Copyright
©The Author(s) 2016.
World J Pharmacology. Sep 9, 2016; 5(3): 59-67
Published online Sep 9, 2016. doi: 10.5497/wjp.v5.i3.59
Published online Sep 9, 2016. doi: 10.5497/wjp.v5.i3.59
Figure 1 Chromatogram amplification showing the sevoflurane peak at 2.
7 min in the different dilutions at -10 °C (A), 4 °C (B) and 25 °C (C).
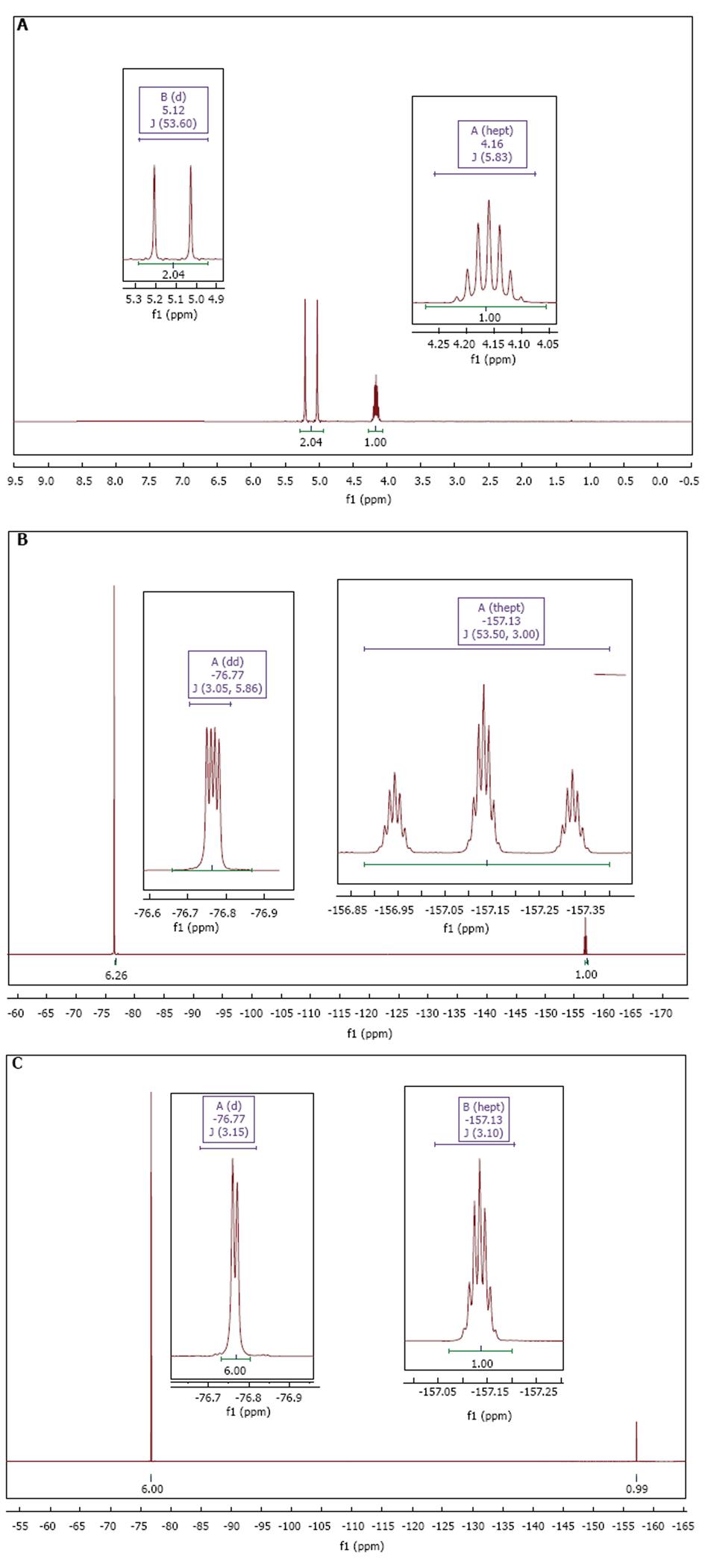
Figure 2 Undiluted sevoflurane nuclear magnetic resonance spectra.
A: 1H-NMR; B: Coupled 19F-NMR; C: Proton-decoupled 19F-NMR using a Waltz16 CPD pulse sequence. NMR: Nuclear magnetic resonance; CPD: Composite proton-decoupling.
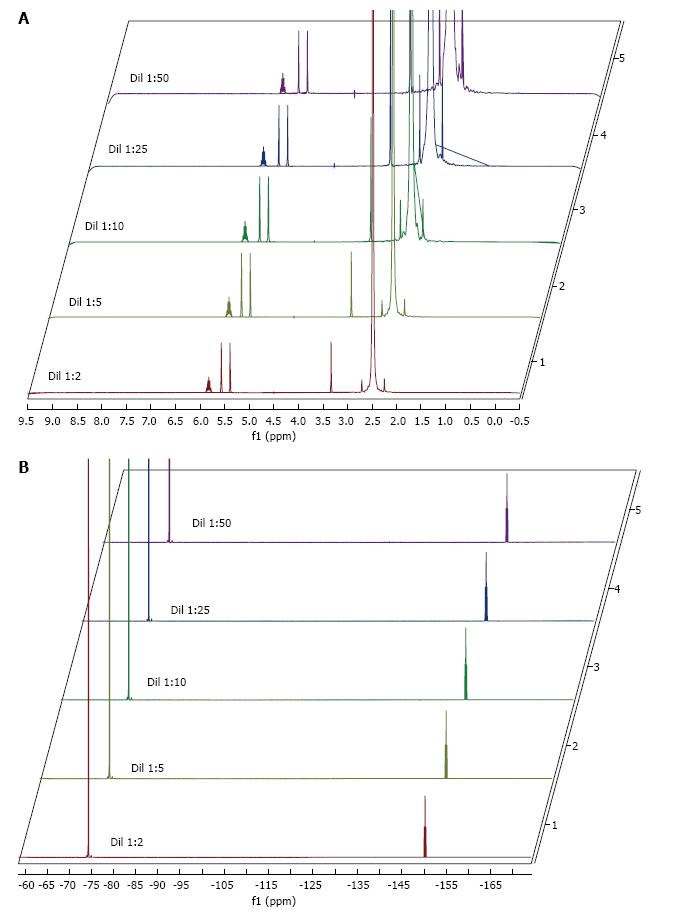
Figure 3 1H-nuclear magnetic resonance (A) and 19F-nuclear magnetic resonance (B) of solutions kept at all temperatures.
The images have been adjusted and sevoflurane peaks assigned the same height so that dimethyl sulfoxide and water signals in the 1H spectrum increase inversely to concentration.
- Citation: Fernández-Ginés FD, García-Muñoz S, Mateo-Carrasco H, Rincón-Cervera M&, Cortiñas-Sáenz M, Morales-Molina JA, Fernández-Sánchez C, Expósito-López JM, Rodríguez-García I. Innovate combination of sevoflurane dilution in dimethyl sulfoxide: A stability study by gas chromatography and nuclear magnetic resonance. World J Pharmacology 2016; 5(3): 59-67
- URL: https://www.wjgnet.com/2220-3192/full/v5/i3/59.htm
- DOI: https://dx.doi.org/10.5497/wjp.v5.i3.59