studies, there was insufficient evidence to judge that robust blinding of participants and personnel occurred. In the study conducted by Somi et al[14] (2014) no mention of blinding was made. This led us to judge these studies as having as high risk of bias and thus further reduced reliability of reported results.
There was heterogeneity of the trials with respect to duration, type of active comparator drug and background therapy, as well as carnitine formulation and dose. Two studies used carnitine in complex with orotic acid (carnitine-orotate); thus establishing a true effect of L-carnitine alone on outcomes measures was not possible. Doses varied widely, from 500 mg daily to 2.25 grams daily, resulting in differential exposure to active L-carnitine among patients in each individual study. The NAFLD patient populations studied were also heterogenous, including patients both with and without diabetes, patients with different stages of NAFLD and geographical differences in populations. However, all 5 studies reported reductions in ALT, suggesting a consistent beneficial effect of L-carnitine on hepatocellular injury across populations.
As a marker of treatment response, ALT appears to be reliable, and has been closely correlated to objectively measured reductions in liver fat using histological and imaging methods in NAFLD post-intervention[15,16]. In phase 2 RCTs, ALT continues to be used as a surrogate marker of disease activity in addition to estimation of fat[17]. The overall significant reduction in ALT following intervention in the included studies suggests a global reduction in hepatocellular injury in patients treated with L-carnitine. Indeed, Malaguarnera et al[10] (2010) demonstrated an improvement in all histological parameters in their biopsied NAFLD cohort following L-carnitine therapy, including steatosis, inflammatory activity and fibrosis. Steatosis reduction was confirmed using CT with liver attenuation index by Bae et al[11] (2015).
With respect to translation into clinical practice, none of the 5 trials evaluated plasma levels of Trimethylamine-N-Oxide, a metabolite of L-carnitine associated with increased risk of atherosclerosis. In a population already at high risk for cardiovascular outcomes, the safety profile of L-carnitine supplementation would require rigorous assessment in this context. However, the evidence base for a direct adverse link between L-carnitine supplementation and cardiovascular events is to our knowledge limited. On the contrary, a meta-analysis of 13 placebo-controlled trials including 3629 patients evaluated the clinical impact of L-carnitine supplementation in patients with ischaemic heart disease and concluded that carnitine administration was associated with clinical benefit, including reduced mortality and reduction in onset of cardiac arrhythmias and angina[18]. A recently published study further concluded that although 24-wk of L-carnitine supplementation increased plasma Trimethylamine-N-Oxide concentrations, no changes in lipid profile or other serum biomarkers of atherosclerosis were seen[19].
The evidence collated from studies included in this review forms a compelling argument for further robust, randomised trial data evaluating mechanisms of action of L-carnitine on liver and muscle tissue in a NAFLD phenotype, as well as its effect on validated outcome measures such as liver histology, magnetic resonance imaging and spectroscopy. Further, effects of L-carnitine supplementation on metabolic outcomes in NAFLD require specific attention, for example through utilising gold-standard measures of liver-specific and whole-body insulin sensitivity such as the euglycaemic hyperinsulinaemic clamp technique. As a naturally occurring, broadly applicable, safe and cost-effective agent, L-carnitine could overcome traditional barriers to translation to become routinely available for patients in clinical practice as an adjunctive treatment for NAFLD.
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of liver disease worldwide, affecting approximately 25% of the general population. To date, there are no licensed disease-modifying treatments to attenuate global burden of NAFLD. A growing body of evidence suggests that NAFLD is characterised at a cellular level by impaired mitochondrial fat oxidation. L-carnitine, a naturally occurring nutrient, is a key mediator of mitochondrial fuel selection and promotes lipid oxidation. In this article, we synthesise available evidence of a role for L-carnitine supplementation in the treatment of NAFLD.
L-carnitine has gained traction in recent years as a potential tool for the treatment of metabolic disorders including type 2 diabetes and heart disease. At the nexus of glucose and lipid metabolism, L-carnitine promotes mitochondrial lipid oxidation and enhances tissue metabolic flexibility. It may confer protective effects in NAFLD through these mechanisms. There is a critical unmet need for broadly applicable, population based treatment in NAFLD, which inspired this narrative and quantitative synthesis.
In this study, we aimed to systematically review randomised trials reporting effects of dietary L-carnitine supplementation on liver biochemistry, liver fat and insulin sensitivity in NAFLD.
Ovid MEDLINE, Ovid Embase, PubMed, Web of Science and the Cochrane Library were searched from their inception until April 2019. Outcome measures included serum concentrations of alanine and aspartate aminotransferase (ALT and AST), liver fat and insulin sensitivity assessed by the homeostasis model of insulin resistance (HOMA-IR). A random effects meta-analysis was performed for, ALT, AST and HOMA-IR measures separately. Between-study heterogeneity was measured using I2 statistics. A protocol for the systematic review was published a priori in the PROSPERO database (Reference: CRD42018107063).
Results from the synthesised evidence suggest that L-carnitine is associated with a significant reduction in serum alanine aminotransferase (ALT), the most commonly used biomarker of hepatocellular injury. In two robust, high-quality randomised trials, L-carnitine supplementation reduced liver fat significantly. Further, in subgroup analysis of studies assessing insulin resistance, L-carnitine supplementation was associated with a significant reduction in the HOMA-IR.
We present an argument for further robust, randomised trial data evaluating mechanisms of action of L-carnitine on liver and muscle tissue in NAFLD populations. Currently availabile evidence suggests that as a naturally occurring, broadly applicable and cost-effective agent, L-carnitine could be an effective tool for patients in clinical practice as an adjunctive treatment for NAFLD. However, we emphasise that further research using robust and validated endpoints is required to consolidate existing evidence of benefit.
Micronutrient supplementation presents a novel and exciting avenue for the treatment of population-level diseases, including NAFLD. A broader impact of L-carnitine on metabolic health (including improved insulin sensitivity) could have implications beyond NAFLD alone and its effect in other metabolically challenged populations deserves attention. Ultimately, further well-conducted prospective, randomised data will be required to translate a speculative benefit of L-carnitine in NAFLD into the clinical sphere.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Andreone P, Basaranoglu M, Cheng TH, Enosawa S S-Editor: Wang J L-Editor: A E-Editor: Qi LL
| 1. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1516] [Cited by in F6Publishing: 1764] [Article Influence: 196.0] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6285] [Article Influence: 785.6] [Reference Citation Analysis (0)] |
| 3. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 625] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 4. | DeWeerdt S. Disease progression: Divergent paths. Nature. 2017;551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 783] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 6. | Xu Y, Jiang W, Chen G, Zhu W, Ding W, Ge Z, Tan Y, Ma T, Cui G. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv Clin Exp Med. 2017;26:333-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Croci I, Byrne NM, Choquette S, Hills AP, Chachay VS, Clouston AD, O'Moore-Sullivan TM, Macdonald GA, Prins JB, Hickman IJ. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut. 2013;62:1625-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13:2051-2060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 362] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18487] [Cited by in F6Publishing: 21270] [Article Influence: 1636.2] [Reference Citation Analysis (2)] |
| 10. | Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M, Avitabile T, Li Volti G, Galvano F. L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis--a randomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Bae JC, Lee WY, Yoon KH, Park JY, Son HS, Han KA, Lee KW, Woo JT, Ju YC, Lee WJ, Cho YY, Lee MK. Improvement of Nonalcoholic Fatty Liver Disease With Carnitine-Orotate Complex in Type 2 Diabetes (CORONA): A Randomized Controlled Trial. Diabetes Care. 2015;38:1245-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Alavinejad P, Zakerkish M, Hajiani E, Hashemi SJ, Chobineh M, Moghaddam E. Evaluation of L-Carnitine Efficacy in the Treatment of Non-Alcoholic Fatty Liver Disease among Diabetic Patients: A Randomized Double Blind Pilot Study (2016). J Gastroenterol Hepatol Res. 2016;5; 2191-2195. [Cited in This Article: ] |
| 13. | Hong ES, Kim EK, Kang SM, Khang AR, Choi SH, Park KS, Jang HC, Lim S. Effect of carnitine-orotate complex on glucose metabolism and fatty liver: a double-blind, placebo-controlled study. J Gastroenterol Hepatol. 2014;29:1449-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Somi MH, Fatahi E, Panahi J, Havasian MR, Judaki A. Data from a randomized and controlled trial of LCarnitine prescription for the treatment for Non- Alcoholic Fatty Liver Disease. Bioinformation. 2014;10:575-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, Sanyal AJ, Tonascia J; Non-alcoholic Steatohepatitis Clinical Research Network (NASH CRN). Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 506] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 17. | Harrison SA, Rinella ME, Abdelmalek MF, Trotter JF, Paredes AH, Arnold HL, Kugelmas M, Bashir MR, Jaros MJ, Ling L, Rossi SJ, DePaoli AM, Loomba R. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 18. | DiNicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O'Keefe JH. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clin Proc. 2013;88:544-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Samulak JJ, Sawicka AK, Hartmane D, Grinberga S, Pugovics O, Lysiak-Szydlowska W, Olek RA. L-Carnitine Supplementation Increases Trimethylamine-N-Oxide but not Markers of Atherosclerosis in Healthy Aged Women. Ann Nutr Metab. 2019;74:11-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |