Peer-review started: October 6, 2015
First decision: December 4, 2015
Revised: December 19, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: March 9, 2016
Macular edema such as diabetic macular edema (DME) and diabetic retinopathy are devastating back-of-the-eye retinal diseases leading to loss of vision. This area is receiving considerable medical attention. Posterior ocular diseases are challenging to treat due to complex ocular physiology and barrier properties. Major ocular barriers are static (corneal epithelium, corneal stroma, and blood-aqueous barrier) and dynamic barriers (blood-retinal barrier, conjunctival blood flow, lymph flow, and tear drainage). Moreover, metabolic barriers impede posterior ocular drug delivery and treatment. To overcome such barriers and treat back-of-the-eye diseases, several strategies have been recently developed which include vitreal drainage, laser photocoagulation and treatment with biologics and/or small molecule drugs. In this article, we have provided an overview of several emerging novel strategies including nanotechnology based drug delivery approach for posterior ocular drug delivery and treatment with an emphasis on DME.
Core tip: Macular edema such as diabetic macular edema (DME) and diabetic retinopathy are devastating back-of-the-eye retinal diseases leading to loss of vision. The standard treatments of DME include laser photocoagulation, vitrectomy, intravitreal injections of anti-vascular endothelial growth factor biologics and steroids. In this article we have provided an overview of several emerging novel strategies including nanotechnology based drug delivery approacher for posterior ocular drug delivery and treatment with emphasis on DME.
- Citation: Trinh HM, Joseph M, Cholkar K, Pal D, Mitra AK. Novel strategies for the treatment of diabetic macular edema. World J Pharmacol 2016; 5(1): 1-14
- URL: https://www.wjgnet.com/2220-3192/full/v5/i1/1.htm
- DOI: https://dx.doi.org/10.5497/wjp.v5.i1.1
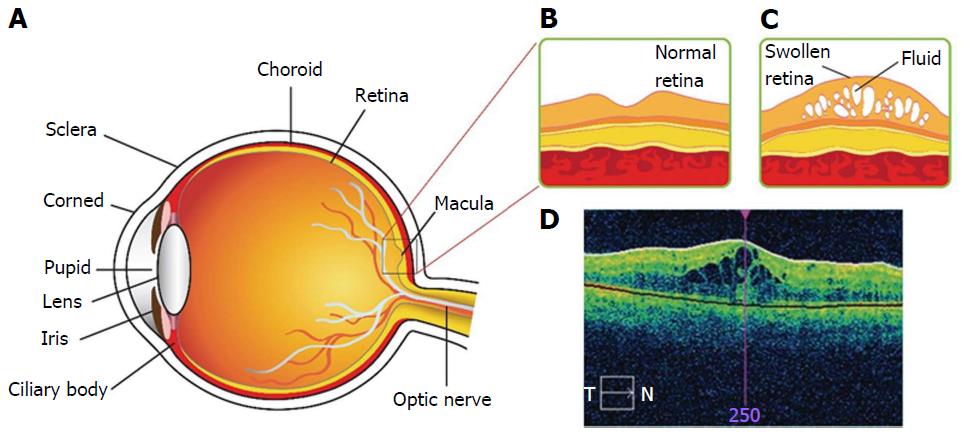
Diabetic macular edema (DME) is a chronic back-of-the-eye disease that may lead to vision loss. DME causes retina thickening due to accumulation of fluid in the center of macula (Figure 1)[1]. Chronic diseases such as diabetes, non-proliferative and proliferative diabetic retinopathy (DR) are significant factors for the onset of DME[2,3]. The exact mechanism by which diabetes leads to retinopathy is not well-delineated. However, several theories have been postulated in the literature. DR may develop due to excessive growth of leaky vascularization in the retina. According to National Eye Institute, DR progresses in four stages[4]. In brief, stage 1 aka mild non-proliferative retinopathy, is the initial stage where tiny abnormal blood vessels or micro aneurysms are developed. Such blood vessels appear as balloon-like swelling in the retina. With disease progression, stage 2 aka moderate non-proliferative retinopathy ensures blockage of blood vessels that supplies nutrition to retina. Severe non-proliferative retinopathy aka stage 3 is diagnosed with blockage of blood vessels thereby depriving blood flow to the retina. Under such conditions retina lacks oxygen and nutrients supply. Moreover, several cellular signals (particularly HIF-α) are triggered that cause development of new vasculature to compensate oxygen and nutrient supply. Proliferative retinopathy is termed as the final stage or the advanced stage of DR. The new abnormal blood vessels developed are fragile, and leaky. Such development is termed as neovascularization. Several factors can add to severity of DME depending on the degree of DR, length of time the subject is diabetic, type of diabetes, hypertension, fluid retention, hypoalbuminemia and hyperlipidemia. Advent of microscopic techniques such as fundus contact lens bio-microscopy or funduscopic examination are proven to aid DME diagnosis. It can be diagnosed with ocular clinical conditions such as retinal thickening within 500 μm and/or hard exudates within 500 μm or in one disk diameter from the center of macula[5].
Pathogenesis of DME is not clearly delineated in the literature. However, DME is a complex multifactorial ocular disease[6]. Blood retinal barrier (BRB) is an essential structure that regulates normal visual function. Such a physiologic barrier also regulates fluid and solute movement in and out of retina[7]. BRB is comprised of inner and outer BRB[8,9]. The inner BRB is composed of tight junctions between retinal capillary endothelial cells while the outer BRB tight junctions exist between retinal pigment epithelial cells[7]. The breakdown of inner BRB results in vasogenic edema, neural tissue impairment and ultimately vision loss, if not treated[10]. Disruption of BRB is one of the common factors for DME development[11,12].
Many macro and microvascular factors along with various pathways are involved in retinal thickening, disruption of BRB and loss of pericytes[13].
Macro-vascular factors include Starling’s law for edema, oxygen tension and shear stress.
Starling’s law and macular edema: According to the Starling’s law, hydrostatic blood and osmotic pressures of tissue fluid are responsible for vasogenic edema. It appears to maintain the gradients between two forces involving fluid movement between inner and outer retinal layers which is crucial to prevent DME[10]. This law is based on water accumulation caused by decreasing osmotic pressure gradient between vessel and tissue. Current strategies for DME such as vitrectomy, laser, anti-vascular endothelial growth factor (anti-VEGF) or steroids can lower osmotic pressure gradient and vascular permeability to prevent water accumulation.
Oxygen tension: In diabetic patients, the level of oxygen is reduced in the macular region. Consequently hypoxia induces VEGF expression[14,15] resulting in enhanced vascular permeability. Elevation of oxygen tension causes compensatory vasoconstriction of the retinal vessels which can reduce hydrostatic pressure, resulting in macula edema[13,16,17]. Stefánsson[18] has explained how and why vitrectomy and photocoagulation can have effects on DME and other neovascularization retinopathies due to improved ocular oxygen tension.
Shear stress: The damage of endothelial cells and decoupling caused by shear stress over time can lead to alterative fluid flow in edema. Increase in shear stress also elevates nitric oxide (NO) production, which may result in vasodilatation and elevated hydrostatic pressure[19].
Endothelial dysfunction and vascular damage due to hyperglycemia: Endothelial cells play a vital role in maintaining the structure, vascular tone and prevention of platelet and leucocyte adhesion onto vessel wall. These cells are responsible for production of vasoconstriction, a dilatation and various inflammatory mediators such as intracellular adhesion molecule, leucocyte adhesion molecule, and vascular cell adhesion molecule[20-22]. While endothelial progenitor cells play a role in repair of damaged vessels, number of these cells are highly reduced under hyperglycemic conditions[23,24].
BRB: Since endothelial cells play an important role in maintaining the integrity of BRB, damage in endothelial cells leads to disruption and leakiness of vascular beds. This increased permeability leads to accumulation of extracellular fluid. It increases the oncotic pressure due to influx of protein from blood vessels to inner retina[25,26].
Growth factors: Growth factors regulate angiogenesis by stimulating endothelial cell proliferation, migration, and survival. These factors have profound influence in many ocular diseases such as DME, DR and neo-vascular age-related macular degeneration (AMD)[27-29]. Growth factors including VEGF, placental growth factor, and hepatocyte growth factor are responsible for increased vascular permeability. VEGF appears to be an important factor for endothelial cell migration, proliferation and survival.
Inflammation: Inflammation plays a crucial role in DME pathogenesis. Leucocytes naturally adhere to vascular endothelium (leukostasis) and have the ability to create toxic superoxide radicals and enzymes[30]. Leukostasis induce vascular permeability and impair endothelial cells by producing enzymes, cytokine and free radicals[31,32]. Also inflammation motivates the occludin phosphorylation which regulates tight junction and barrier function resulting in the breakdown of BRB[33-35].
Oxidative stress: Diabetes can cause oxidative stress leading to elevated levels of NO, superoxide, peroxynitrite development and VEGF expression, all of which may alter vascular permeability and BRB breakdown[36-38].
Others factors include matrix metallo proteinases, protein kinase C, carbonic anhydrase, and angiotensin-II that have direct or indirect role in enhancing vascular permeability that results in DME[9,39-42]. Moreover, several pivotal pathways have been implicated in DME such as angiogenesis, inflammatory and oxidative stress pathways[9,11,13,43]. Chronic hypertension and hyperglycemia cause blood vessels to become more porous allowing fluid, lipid sand erythrocytes escape. Such leakage and accumulation only cause vascular basement membrane thickening, free radical formation, non-enzymatic glycosylation and pericyte death[44]. Moreover, increased vascular permeability and capillary dropout may cause inadequate blood supply to retina.
The current treatment strategies for DME has been summarized in Figure 2 and discussed in detail as followings.
Despite the fact that anti-VEGF [bevacizumab, ranibizumab (RBZ) and pegaptanib] and VEGF trap (aflibercept) have emerged as treatment options for back-of-the-eye diseases, laser (focal or/and grid) photocoagulation surfaced as another treatment option for DME[45]. A recent study conducted on non-center involved (CI) DME subjects involves focal laser photocoagulation. In this study 29 eyes with non-CI received focal laser coagulation and 20 eyes with no treatment served as control. Photocoagulation treated eyes demonstrated a 5 letter gain in visual acuity in 21% subjects relative to 5% of control eyes[46]. Interestingly, this study indicated a decrease in inner and outer zone, central subfield thickness (CST) and reduction in total macula volume relative to control group[46].
Modern laser technologies and applications have been employed to treat DME. Such laser technologies include pattern scan laser photocoagulator (OptiMedica Corp, Santa Clara, CA) and NAVILAS (OD-OS Teltow, Inc. Germany). The laser beam delivery systems have short pulse duration that reduce heat thereby minimizes thermal damage at the site of application leading to patient compliance[47,48]. Other techniques such as subthreshold diode micro-pulse, navigated laser photocoagulation, pan retina photocoagulation and conventional single-spot laser application have been demonstrated to be more effective and safe to retina relative to conventional laser photocoagulation[47].
Although laser photocoagulation provides certain advantages, the associated drawbacks lessen enthusiasm and patient compliance. Drawbacks include destruction of photoreceptors due to laser photocoagulation, retinal scar formation and impedance of visual prognosis[49,50]. However, laser photocoagulation may be beneficial in DME subjects who do not respond to drug treatments[50]. Recently, a combination of intravitreal drug administration with laser photocoagulation have been investigated. Such treatment appears to be promising[45,51]. However, further studies may be required to establish the clinical benefit of the combination approach.
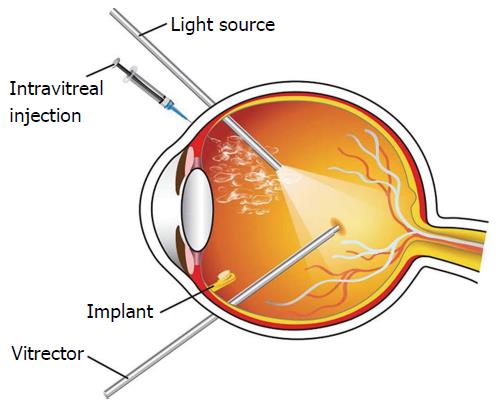
Vitreous plays an important role in the progression of DME. Studies demonstrated that improvement in vision for DME subjects may be achieved by induction of posterior vitreous detachment (PVD), pars plana vitrectomy (PPV), removal of internal limiting membrane (ILM) or taut posterior cortex[52-56]. However, the exact mechanism for vision restoration in DME with vitrectomy is yet to be delineated. Recent studies suggests that exclusion of vitreous gel may reduce the concentration of DME-promoting factors, alter vascular permeability and enhance retinal oxygen supply[11,57]. Vitrectomy may also improve vasoconstriction by lowering tissue pressure and elevating hydrostatic pressure gradient between the vascular and tissue compartments[18]. Moreover, vitrectomy improves vaso-permeability of the retinal endothelial cells and restore visual acuity. In a cohort study of vitrectomy in DME subjects, 87 eyes were evaluated visual acuity 20/63-20/400 including 54% ILM peeling, 61% epiretinal membrane peeling, and 40% panretinal photocoagulation[57]. Vitrectomy significantly reduced retinal thickness and improved visual acuity. However, vitrectomy is associated with side effects such as elevated intraocular pressure (IOP), vitreal hemorrhage, endophthalmitis, retinal detachment, induction of iris neovascularization and cataract formation[58]. Several randomized, controlled trials were conducted to investigate the side effects of vitrectomy on DME[59-65]. Such studies compared vitrectomy with laser, intravitreal steroid injection, and combinations. Vitrectomy may be applicable in DME subjects demonstrating epiretinal membrane and/or vitreomacular traction[66].
Macromolecular therapy: VEGF plays an important role in retinal vascular permeability, breakdown of BRB and formation of macular edema. The current gold standard therapy for DME treatment is administering anti-VEGF agents[67-69]. VEGF inhibitors have demonstrated beneficial effects in DME treatment[70-74]. Current VEGF inhibitors include aflibercept (Eylea), RBZ (Lucentis), pegaptanib (Macugen), and bevacizumab (Avastin). RBZ and aflibercept are approved by Food and Drug Administration (FDA) for DME. Other anti-VEGF agents are also being considered due to cost effectiveness[75,76].
RBZ is a monoclonal antibody, approved for DME[77]. It has strong binding affinity to VEGF-A and blocks all isoforms of VEGF-A. Nguyen et al[78] demonstrated long term effects of RBZ in diabetic patients with DME. In this study, subjects were treated with RBZ, focal or grid laser or combination. The mean best-corrected visual acuity indicated that RBZ had significant effect to control edema in DME subjects. Moreover, a combination treatment with RBZ and focal/grid laser lowers edema residues also. Similarly, a clinical study, RIDE/RISE of RBZ demonstrated significant improvement in macula edema, accompanied by slow progress of vision loss in DME subjects[79-82].
Bevacizumab is full-length humanized monoclonal antibody with approximately three times larger molecular weight and size than RBZ. Bevacizumab also obtained FDA approval for the treatment of glioblastoma and colorectal cancer. However, it is being used as an “off-label” drug for DME treatment due to low cost. Several studies have reported bevacizumab to significantly improve macula edema and restore vision atleast partially in DME subjects[83-89]. Intravitreal injections of bevacizumab alone or in combinations with triamcinolone or photocoagulation were investigated. Interestingly, a combination of intravitreal bevacizumab and triamcinolone acetonide (TA) produced marginal improvement over bevacizumab alone in DME[90].
Aflibercept: (Eylea; Regeneron) aka VEGF Trap for eye is a soluble protein composed of binding domain for human VEGF receptor 1 (VEGFR1), 2 and Fc domain of human immunoglobulin G1[91,92]. Eylea has 100 times higher binding affinity to VEGF isoforms relative to bevacizumab or RBZ[93]. Moreover, aflibercept binds to special P1GF and VEGF-B and inhibits the activation of VEGFR1[93]. Korobelnik et al[91] conducted VISTADME and VIVIDDME phase three studies to compare the efficacy and safety of intravitreal aflibercept at 4 and 8 wk after initial monthly doses and laser treatment. Aflibercept demonstrated significant improvement in visual acuity over laser treatment. These results suggest that aflibercept is safe and well-tolerated. The most best corrected visual acuity (BCVA) can be achieved with aflibercept[94]. Many other studies such as VIBRANT, COPERNICUS, and GALILEO have reported significant benefits for aflibercept with better visual acuity[95-98]. Aflibercept did not cause significant difference at mild level of initial visual acuity relative to bevacizumab and RBZ. In fact, aflibercept can improve vision more effectively at worse level of initial visual acuity[76,92].
Pegaptanib (Macugen) is a ribonucleic acid aptamer which was the first anti-VEGF approved by FDA for AMD. Macugen is another “off-label” drug for DME and has selective target to VEGF 165[99]. Several studies demonstrated pegaptanib to be safe, well-tolerated and superior efficacy in DME treatment[100-104].
Small molecule: Rapamycin or sirolimus is an immunosuppressive drug with anti-inflammation, antiangiogenic, antifibrotic, and antifungal properties. Sirolimus blocks interleukin-2-mediated signaling pathway and reduce VEGF production by inhibiting S6K1 phosphorylation[105-108]. Recently subconjunctival and intravitreal injections of sirolimus are applied for the treatment of DME, AMD and non-infectious uveitis patients which appear to be well tolerated[109-112]. Efficacy studies with sirolimus in DME subjects have also been conducted[109]. Moreover, aqueous nanomicellar topical drop of sirolimus has been developed. These nanomicellar constructs have been demonstrated to deliver sirolimus in high concentrations to back-of-the-eye tissues [retina/choroid] with topical drop[105].
Inflammation plays a crucial role in DME pathogenesis. Though exact mechanism of corticoid action is unclear its use as anti-inflammatory agent is well recognized. It decreases VEGF activity and shows beneficial effects in DME[5,113-117]. Steroids may inhibit inflammatory cytokine production, leukostasis, and phosphorylation of cell-junction proteins[118].
TA is a synthetic steroid, recommended for DME treatment. TA displays anti-inflammatory and anti-angiogenic properties[119], improves tight-junctional levels between endothelial cells and reduces vascular leakage[120]. The widespread biological effects and large therapeutic window of intravitreal TA (IVTA) in the treatment of various ocular disorder is well known. It is prescribed as an “off-label” drug for DME and DR[121-124]. Several studies have been conducted to compare the safety and efficacy between IVTA and other treatments[67,90,125-128]. In a meta-analysis of randomized controlled trials study, IVTA demonstrated better vision acuity relative to standard care for ocular inflammation[126]. Moreover, IVTA administrations demonstrated short-term efficacy in retinal vein occlusion[129]. However intravitreal administration of IVTA, can also elevate IOP, accelerate cataract formation and cause other associated side effects such as endophthalmitis and pseudoendophthalmitis[130-134]. To overcome such side effects, recently aqueous nanomicellar topical drop of dexamethasone has been reported from our laboratory[135,136] that delivers therapeutic levels of the steroid to both anterior and posterior ocular tissues. Other studies for DME with corticoids include biodegradable dexamethasone implant (Ozurdex), surgically implantable reservoir of fluocinolone (Retisert), the dexamethasone intravitreal implant (Posidurex), and non-bioerodible injectable fluocinolone polymer (Iluvien)[137-143].
Ophthalmic complications associated with diabetes are the leading cause of blindness in adults. In recent years, several formulations utilizing nanotechnology, anti-VEGF, VEGF trap, and implants for treating DME and other back-of-the-eye diseases are emerging. In addition, several combination therapies that involve two or more therapies together are being administered. Most of these drugs and combination therapies are either FDA approved or are in clinical trials and have shown tremendous improvement in vision to DME patients[78,144,145]. The following sections discuss different emerging formulations for treatment of DME.
Inhibition of VEGF has been indicated in AMD in recent years. Studies have shown that inhibition of VEGF can also be an effective interaction in the treatment and management of DME. Furthermore, intravitreal injection of anti-VEGF therapeutics (RBZ and aflibercept) was compared with laser monotherapy for treatment of DME on 1978 patients. Anti-VEGF therapeutics appeared to be statistically and clinically more superior to laser monotherapy[146]. Nguyen et al[82] conducted a phase III randomized trial on 377 adult patients with vision loss due to DME. This study was conducted to evaluate efficacy and safety of RBZ administered at different dosages. Results indicated that after 24 mo of treatment 18.1% of sham patients gained more than 15 letters compared to 44.8% of patients treated with 0.3 mg of RBZ[82]. In addition RBZ showed rapid and sustainably improved vision with lower risk for further vision loss. This intervention significantly improved macular edema for DME patients[82].
Combination formulations are also emerging in the treatment of diseases associated with posterior segment of the eye. Combined regimens are utilized where retinopathies are not responding to one particular therapeutics strategy[147]. Liegl et al[51] conducted a study to evaluate a combination of laser photocoagulation and RBZ in the treatment of DME over one year period. One group receives combination therapy which involved 3 mo RBZ injections followed by laser photocoagulation. The second group is treated with RBZ injections only. BCVA is measured in both groups after treatment. An improvement in BCVA letter sore from 6.31 to 8.41 on both groups is observed. However, patients in monotherapy group require repeated RBZ injections (84%) relative to combined therapy (35%)[51]. These findings suggest that number of injections is significantly reduced with combination therapy. This may be beneficial to subjects since frequent intravitreal injections may result in local ocular complications such as endophthalmitis, retinal hemorrhage, retinal detachment and patient noncompliance[148,149].
In addition to antibody therapeutics for treatment of DME, some promising strategies such as non-antibody drug products that have been used in the treatment and management of DME. Fluocinolone acetonide (FAc) (ILUVIEN®) was approved in 2014 by FDA for the treatment of DME. A long term follow-up study is conducted on DME subjects after receiving FAc intravitreal implant[150]. In this study subjects not responding to laser photocoagulation or anti-VEGF therapy were treated with FAc implant in one eye and anti-VEGF therapy in the contralateral eye[150]. Intravitreal FAc implant eye produced reduction in central macular thickness from 642 μm to 364 μm in the first month. On the contrary, eye treated with anti-VEGF therapy was unresponsive[150]. Similarly, another study that was conducted with FAc in chronic DME patients[151]. Results indicated an improvement of more than 15 letters on 34.0% patients treated with FAc compared to 13.4% on sham[151]. Such results provide an option for clinicians to treat subjects who do not respond to laser or anti-VEGF therapy. Moreover, FAc implant provides a long term sustained drug release of 0.2 μg/d for up to 3 years which can be more patient compliant therapy[150,151].
In this non-randomized, multicenter study, 2603 patients with macular edema and DME, Adelman et al[152] conducted a study to compare efficacy of anti-VEGF with triamcolone monotherapy and laser treatments. Despite the fact that all treatments revealed some improvement in visual acuity, anti-VEGF treatment showed the most improvement. However, treatment with PPV and ILM peeling exhibited improvement in vision acuity greater than anti-VEGF alone[152]. Consequently, this result indicates that treatment with ILM peeling and vitrectomy may be a better option to treat DME compared to other therapies.
Misra et al[153] have developed an insulin therapy that can be delivered to the retina. This is a sub-conjuctivally implantable hydrogel with thermosensitive and biodegradable properties for sustained delivery of insulin to the retina. Hydrogels are synthesized with UV photo-polymerization of N-isopropylacrylamide monomer and dextran containing biodegradable oligolactate-(2-hydroxyetheyl methacrylate) units. Insulin loading efficiency was very high (98%)[153]. In vitro studies demonstrated that hydrogels were nontoxic when subjected to R28 retinal cells and can release active insulin for 7 d[153]. Such hydrogel implant may be utilized to load other macromolecular drugs intended to treat back-of-the-eye diseases.
Similarly, studies have been conducted to evaluate efficacy of combined treatments in DME. Vitrectomy combined with triamcinolone acetonide injection (IVTA) and macular laser photocoagulation was studied by Kim et al[147] for the treatment of non-tractional DME. This study was performed on 28 patients, who were sequentially subjected to vitrectomy, IVTA and macular laser photocoagulation. BCVA and CST were observed before vitrectomy, 1, 3, and 6 mo after the treatment. Results indicated substantial improvement in BCVA from 0.44 to 0.34 and from 433.3 to 310.1 for CST. These results suggest that combination of vitrectomy, IVTA and laser photocoagulation may be indicated in the treatment of DME.
Enzymatic vitrectomy for DME patients has recently been explored[154]. Diaz-Llopis et al[155] investigated the role of enzymatic vitrectomy through intravitreal injection of autologous plasmin enzyme in management of DME and DR. In a clinical study 63 eyes were treated with intravitreal injection of autologous plasmin enzyme and reexamined after one month for central macular thickness, BCVA and hyaloid. A second injection of this enzyme was administered to patients who did not develop PVD. Results indicated a massive improvement in central macular thickness by 100% and BCVA by 89%. However, PVD was observed to be 38% after first injection, which then improved to 51% after second injection[155]. Enzymatic vitrectomy is still new in the world of ophthalmology and further studies are required to understand the mechanism of action, efficacy and safety. Enzymatic vitrectomy may be considered as an alternative therapy for treatment of DME.
In a study with nine patients who had persistent DME, Zucchiatti et al[156] evaluated the effect of single injection of dexamethasone implant (0.7 mg) over 6 mo period. Results indicated a significant improvement in BCVA and central retina thickness which was sustained over 4 mo. A similar study was performed in DME patients with vitrectomized eyes for 26 wk by Boyer et al[139] to evaluate safety and efficacy of dexamethasone. A significant improvement in BCVA and central retinal thickness were maintained throughout the treatment period. In comparison, dexamethasone implant appeared to achieve superior outcomes in terms of BCVA, CMT with fewer injections compared to bevacizumab by Gillies et al[157]. Both treatments indicated excellent progress on vision impairment score. However, 11% of patients treated with dexamethasone implant lost 10 letters or more due to cataract formation[157]. FDA approved dexamethasone implant (Ozurdex) in the treatment of DME in 2014. This implant was previously approved for the treatment of non-infectious uveitis affecting posterior segment of the eye. Table 1 summarizes major clinical trials that have been performed to study biologics, steroid and implants in DME.
| Trade name | Generic name | Study | Main conclusion | Ref. |
| Lucentis | Ranibizumab | RISE/RIDE | Ranibizumab improved vision and macular edema in DME patients | [82] |
| Eylea | Aflibercept | VISTA/VIVID | Intravitreal injection of aflibercept was shown to be superior compared to laser therapy in treatment of DME | [91] |
| Ozurdex | Dexamethasone implant | MEAD | Dexamethasone implant were well tolerated and improved BCVA in DME patients | [168] |
| Iluvien | Fluocinolone acetonide | FAME | Both low and high dose of Fluocinolone acetonide exhibited improved BCVA in treatment of DME | [169] |
As described earlier, DME is a back-of-the-eye disease. For local drug delivery, sub-conjunctival or intravitreal route of administration may be recommended. Since frequent injections are needed to maintain therapeutic levels, may cause complications such as retinal detachment, endophthalmitis, pseudoendophthalmitis and retina hemorrhage. Nanoparticle mediated sustained release formulations may lower frequent injections, and improve efficacy leading to reduced side effects and improved patient compliance.
Recently, several groups have developed topical formulations for delivery to the retina. Cholkar et al[135] have reported a topical administration of mixed nanomicelle formulation (MNF) loaded with dexamethasone[136] rapamycin (sirolimus) and cyclosporine[158] for back-of-the-eye delivery[105]. MNF was found to be safe when tested on human retinal pigment epithelial cells (D407) and rabbit primary corneal epithelial cells in vitro. MNF can provide high drug loading and entrapment efficiency with an average size of 10.84 ± 0.11 nm. Furthermore, in vivo studies exhibited higher rapamycin concentration of 362.35 ± 56.17 ng/g in retina-choroid area but no drug was found in the lens or vitreous humor[105]. With these results, the topical administration may provide patient compliance since no injections are involved.
In addition, Fujisawa et al[159] have explored liposomal diclofenac eye drop formulation targeted to retina along with the aid of surface modification of liposomes. Liposomal formulation was prepared by using calcium acetate gradient method which increased entrapment efficiency from 51.3% (obtained by using hydration method) to 97%[159]. The researchers have utilized liposome surface modification with poly vinyl alcohol (PVA) or its derivatives (PVA-R) and observed that particle size of liposome with PVA modification to are 135 nm and 177 nm with PVA-R. In vivo studies performed on Japanese albino rabbits indicated an enhancement in accumulation of diclofenac in the retina-choroid by 1.8 fold with surface modified liposome relative to unmodified liposomes[159]. Higher entrapment efficiency may result in longer drug release. This delivery system may be suitable for the treatment of DME and other diseases associated with posterior segment of the eye.
RNA has been widely used as a therapeutic agent for treatment of wide variety of diseases. It involves modification, engineering, and/or assembly of organized materials at nanometer scale. The 117-nucleotide (nt) RNA, known as packaging RNA (pRNA) of bacteriophage and small interfering RNA (siRNA) have been widely applied in the treatment of cancer, viral infection, genetic diseases, and other diseases. Recently, Feng et al[160] have reported ocular delivery of pRNA (pRNA-3WJ and pRNA-X) nanoparticles and investigated distribution and clearance after subconjunctival injection. pRNA-3WJ and pRNA-X-nanoparticles labelled with Alexa647 and dsRNA were prepared and administered to mice by subconjunctival injection. It was observed that nanoparticles (pRNA-3WJ, pRNA-X and dsRNA) were distributed in corneal, sclera, and conjunctiva cells, but pRNA-X was found only in retina cells. This study suggests that RNA therapy for ocular diseases including back-of-the-eye delivery is feasible.
Similarly gene therapy for the treatment of inherited and acquired ocular diseases has been rapidly evolving in recent years. A major challenge for gene therapy is to overcome barriers associated with ocular gene delivery. This can be achieved by developing a suitable nanotechnology platform that can cross ocular barriers and deliver genes at target site. A polymer (natural or synthetic) or peptides have been employed to encapsulate DNA in polymer or peptide compacted DNA gene delivery nanoparticles[161]. Safety of compacted DNA nanoparticles for ocular delivery has also been investigated by Ding et al[162]. Polyethylene glycol substituted lysine peptide (CK30PEG) compacted DNA nanoparticles encapsulating EGFP vector were subretinally injected in mice at different dosages. Retina was observed at 1, 2, 4, 7 d post injection for any inflammatory signs or mediators. No inflammatory response was observed in the retina[162]. In addition, chitosan DNA nanoparticles for retinal gene delivery have been reported by Mitra et al[163]. Results indicate that compacted DNA nanoparticles may be exploited as gene therapies for treatment of the posterior diseases and particularly with RPE.
Carbon nanotubes are nanometer-scale tube-like cylindrical nanostructure. These cylindrical carbon molecules have unusual properties, which are valuable for nanotechnology, particularly in drug delivery. Nanotubes have also been explored for therapeutic delivery at back-of-the-eye. Panda et al[164] studied self-assembly dipeptide phenylalanine-α, β-hydrophenylalanine nanotubes for sustained intravitreal delivery of targeted tyrosine kinase inhibitor (pazopanib). The nanotube has a diameter and length of 15-30 nm and 1500 nm respectively. The nanotubes can be injected using 33G needle. Nanotubes loaded with a 25% w/w pazopanib were found to be nontoxic in in vitro studies. In vivo investigation was performed with pazopanib loaded nanotube for 15 d and the drug was observed in vitreous humor, retina and choroid RPE at 4.5, 5 and 2.5 times respectively compared to pazopanib solution[164]. These results suggest that nanotubes can be applied as a delivery system which may sustain higher drug concentration in ocular tissues.
Biodegradable polymers have been extensively utilized for the preparation of nanoparticles in drug delivery. Also nanoparticle in gel formulation of steroids has been reported for the treatment of macular edema by Boddu et al[165]. In this formulation PLGA (50:50 and 65:35) nanoparticles loaded with dexamethasone, hydrocortisone acetate, and prednisolone acetate were prepared by water in oil emulsion and then suspended in thermosensitive gel. Results indicated that entrapment efficiency for dexamethasone, hydrocortisone acetate and prednisolone acetate were 77.3%, 91.3% and 92.3% respectively. Drug release studies indicated no burst release and release kinetics followed zero order[165]. Nanoparticles suspended in thermosensitive gel may provide sustained release of drug at retina-choroid and may be exploited for DME and other ocular diseases.
A quench technology where nanoparticles in porous microparticles (NPinPMP) were prepared by superficial infusion and pressure for sustained bevacizumab delivery. The protein was coated with PLA nanoparticles and then mixed with PLGA microparticles. The particles were allowed to pass through supercritical carbon dioxide gas[166]. This allows expansion of PLGA matrix but not PLA matrix. Hence it creates porous PLGA microparticles in which encapsulated bevacizumab PLA nanoparticles are incorporated to generate NPinPMP. In vitro study indicated sustained release of bevacizumab for 4 mo with no change in conformation and activity[166]. Therefore, this formulation may be utilized with other protein therapeutics for the treatment of back-of-the-eye diseases and may reduce frequent injections to maintain therapeutic levels. However, size of microparticles may be controlled for intravitreal injections.
In addition, tailor made pentablock copolymer based formulation for sustained ocular delivery of protein therapeutics was extensively investigated by Patel et al[148,167]. Biodegradable pentablock copolymers (FDA approved) were synthesized by ring opening polymerization method using different monomers[148,167]. In vitro studies confirmed that polymers and monomers are safe and biocompatible when tested in ocular cell lines (APRE-19, SIRC, HCEC and RAW-264.7)[148,167]. Furthermore, pentablock nanoparticles loaded with FITC-BSA, IgG and bevacizumab were tested for particle size distribution which ranges between 320 and 355 nm. The entrapment efficiency, however, widely varied from 35% to 70%. In vitro studies indicate 40 d release of FITC-BSA and 60 d for IgG when nanoparticles are suspended in gel[167]. This IgG has similar molecular weight as bevacizumab, which can be delivered at the back-of-the-eye for the treatment of posterior diseases. Therefore, this formulation may be adopted to prepare other anti-VEGF therapies which can be delivered to the posterior ocular segment for DME and other retinal diseases.
DME is a disease associated with the posterior segment of the eye; therefore, it poses a significant delivery challenge. A significant portion of the drug may not reach back-of-the-eye due to associated barriers such as BRB, blood aqueous barrier, and vitreous barrier. Consequently only a small amount of drug reaches the back of eye. In order to maintain therapeutic drug levels, generally frequent intravitreal injections are required, which are not patient compliance and may cause other complications. In addition, delivery system that can sustain drug release for a prolonged period of time should be developed so that injection frequency can be minimized/avoided.
DME is a chronic disease leading to declined visual acuity and vision loss. It is a complex multifactorial disease which involves multiple pathways involving vision loss. At present, several novel drug delivery and treatment strategies have been developed to improve visual acuity and restore vision. The standard treatments of DME include laser photocoagulation, vitrectomy, intravitreal injections of anti-VEGF biologics and steroids. Because of destruction of photoreceptors due to laser photocoagulation, retinal scar formation and impedance of visual prognosis, it may be utilized in combination with vitrectomy or intravitreal injection. Moreover, the current understanding of DME pathophysiology has revealed a new therapy which includes targeted chemical mediators such as VEGF and inflammatory agents. The completion of several randomized, controlled trials in the long term may provide new therapeutics and novel delivery systems for the back-of-the-eye diseases.
P- Reviewer: Charoenphandhu N, Masaki T S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Bandello F, Battaglia Parodi M, Tremolada G, Lattanzio R, De Benedetto U, Iacono P. Steroids as part of combination treatment: the future for the management of macular edema? Ophthalmologica. 2010;224 Suppl 1:41-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Varma R, Bressler NM, Doan QV, Gleeson M, Danese M, Bower JK, Selvin E, Dolan C, Fine J, Colman S. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132:1334-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Centers for Disease Control and Prevention. National Diabetes Statistics Report 2014. [accessed. 2015;Sept 1] Available from: http: //www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Cited in This Article: ] |
| 4. | National Eye Institute. Facts About Diabetic Eye Disease. Available from: https: //nei.nih.gov/health/diabetic/retinopathy. [Cited in This Article: ] |
| 5. | Romero-Aroca P. Managing diabetic macular edema: The leading cause of diabetes blindness. World J Diabetes. 2011;2:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 96] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (2)] |
| 6. | Frank RN. DR and systemic factors. Middle East Afr J Ophthalmol. 2011;22:151-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21 Suppl 6:S3-S9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 8. | Cholkar KDS, Pal D, Mitra AK. Eye: Anatomy, Physiology and Barriers to Drug Delivery, 2013. . [Cited in This Article: ] |
| 9. | Wenick AS, Bressler NM. Diabetic macular edema: current and emerging therapies. Middle East Afr J Ophthalmol. 2012;19:4-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 444] [Article Influence: 40.4] [Reference Citation Analysis (1)] |
| 11. | Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 12. | Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006;142:405-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol. 2010;88:279-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Sotoodehnejadnematalahi F, Burke B. Human activated macrophages and hypoxia: a comprehensive review of the literature. Iran J Basic Med Sci. 2014;17:820-830. [PubMed] [Cited in This Article: ] |
| 15. | Pham I, Uchida T, Planes C, Ware LB, Kaner R, Matthay MA, Clerici C. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1133-L1142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 649] [Cited by in F6Publishing: 662] [Article Influence: 73.6] [Reference Citation Analysis (6)] |
| 17. | Lange CA, Stavrakas P, Luhmann UF, de Silva DJ, Ali RR, Gregor ZJ, Bainbridge JW. Intraocular oxygen distribution in advanced proliferative DR. Am J Ophthalmol. 2011;152:406-412.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Stefánsson E. Ocular oxygenation and the treatment of DR. Surv Ophthalmol. 2006;51:364-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of DR. Diabetes. 1995;44:603-607. [PubMed] [Cited in This Article: ] |
| 20. | Altabas V. Diabetes, Endothelial Dysfunction, and Vascular Repair: What Should a Diabetologist Keep His Eye on? Int J Endocrinol. 2015;2015:848272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Georgescu A. Vascular dysfunction in diabetes: The endothelial progenitor cells as new therapeutic strategy. World J Diabetes. 2011;2:92-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853-876. [PubMed] [Cited in This Article: ] |
| 23. | Huang PH, Chen JS, Tsai HY, Chen YH, Lin FY, Leu HB, Wu TC, Lin SJ, Chen JW. Globular adiponectin improves high glucose-suppressed endothelial progenitor cell function through endothelial nitric oxide synthase dependent mechanisms. J Mol Cell Cardiol. 2011;51:109-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, Liu PL, Chen YL, Chen JW. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | Sikorski BL, Malukiewicz G, Stafiej J, Lesiewska-Junk H, Raczynska D. The diagnostic function of OCT in diabetic maculopathy. Mediators Inflamm. 2013;2013:434560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Soliman W, Sander B, Jørgensen TM. Enhanced optical coherence patterns of diabetic macular oedema and their correlation with the pathophysiology. Acta Ophthalmol Scand. 2007;85:613-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Dabhi B, Mistry KN, Patel H, Lal S. Vascular endothelial growth factor insertion/deletion gene polymorphism in West Indian patients of type 2 diabetes and diabetic nephropathy. Indian J Biochem Biophys. 2015;52:209-212. [PubMed] [Cited in This Article: ] |
| 28. | Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18:502-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Shams N, Ianchulev T. Role of vascular endothelial growth factor in ocular angiogenesis. Ophthalmol Clin North Am. 2006;19:335-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 25] [Reference Citation Analysis (0)] |
| 30. | Patel N. Targeting leukostasis for the treatment of early DR. Cardiovasc Hematol Disord Drug Targets. 2009;9:222-229. [PubMed] [Cited in This Article: ] |
| 31. | Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal. 2011;15:1271-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Miyamoto K, Ogura Y. [DR and leukocytes--feasibility of anti-leukostasis therapy for DR]. Nihon Rinsho. 2002;60 Suppl 10:183-188. [PubMed] [Cited in This Article: ] |
| 33. | Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Bennett J, Basivireddy J, Kollar A, Biron KE, Reickmann P, Jefferies WA, McQuaid S. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J Neuroimmunol. 2010;229:180-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 35. | Morgan L, Shah B, Rivers LE, Barden L, Groom AJ, Chung R, Higazi D, Desmond H, Smith T, Staddon JM. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience. 2007;147:664-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4561] [Cited by in F6Publishing: 4192] [Article Influence: 246.6] [Reference Citation Analysis (0)] |
| 37. | Awata T, Neda T, Iizuka H, Kurihara S, Ohkubo T, Takata N, Osaki M, Watanabe M, Nakashima Y, Sawa T. Endothelial nitric oxide synthase gene is associated with diabetic macular edema in type 2 diabetes. Diabetes Care. 2004;27:2184-2190. [PubMed] [Cited in This Article: ] |
| 38. | El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003;162:1995-2004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 39. | Gálvez MI. Protein kinase C inhibitors in the treatment of DR. Review. Curr Pharm Biotechnol. 2011;12:386-391. [PubMed] [Cited in This Article: ] |
| 40. | Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, Bilous R, Chaturvedi N. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385-1393. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56:2380-2387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, Fujimoto JG, Avery RL, Arrigg PG, Bursell SE. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 43. | Singh A, Stewart JM. Pathophysiology of diabetic macular edema. Int Ophthalmol Clin. 2009;49:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Jain A, Varshney N, Smith C. The evolving treatment options for diabetic macular edema. Int J Inflam. 2013;2013:689276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Park YG, Kim EY, Roh YJ. Laser-based strategies to treat diabetic macular edema: history and new promising therapies. J Ophthalmol. 2014;2014:769213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Perente I, Alkin Z, Ozkaya A, Dardabounis D, Ogreden TA, Konstantinidis A, Kyratzoglou K, Yazici AT. Focal laser photocoagulation in non-center involved diabetic macular edema. Med Hypothesis Discov Innov Ophthalmol. 2014;3:9-16. [PubMed] [Cited in This Article: ] |
| 47. | Kozak I, Luttrull JK. Modern retinal laser therapy. Saudi J Ophthalmol. 2015;29:137-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Chalam KV, Murthy RK, Brar V, Radhakrishnan R, Khetpal V, Grover S. Evaluation of a Novel, Non Contact, Automated Focal Laser with Integrated (NAVILAS) Fluorescein Angiography for Diabetic Macular Edema. Middle East Afr J Ophthalmol. 2012;19:158-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Romero-Aroca P, Reyes-Torres J, Baget-Bernaldiz M, Blasco-Suñe C. Laser treatment for diabetic macular edema in the 21st century. Curr Diabetes Rev. 2014;10:100-112. [PubMed] [Cited in This Article: ] |
| 50. | Romero-Aroca P. Is laser photocoagulation treatment currently useful in diabetic macular edema? Med Hypothesis Discov Innov Ophthalmol. 2015;4:5-8. [PubMed] [Cited in This Article: ] |
| 51. | Liegl R, Langer J, Seidensticker F, Reznicek L, Haritoglou C, Ulbig MW, Neubauer AS, Kampik A, Kernt M. Comparative evaluation of combined navigated laser photocoagulation and intravitreal ranibizumab in the treatment of diabetic macular edema. PLoS One. 2014;9:e113981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Iwase T, Oveson BC. Long-term outcome after vitrectomy for macular edema with retinal vein occlusion dividing into the occlusion site. J Ophthalmol. 2014;2014:198782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 53. | Hartley KL, Smiddy WE, Flynn HW, Murray TG. Pars plana vitrectomy with internal limiting membrane peeling for diabetic macular edema. Retina. 2008;28:410-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Yanyali A, Horozoglu F, Celik E, Nohutcu AF. Long-term outcomes of pars plana vitrectomy with internal limiting membrane removal in diabetic macular edema. Retina. 2007;27:557-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Grigorian R, Bhagat N, Lanzetta P, Tutela A, Zarbin M. Pars plana vitrectomy for refractory diabetic macular edema. Semin Ophthalmol. 2003;18:116-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Rosenblatt BJ, Shah GK, Sharma S, Bakal J. Pars plana vitrectomy with internal limiting membranectomy for refractory diabetic macular edema without a taut posterior hyaloid. Graefes Arch Clin Exp Ophthalmol. 2005;243:20-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | DR Clinical Research Network Writing C; Haller JA, Qin H, Apte RS, Beck RR, Bressler NM, Browning DJ, Danis RP, Glassman AR, Googe JM, Kollman C, Lauer AK, Peters MA, Stockman ME. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087-1093.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 58. | Stefánsson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247:147-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 59. | Doi N, Sakamoto T, Sonoda Y, Yasuda M, Yonemoto K, Arimura N, Uchino E, Ishibashi T. Comparative study of vitrectomy versus intravitreous triamcinolone for diabetic macular edema on randomized paired-eyes. Graefes Arch Clin Exp Ophthalmol. 2012;250:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Hoerauf H, Brüggemann A, Muecke M, Lüke J, Müller M, Stefánsson E, Hammes HP, Weiss C. Pars plana vitrectomy for diabetic macular edema. Internal limiting membrane delamination vs posterior hyaloid removal. A prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2011;249:997-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Kumar A, Sinha S, Azad R, Sharma YR, Vohra R. Comparative evaluation of vitrectomy and dye-enhanced ILM peel with grid laser in diffuse diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:360-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Yanyali A, Horozoglu F, Celik E, Ercalik Y, Nohutcu AF. Pars plana vitrectomy and removal of the internal limiting membrane in diabetic macular edema unresponsive to grid laser photocoagulation. Eur J Ophthalmol. 2006;16:573-581. [PubMed] [Cited in This Article: ] |
| 63. | Patel JI, Hykin PG, Schadt M, Luong V, Bunce C, Fitzke F, Gregor ZJ. Diabetic macular oedema: pilot randomised trial of pars plana vitrectomy vs macular argon photocoagulation. Eye (Lond). 2006;20:873-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Stolba U, Binder S, Gruber D, Krebs I, Aggermann T, Neumaier B. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol. 2005;140:295-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Thomas D, Bunce C, Moorman C, Laidlaw DA. A randomised controlled feasibility trial of vitrectomy versus laser for diabetic macular oedema. Br J Ophthalmol. 2005;89:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Simunovic MP, Hunyor AP, Ho IV. Vitrectomy for diabetic macular edema: a systematic review and meta-analysis. Can J Ophthalmol. 2014;49:188-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Aksoy S, Yilmaz G, Akkoyun I, Yazici AC. Comparison of intravitreal bevacizumab and triamcinolone acetonide theraphies for diffuse diabetic macular edema. Int J Ophthalmol. 2015;8:550-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 68. | Keating GM. Aflibercept: A Review of Its Use in Diabetic Macular Oedema. Drugs. 2015;75:1153-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Lim LT, Chia SN, Ah-Kee EY, Chew N, Gupta M. Advances in the management of diabetic macular oedema based on evidence from the DR Clinical Research Network. Singapore Med J. 2015;56:237-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Stefanini FR, Badaró E, Falabella P, Koss M, Farah ME, Maia M. Anti-VEGF for the management of diabetic macular edema. J Immunol Res. 2014;2014:632307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Ford JA, Lois N, Royle P, Clar C, Shyangdan D, Waugh N. Current treatments in diabetic macular oedema: systematic review and meta-analysis. BMJ Open. 2013;3 pii:e002269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 72. | Stewart MW. Critical appraisal of ranibizumab in the treatment of diabetic macular edema. Clin Ophthalmol. 2013;7:1257-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013;4:151-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 74. | Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev. 2014;10:CD007419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 75. | Régnier SA, Malcolm W, Haig J, Xue W. Cost-effectiveness of ranibizumab versus aflibercept in the treatment of visual impairment due to diabetic macular edema: a UK healthcare perspective. Clinicoecon Outcomes Res. 2015;7:235-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1033] [Cited by in F6Publishing: 1030] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 77. | Krispel C, Rodrigues M, Xin X, Sodhi A. Ranibizumab in diabetic macular edema. World J Diabetes. 2013;4:310-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, Boyer D, Heier JS, Abraham P, Thach AB. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146-2151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 339] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 79. | Domalpally A, Ip MS, Ehrlich JS. Effects of intravitreal ranibizumab on retinal hard exudate in diabetic macular edema: findings from the RIDE and RISE phase III clinical trials. Ophthalmology. 2015;122:779-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 80. | Bressler NM, Varma R, Suñer IJ, Dolan CM, Ward J, Ehrlich JS, Colman S, Turpcu A. Vision-related function after ranibizumab treatment for diabetic macular edema: results from RIDE and RISE. Ophthalmology. 2014;121:2461-2472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 81. | Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 554] [Cited by in F6Publishing: 595] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 82. | Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1019] [Cited by in F6Publishing: 1135] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 83. | Liu XD, Zhou XD, Wang Z, Shen HJ. Comparison of intravitreal bevacizumab with macular photocoagulation for treatment of diabetic macular edema: a systemic review and Meta-analysis. Int J Ophthalmol. 2014;7:1048-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 84. | Poku E, Rathbone J, Wong R, Everson-Hock E, Essat M, Pandor A, Wailoo A. The safety of intravitreal bevacizumab monotherapy in adult ophthalmic conditions: systematic review. BMJ Open. 2014;4:e005244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 85. | Jin ZY, Zhu D, Tao Y, Wong IY, Jonas JB. Meta-analysis of the effect of intravitreal bevacizumab versus intravitreal triamcinolone acetonide in central retinal vein occlusion. J Ocul Pharmacol Ther. 2013;29:826-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130:972-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 87. | Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two-year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina. 2012;32:314-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, Ahmadieh H, Dehghan MH, Azarmina M, Moradian S. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116:1142-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 89. | Soheilian M, Ramezani A, Bijanzadeh B, Yaseri M, Ahmadieh H, Dehghan MH, Azarmina M, Moradian S, Tabatabaei H, Peyman GA. Intravitreal bevacizumab (avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina. 2007;27:1187-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 90. | Liu X, Zhou X, Wang Z, Li T, Jiang B. Intravitreal bevacizumab with or without triamcinolone acetonide for diabetic macular edema: a meta-analysis of randomized controlled trials. Chin Med J (Engl). 2014;127:3471-3476. [PubMed] [Cited in This Article: ] |
| 91. | Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247-2254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 555] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 92. | Available from: http: //www.eylea.us. [Cited in This Article: ] |
| 93. | Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 735] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 94. | Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, Berliner AJ, Gao B, Zeitz O, Ruckert R. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 95. | Campochiaro PA, Clark WL, Boyer DS, Heier JS, Brown DM, Vitti R, Kazmi H, Berliner AJ, Erickson K, Chu KW. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015;122:538-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 96. | Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U, Lorenz K, Honda M, Vitti R, Berliner AJ. Intravitreal Aflibercept Injection for Macular Edema Resulting from Central Retinal Vein Occlusion: One-Year Results of the Phase 3 GALILEO Study. Ophthalmology. 2014;121:202-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 97. | Evoy KE, Abel SR. Aflibercept: newly approved for the treatment of macular edema following central retinal vein occlusion. Ann Pharmacother. 2013;47:819-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Brown DM, Heier JS, Clark WL, Boyer DS, Vitti R, Berliner AJ, Zeitz O, Sandbrink R, Zhu X, Haller JA. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 99. | Kourlas H, Schiller DS. Pegaptanib sodium for the treatment of neovascular age-related macular degeneration: a review. Clin Ther. 2006;28:36-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 100. | Ishibashi T, Yuzawa M, Yoshimura N, Ohji M, Ishida S, Isogawa N, Esaka E. [Japan phase 3 study of pegaptanib sodium in patients with diabetic macular edema]. Nippon Ganka Gakkai Zasshi. 2014;118:773-782. [PubMed] [Cited in This Article: ] |
| 101. | Sivaprasad S, Browning RC, Starita C. An open-label, one-year, noncomparative study to evaluate the safety and tolerability of intravitreal pegaptanib sodium in patients with diabetic macular edema. Clin Ophthalmol. 2014;8:1565-1571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 102. | Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011;118:1107-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 103. | Wroblewski JJ, Wells JA, Gonzales CR. Pegaptanib sodium for macular edema secondary to branch retinal vein occlusion. Am J Ophthalmol. 2010;149:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Querques G, Bux AV, Martinelli D, Iaculli C, Noci ND. Intravitreal pegaptanib sodium (Macugen) for diabetic macular oedema. Acta Ophthalmol. 2009;87:623-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Cholkar K, Gunda S, Earla R, Pal D, Mitra AK. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS PharmSciTech. 2015;16:610-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 106. | Frost P, Berlanger E, Mysore V, Hoang B, Shi Y, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors induce tumor cell apoptosis in vivo primarily by inhibiting VEGF expression and angiogenesis. J Oncol. 2013;2013:897025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | Stahl A, Paschek L, Martin G, Gross NJ, Feltgen N, Hansen LL, Agostini HT. Rapamycin reduces VEGF expression in retinal pigment epithelium (RPE) and inhibits RPE-induced sprouting angiogenesis in vitro. FEBS Lett. 2008;582:3097-3102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 108. | Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, Benjamin LE. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol. 2009;29:1172-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 109. | National Eye Institute (NEI). Palomid 529 in Patients With Neovascular Age-Related Macular Degeneration. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: https: //clinicaltrials.gov/ct2/show/NCT01271270?term=NCT01271270&rank=1 NLM Identifier: NCT01271270. [Cited in This Article: ] |
| 110. | Nguyen QD, Ibrahim MA, Watters A, Bittencourt M, Yohannan J, Sepah YJ, Dunn JP, Naor J, Shams N, Shaikh O. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J Ophthalmic Inflamm Infect. 2013;3:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 111. | Dugel PU, Blumenkranz MS, Haller JA, Williams GA, Solley WA, Kleinman DM, Naor J. A randomized, dose-escalation study of subconjunctival and intravitreal injections of sirolimus in patients with diabetic macular edema. Ophthalmology. 2012;119:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Krishnadev N, Forooghian F, Cukras C, Wong W, Saligan L, Chew EY, Nussenblatt R, Ferris F, Meyerle C. Subconjunctival sirolimus in the treatment of diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2011;249:1627-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 113. | Bandello F, Casalino G, Loewenstein A, Goldstein M, Pelayes D, Battaglia Parodi M. Pharmacological approach to diabetic macular edema. Ophthalmic Res. 2014;51:88-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Stewart MW. Corticosteroid use for diabetic macular edema: old fad or new trend? Curr Diab Rep. 2012;12:364-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 115. | Wolfensberger TJ, Gregor ZJ. Macular edema--rationale for therapy. Dev Ophthalmol. 2010;47:49-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 117. | Nauck M, Roth M, Tamm M, Eickelberg O, Wieland H, Stulz P, Perruchoud AP. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol. 1997;16:398-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 170] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 118. | Das A, McGuire PG, Rangasamy S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology. 2015;122:1375-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 337] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 119. | Veritti D, Di Giulio A, Sarao V, Lanzetta P. Drug safety evaluation of intravitreal triamcinolone acetonide. Expert Opin Drug Saf. 2012;11:331-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Sears JE, Hoppe G. Triamcinolone acetonide destabilizes VEGF mRNA in Müller cells under continuous cobalt stimulation. Invest Ophthalmol Vis Sci. 2005;46:4336-4341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Tao Y, Jonas JB. Intravitreal triamcinolone. Ophthalmologica. 2011;225:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Jonas JB. Intravitreal triamcinolone acetonide for DR. Dev Ophthalmol. 2007;39:96-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | Jonas JB. Intravitreal triamcinolone acetonide: a change in a paradigm. Ophthalmic Res. 2006;38:218-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 124. | Jonas JB, Kreissig I, Kamppeter B, Degenring RF. [Intravitreal triamcinolone acetonide for the treatment of intraocular edematous and neovascular diseases]. Ophthalmologe. 2004;101:113-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 125. | Yumusak E, Buyuktortop N, Ornek K. Early results of dexamethasone implant, ranibizumab, and triamcinolone in macular edema due to branch retinal vein occlusion. Eur J Ophthalmol. 2015;26:54-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Bucolo C, Grosso G, Drago V, Gagliano C. Intravitreal triamcinolone acetonide in the treatment of ophthalmic inflammatory diseases with macular edema: a meta-analysis study. J Ocul Pharmacol Ther. 2015;31:228-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 127. | DR Clinical Research N; Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 128. | DR Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447-1449, 1449.e1-e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 379] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 129. | Yoo SG, Kim JH, Lee TG, Kim CG, Kim JW. Short-term efficacy of intravitreal triamcinolone acetonide for macular edema secondary to retinal vein occlusion that is refractory to intravitreal bevacizumab. Indian J Ophthalmol. 2015;63:25-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Thompson JT. Cataract formation and other complications of intravitreal triamcinolone for macular edema. Am J Ophthalmol. 2006;141:629-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 131. | Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology. 2005;112:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 132. | Moshfeghi DM, Kaiser PK, Bakri SJ, Kaiser RS, Maturi RK, Sears JE, Scott IU, Belmont J, Beer PM, Quiroz-Mercado H. Presumed sterile endophthalmitis following intravitreal triamcinolone acetonide injection. Ophthalmic Surg Lasers Imaging. 2005;36:24-29. [PubMed] [Cited in This Article: ] |
| 133. | Bhavsar AR, Ip MS, Glassman AR. The risk of endophthalmitis following intravitreal triamcinolone injection in the DRCRnet and SCORE clinical trials. Am J Ophthalmol. 2007;144:454-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 134. | Dodwell DG, Krimmel DA, de Fiebre CM. Sterile endophthalmitis rates and particle size analyses of different formulations of triamcinolone acetonide. Clin Ophthalmol. 2015;9:1033-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 135. | Cholkar K, Hariharan S, Gunda S, Mitra AK. Optimization of dexamethasone mixed nanomicellar formulation. AAPS PharmSciTech. 2014;15:1454-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 136. | Earla R, Boddu SH, Cholkar K, Hariharan S, Jwala J, Mitra AK. Development and validation of a fast and sensitive bioanalytical method for the quantitative determination of glucocorticoids--quantitative measurement of dexamethasone in rabbit ocular matrices by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2010;52:525-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 137. | Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, Garretson B, Gupta A, Hariprasad SM, Bailey C. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125-2132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 138. | Pearson PA, Comstock TL, Ip M, Callanan D, Morse LS, Ashton P, Levy B, Mann ES, Eliott D. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118:1580-1587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 139. | Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, Liu CC, Lou J, Whitcup SM. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 140. | Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, Welty D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 141. | Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, Whitcup SM. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 142. | Campochiaro PA, Hafiz G, Shah SM, Bloom S, Brown DM, Busquets M, Ciulla T, Feiner L, Sabates N, Billman K. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010;117:1393-1399.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 143. | Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 144. | Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078-1086.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 145. | Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, Abraham P, Campochiaro PA. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116:2175-2181.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 146. | Régnier S, Malcolm W, Allen F, Wright J, Bezlyak V. Efficacy of anti-VEGF and laser photocoagulation in the treatment of visual impairment due to diabetic macular edema: a systematic review and network meta-analysis. PLoS One. 2014;9:e102309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 147. | Kim JH, Kang SW, Ha HS, Kim JR. Vitrectomy combined with intravitreal triamcinolone acetonide injection and macular laser photocoagulation for nontractional diabetic macular edema. Korean J Ophthalmol. 2013;27:186-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 148. | Patel SP, Vaishya R, Yang X, Pal D, Mitra AK. Novel thermosensitive pentablock copolymers for sustained delivery of proteins in the treatment of posterior segment diseases. Protein Pept Lett. 2014;21:1185-1200. [PubMed] [Cited in This Article: ] |
| 149. | Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol. 2010;21:178-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 150. | Bertelmann T, Schulze S. Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant. Ophthalmol Ther. 2015;4:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 151. | Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG, Weber M, Danis RP, Kuppermann BD, Bailey C. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology. 2014;121:1892-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 152. | Adelman R, Parnes A, Michalewska Z, Parolini B, Boscher C, Ducournau D. Strategy for the management of diabetic macular edema: the European vitreo-retinal society macular edema study. Biomed Res Int. 2015;2015:352487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 153. | Misra GP, Singh RS, Aleman TS, Jacobson SG, Gardner TW, Lowe TL. Subconjunctivally implantable hydrogels with degradable and thermoresponsive properties for sustained release of insulin to the retina. Biomaterials. 2009;30:6541-6547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 154. | Arevalo JF. Diabetic macular edema: Current management 2013. World J Diabetes. 2013;4:231-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 155. | Diaz-Llopis M, Udaondo P, Millán JM, Arevalo JF. Enzymatic vitrectomy for DR and diabetic macular edema. World J Diabetes. 2013;4:319-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 156. | Zucchiatti I, Lattanzio R, Querques G, Querques L, Del Turco C, Cascavilla ML, Bandello F. Intravitreal dexamethasone implant in patients with persistent diabetic macular edema. Ophthalmologica. 2012;228:117-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 157. | Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, Aroney C, McAllister IL, Fraser-Bell S. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121:2473-2481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 158. | Cholkar K, Gilger BC, Mitra AK. Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery. Transl Vis Sci Technol. 2015;4:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 159. | Fujisawa T, Miyai H, Hironaka K, Tsukamoto T, Tahara K, Tozuka Y, Ito M, Takeuchi H. Liposomal diclofenac eye drop formulations targeting the retina: formulation stability improvement using surface modification of liposomes. Int J Pharm. 2012;436:564-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 160. | Feng L, Li SK, Liu H, Liu CY, LaSance K, Haque F, Shu D, Guo P. Ocular delivery of pRNA nanoparticles: distribution and clearance after subconjunctival injection. Pharm Res. 2014;31:1046-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 161. | Conley SM, Naash MI. Nanoparticles for retinal gene therapy. Prog Retin Eye Res. 2010;29:376-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 162. | Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS One. 2009;4:e7410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 163. | Mitra RN, Han Z, Merwin M, Al Taai M, Conley SM, Naash MI. Synthesis and characterization of glycol chitosan DNA nanoparticles for retinal gene delivery. ChemMedChem. 2014;9:189-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 164. | Panda JJ, Yandrapu S, Kadam RS, Chauhan VS, Kompella UB. Self-assembled phenylalanine-α,β-dehydrophenylalanine nanotubes for sustained intravitreal delivery of a multi-targeted tyrosine kinase inhibitor. J Control Release. 2013;172:1151-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 165. | Boddu SH, Jwala J, Vaishya R, Earla R, Karla PK, Pal D, Mitra AK. Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J Ocul Pharmacol Ther. 2010;26:37-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 166. | Yandrapu SK, Upadhyay AK, Petrash JM, Kompella UB. Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab. Mol Pharm. 2013;10:4676-4686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 167. | Patel SP, Vaishya R, Mishra GP, Tamboli V, Pal D, Mitra AK. Tailor-made pentablock copolymer based formulation for sustained ocular delivery of protein therapeutics. J Drug Deliv. 2014;2014:401747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 168. | Boyer DS, Yoon YH, Belfort R, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 546] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 169. | Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626-635.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |